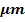Advances in Animal and Veterinary Sciences
Research Article
Effect of Using Different Concentrations of the Aqueous Extract for Thymus Leaves in some Physiological, Histological and Immunological Traits for Broiler Chicks
Jassim Kassim Menati1, Nihad Abdul-Lateef Ali2*, Hassan Saad Abidelhuseen3
1University of Al-Muthana, College of Agriculture; 2University of AL-Qasim Green, College of Agriculture; 3University of Baghdad, College of Agriculture, Iraq.
Abstract | This experiment was conducted at poultry field in the Agriculture College- Al-Muthana University during the period from 15/10 /2015 until 22/ 11/ 2015. It was used 240 of broiler chicks Ross 308, chicks were randomly distributed to four experimental treatments, where added the aqueous extract of thyme leaves at levels (0, 2, 4, 6 ml/L) drinking water. All of the histological traits were studied such as the villus height, the crypts depth , the percentage of the villus height to the crypts depth and immunological traits are both the immunity directed against Newcastle disease and the test of the relative hypersensitivity in thewattles and relative weight and the Fabricia gland index and the blood traits are both the number of red and white blood cells and volume of the blood packing cells (Haematocrit) and Hemoglobin concentration, and biochemical traits, such as concentration of glucose, cholesterol, triglyceride, globulin, albumin, and total protein. The results showed a significant improvement (P≤0.05) for the treatment of the aqueous extract of high concentration thyme leaves (6 ml/L drinking water) in all studied traits compared to all study treatment
Keywords | Aqueous extract, Thymus vulgaris, Small intestine tissue, Immunological, Blood traits, Broiler chicks.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 10, 2018; Accepted | August 12, 2018; Published | September 10, 2018
*Correspondence | Nihad Abdul-Lateef Ali, University of AL-Qasim Green, College of Agriculture, Iraq; Email: agricnano.egypt.2017@gmail.com
Citation | Menati JK, Ali NAL, Abidelhuseen HS (2018). Effect of using different concentrations of the aqueous extract for thymus leaves in some physiological, histological and immunological traits for broiler chicks. Adv. Anim. Vet. Sci. 6(10): 406-412.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.10.406.412
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Menati et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Thyme is a famous plant of the platoon Lamiaceae; it is widely cultivated in the Mediterranean countries, it characterized by a smell good with a hot and slightly bitter taste (Eqbal and Abdullah 2017). It is cultivated widely seeped in northern Iraq, it one of the most important medicinal plants, is a natural source of antioxidants (Al allaf, 2009). The most important of which are phenols and flavonoids (Barnes et al., 2002). Thyme is one of aromatic and medicinal plants which are used in food to prevent self-oxidation (Seo and Jeong, 2015).The leaves consider (containing 5-25% volatile oils) and the apical meristem are the most effective parts of thyme, and the most important compounds are effective phenolic compounds and Thymol and Carfcrol (Wright, 2000).Resin materials and linoleic acid (Watt, 1995), which have a role in preventing the growth of pathogenic microorganisms as they inhibit the growth of positive and negative germs (Priccaglia and Marotti, 1991).When Thymol concentration is 38-60% as for the effects of leaves of thyme plant on glucose, a study should be indicated (Amin Agha, 2002). Bulokbasi and Erhan (2007) demonstrated the possibility of using thyme as an antimicrobial agent for Escherichia coli when adding thyme leaves to white chicken feed, which resulted in a decrease in the number of bacteria in the contents of the waste, The use of thyme in broiler chicks rations significantly reduced the number of microbiological organisms due to increased content of thymol compounds (Haselmeyer et al., 2015).
This study is conducted to investigate the effect of aqueous extracts on thyme leaves in some physiological, histological and immunological traits of broiler chicks Ross 308.
MATERIALS AND METHODS
Design of The Experiment
This experiment was conducted at poultry field in the Agriculture College- Al-Muthana University during the period from 15/10 /2015 until 22/ 11/ 2015. It was used 240 of broiler chicks Ross 308, with one day age and 40 g weight. The chicks were breaded in a hall with 40 m x 10 m dimension in four-floor cages. Each floor contains a cage of 1.5 x 1 m, the chicks were randomly distributed in three experimental treatments with 60 chicks per treatment and three replicates of treatment (20 chick / replicate) as following:
First treatment: (treatment of control without any addition).
The second treatment: add of the aqueous extract of thyme leaves with a concentration of (2 ml extract / liter) drinking water.
The third treatment: add aqueous extract of thyme leaves with a concentration of (4 ml extract / liter) drinking water.
The fourth treatment: Add the aqueous extract of thyme leaves with a concentration of (6 ml extract / liter) drinking water.
The chicks were fed until the age of 22 days on the initiator’s ration and from 23 days until the age of marketing 35 days on the growth ration as shown in Table 1, feed was provided in free form.
Table 1: Composition of basal diet
| Ingredient (%) |
Starter diet 1-21 day |
Finisher diet 22-35 day |
| Yellow corn | 44.9 | 53.10 |
| Wheat | 18.0 | 15 |
| Soybean meal (44%) | 33 | 27 |
|
Vegetable oil |
2 | 3 |
| Limestone | 0.8 | 0.6 |
| Dicalcium phosphate | 0.3 | 0.3 |
| Mineral and vitamin premix* | 1 | 1 |
| Total | 100 | 100 |
|
Calculated composition2 |
||
| Metabolizable energy(Kcal /Kg) | 2990 | 3100 |
| Crude protein (%) | 21.92 | 19.70 |
| Methionine | 0.55 | 0.50 |
| Folic acid ( %) | 1.1 | 1.2 |
| Calcium (%) | 0.93 | 0.85 |
| Phosphorus available (%) | 0.48 | 0.45 |
| Lysine (%) | 1.35 | 1.25 |
| Methionine + Cysteine (%) | 0.85 |
0.91 |
*produced by Ghadeer Babylon, calculated according to the chemical composition of feedstuff contained in NRC (1994). ** Each 3.0 kg of the Vit. and Min. premix contains : Vit. A, 12000000 IU; Vit.D3 2500000.IU; Vit.E, 10 g; Vit.K, 2.5 g; Vit.B1, 1.5 g; Vit. B2,5 g; Vit. B6, 1.5 g; Vit. B12, 10 mg; Choline chloride, 1050 g; Biotin, 50 mg;Folic acid, 1 g; Nicotinic acid, 30 g; Capantothenate, 10 g; Zn, 55 g;Cu, 10 g; Fe, 35 g; Co, 250 mg; Se, 150 mg; I, 1 g; Mn, 60 g and anti-oxidant, 10 g.
Preparation of the extraction: The dried thyme leaves were brought from local markets in Muthanna province and tested using a small electric mill to be as powder, and then prepare the extract according to the method of (Hernandez et al., 1994) modified by mixing the amount of dry powder with the amount of distilled water by 1 g: 2 ml using the electric mixer and display the solution to 60°C for one hour and leave the solution for 24 hours at room temperature, The solution was then filtered by several layers of Sterilizer medical gauze to be ready for use in the experiment.
Studied Traits
Histological traits: At the end of the experiment, at the age of 35 days, 3 birds of each replicate were slaughtered by 9 birds per treatment. Three parts of the small intestine were taken (Duodenum, Jejunum, ileum). The length of the cut was 4 cm. After the contents were removed and washed several times with water, the samples were prepared according to (Tako et al., 2004) method. The histological slides were examined using a compound microscope and all measurements were recorded using the Ocular Micrometer with a 400 x magnification force after calibration to the stage micrometer and the villus length, crypts depth and the ratio of villus length to crypts depth (v/c) was estimated. The villus length was measured from the top of the villus until it is associated with the crypts, the crypts depth is the distance of immersing the neighboring villus (Uni, et al., 1999).
Immune traits: The immunological traits were both the Enzyme-linked immunosorbent assay testing (ELISA) according to (Voller et al., 1977) method, the test of hypersensitivity in the wattles (Al defeay, 2000, Al-Murrani et al., 1995). The relative weight of Fabricia’s gland and Fabricia’s index, as indicated by the researchers (Lucio and Hitchner, 1979).
Bloods traits: Blood samples were collected at the end of the fifth week by taking blood samples from the vena cava. Six birds from each treatment were slaughtered and blood collected by 10 ml glass tubes containing no anticoagulant and were placed horizontally to get rid the thrombus (fibrinogen proteins) to study both the number of blood Red (RBC) and White (WBC) cells, volume of packed cell (PCV) (Archer, 1965) and hemoglobin concentration (Hb) (Varley et al., 1980).The other samples were placed in
Table 2: Effect of the use of different concentrations of the aqueous extract of thyme leaves in the villus height, the crypts depth and the percentage of the villus height to the crypts depth in the Duodenum of the broiler chicks ± the standard error.
| Treatments | Duodenum | ||
|
Villus height ( |
Crypts depth ( |
The percentage of the villus height to the crypts depth in the Duodenum | |
| T1 |
d 95.24± 1.13 |
c12.46± 0.02 |
7.64± 0.02 |
| T2 |
c101.96± 2.36 |
b 13.19 ±0.47 |
7.73 ± 0.02 |
| T3 |
b 105.24± 1.89 |
ab13.51± 0.50 |
7.79 ± 0.09 |
| T4 |
a108.82± 1.46 |
a 13.91 ± 0.50 |
7.82 ± 0.11 |
| significant level | * |
* |
N.S |
T1 First treatment: control treatment. T2 Second treatment: 2 ml aqueous extract of the thyme / liter drinking water. T3 Third treatment: 4 ml aqueous extract of the thyme / liter drinking water. T4 Fourth treatment: 6 ml aqueous extract of the thyme / liter drinking water. N.S indicates that there are no significant differences between the averages of treatment. * The different letters within the same column indicate significant differences between totals at the probability level of 0.05.
Table 3: Effect of the use of different concentrations of the aqueous extract of thyme leaves in the villus height, the crypts depth and the percentage of the villus height to the crypts depth in the Jejunum of the broiler chicks ± the standard error.
| Treatments | Jejunum | ||
|
Villus height ( |
Crypts depth ( |
The percentage of the villus height to the crypts depth in the Duodenum | |
| T1 |
d 79.37 ± 0.63 |
b13.85± 0.11 |
b 5.73 ± 0.009 |
| T2 |
c82.32± 0.35 |
ab 14.12 ± 0.04 |
ab5.82 ± 0.011 |
| T3 |
b 85.33± 0.35 |
ab 14.39 ± 0.03 |
ab5.93 ± 0.007 |
| T4 |
a88.47± 0.51 |
a14.72± 0.03 |
a6.01 ± 0.006 |
| significant level | * |
* |
* |
T1 First treatment: control treatment. T2 Second treatment: 2 ml aqueous extract of the thyme / liter drinking water. T3 Third treatment: 4 ml aqueous extract of the thyme / liter drinking water. T4 Fourth treatment: 6 ml aqueous extract of the thyme / liter drinking water* the different letters within the same column indicate significant differences between totals at the probability level of 0.05.
Table 4: Effect of the use of different concentrations of the aqueous extract of thyme leaves in the villus height, the crypts depth and the percentage of the villus height to the crypts depth in the ileum of the broiler chicks ± the standard error.
| Treatments | Ileum | ||
|
Villus height ( |
Crypts depth ( |
The percentage of the villus height to the crypts depth in the Duodenum | |
| T1 |
c 33.54 ± 0.27 |
b 7.42 ± 0.56 |
b 4.52 ± 0.006 |
| T2 |
b 37.69 ± 0.16 |
ab 7.83 ± 0.27 |
ab 4.82 ± 0.005 |
| T3 |
ab 39.28 ± 0.12 |
ab 7.92 ± 0.15 |
ab 4.96 ± 0.005 |
| T4 |
a 41.24 ± 0.15 |
a 8.07 ± 0.18 |
a 5.11 ± 0.004 |
| significant level | * |
* |
* |
T1 First treatment: control treatment. T2 Second treatment: 2 ml aqueous extract of the thyme / liter drinking water. T3 Third treatment: 4 ml aqueous extract of the thyme / liter drinking water. T4 Fourth treatment: 6 ml aqueous extract of the thyme / liter drinking water.* the different letters within the same column indicate significant differences between totals at the probability level of 0.05.
the centrifuge at 3000 cycles / min for 15 minutes. Serum was stored in other sterilized tubes at a temperature of -18 ° C for the purpose of conducting laboratory analyzes and in accordance with instructions attached to kits for the purpose of estimating the concentration of glucose, Cholesterol, triglycerides, globolins (Monika and Warhorn, 2012), albumins (Maxwell et al., 1992) and total protein (Wooton, 1964).
Statistical Analysis
The completely randomized design (CRD) was used to study the effect of different coefficients in the studied traits. Significant differences between the averages were measured by a Duncan test (1955) multidimensional test
Table 5: Effect of the use of different concentrations of the aqueous extract of thyme leaves in the immune response to broiler chicks ± the standard error.
| Treatment | Cellular immunity (DTH) | Enzyme-linked immunosorbent assay (ELISA) | The relative weight of Fabricia's gland | The Fabricia gland index |
| T1 |
c 0.166±0.001 |
c 2434.6 ± 89.37 |
c 0.059 ± 0.001 |
d 1.000 ± 0 |
| T2 |
b 0.198±0.002 |
b 2732.2 ± 56.66 |
b 0.086 ± 0.001 |
c 1.458 ± 0.010 |
| T3 |
ab 0.217±0.001 |
ab 2846.1 ± 40.12 |
ab 0.098 ± 0.001 |
b 1.661 ± 0.012 |
| T4 |
a 0.234±0.001 |
a 2929.8 ± 77.06 |
a 0.112 ± 0.001 |
a 1.898 ± 0.011 |
| Significant level | * |
* |
* |
* |
T1 First treatment: control treatment. T2 Second treatment: 2 ml aqueous extract of the thyme / liter drinking water. T3 Third treatment: 4 ml aqueous extract of the thyme / liter drinking water. T4 Fourth treatment: 6 ml aqueous extract of the thyme / liter drinking water.* the different letters within the same column indicate significant differences between totals at the probability level of 0.05.
Table 6: Effect of the use of different concentrations of the aqueous extract of thyme leaves in both the number of blood cells (white and red) and the packing cells volume (PCV) and concentration of hemoglobin (Hb) in blood of broiler chicks, ± the standard error
| Treatment | Number of red blood cells (106/ml3) | Number of white blood cells (106/ml3) | PCV (%) | Hb (g/100ml) |
| T1 |
c2.19±0.02 |
c20.26±0.09 |
c 26.77±0.13 |
c 8.38±0.05 |
| T2 |
b2.37±0.01 |
b 23.33±0.11 |
b 28.46±0.05 |
b 8.89±0.04 |
| T3 |
ab2.42±0.03 |
b24.51±0.07 |
ab28.94±0.09 |
ab9.22±0.03 |
| T4 |
a2.57±0.01 |
a25.72±0.05 |
a 29.39±0.11 |
a9.64±0.02 |
| Significant level | * |
* |
* |
* |
T1 First treatment: control treatment. T2 Second treatment: 2 ml aqueous extract of the thyme / liter drinking water. T3 Third treatment: 4 ml aqueous extract of the thyme / liter drinking water. T4 Fourth treatment: 6 ml aqueous extract of the thyme / liter drinking water.* the different letters within the same column indicate significant differences between totals at the probability level of 0.05.
Table 7: Effect of the use of different concentrations of the aqueous extract of thyme leaves in the concentration of Glucose, cholesterol, triglyceride, total protein, albumin and globulin in plasma of broiler chicks, ± the standard error
| Treatment | Glucose (mg /100 ml) | Cholesterol (mg /100 ml) | Triglyceride (mg /100 ml) | Total protein (g /100ml) | Albumin (g /100ml) | Globulin (g /100ml) |
| T1 |
c 158.21±0.50 |
a 192.52±0.79 |
a 65.73±0.37 |
c1.18±0.01 |
c1.06±0.01 |
c2.24±0.03 |
| T2 |
bc164.73±0.56 |
b 179.76±0.41 |
b 59.44±0.13 |
b1.37±0.01 |
b1.28±0.01 |
b2.65±0.04 |
| T3 |
ab172.55±1.11 |
b 174.83±0.16 |
bc56.79±0.40 |
b1.46±0.02 |
b 1.35±0.02 |
b2.81±0.01 |
| T4 |
a 184.69±0.93 |
c 166.17±0.33 |
c 53.90±0.05 |
a1.61±0.01 |
a1.51±0.01 |
a3.12±0.01 |
| Significant level | * |
* |
* | * | * |
* |
T1 First treatment: control treatment. T2 Second treatment: 2 ml aqueous extract of the thyme / liter drinking water. T3 Third treatment: 4 ml aqueous extract of the thyme / liter drinking water. T4 Fourth treatment: 6 ml aqueous extract of the thyme / liter drinking water.* the different letters within the same column indicate significant differences between totals at the probability level of 0.05.
under a significant level of 0.05 and 0.01. SAS (2001) was used in statistical analysis.
RESULTS
Histological Traits
Tables (2, 3, 4) show the effect of using different concentrations of thyme extract in both the height of the villus, the depth of the crypts and the percentage of the height of the villus to the depth of the crypts in the parts of the small intestine (Duodenum, Jejunum, ileum) for broiler chicks. It is noted that the treatment T4 was significantly increased (P≤0.05) in the villus height in both the Duodenum and Jejunum compared to the treatment T3 excelled in turn significantly (P≤0.05) compared to treatment T2, which also excelled significantly (P≤0.05). While treatment T4 (P≤0.05) was excelled in the villus height in ileum and depth of crypts in duodenum than in T2, which was significantly excelled (P≤0.05) compared to control treatment.The treatment of T4 was significantly excelled (P≤0.05) compared to the control treatment at the depth of the crypts and the percentage of the height of the villus to the depth of the crypts in both the Jejunum and ileum, There were no significant differences between all the parameters in the ratio of the height of the villus to the depth of the crypts in the Duodenum.
Immune Traits
Table 5 illustrates the effect of using different concentrations of the aqueous extract for thyme leaves in the immune response to broiler chicks. It is noted that the treatment T4 was significantly excelled (P≤0.05) compared to the treatment T2 excelled in turn significantly (P≤0.05) compared to control treatment in both cellular immunity (DTH) and immunity directed against Newcastle disease and the relative weight of Fabricia’s gland, while no significant differences were observed between T3 and T4, As for the Fabricia gland index, the treatment T4 was significantly excelled (P≤0.05) compared to the treatment T3 which excelled in turn significantly (P≤0.05) compared to treatment T2, which was significantly excelled compared to the control treatment.
Bloods Traits
Table 6 shows the effect of using different concentrations of the aqueous extract for thyme leaves in some blood traits for broiler chicks. It is noted that the treatment T4 was significantly excelled (P≤0.05) compared to the treatment T2 excelled in turn significantly (P≤0.05) compared to control treatment in both the number of red blood cells and the packing cells volume (PCV) and concentration of hemoglobin (Hb), The above table indicates that the T4 treatment is significantly excelled (P≤0.05) on both T2 and T3, which is significantly excelled (P≤0.05) on the control treatment.
Table 7 shows the effect of using different concentrations of the aqueous extract for thyme leaves in some of the biochemical traits for plasma of broiler chicks. There is significant decrease in both concentration, cholesterol and triglycerides in T4 treatment compared to T1 and T2 treatments with significant increase (P≤0.05) in the concentration of glucose, albumin, globulin and total protein in the same treatment compared to the two treatments T2 and T3 (P≤0.05) which are significantly excelled compared to control treatment.
DISCUSSION
Histological Traits
The use of the aqueous extract of thyme leaves, improved all the studied tissue traits in the intestines of broiler chicks. The containment of thyme leaves on thymol compounds, which has an important role in improving the health status of birds by increasing their resistance to pathogens and toxins, which led to increased cellular circulation, Which speeds up the regeneration process (Jang et al., 2007).The leaves of thyme, like some medicinal plants contain some effective compounds such as aromatics and vegetable oils, which reduce the various intestinal infections as a result of its high activity to resist pathogenic bacteria, as well as the increase of mature enterocytes of which increases the villus height and the crypts depth and thus increase their absorption efficiency (Zeng et al., 2015).
Immune Traits
Panizzi et al. (1993) noted that thyme has a significant inhibitory effect against pathogenic bacteria compared with antibiotics such as Tetracycline, Gentamycin and Ciprofloxacin due to its containment of some active compounds, essential oils and Thymol,and between Settiner and Krassner (2003) that Thymol has the ability to dissolve the internal membrane of pathogenic bacteria, which is carried out into the bacterial cell, Affecting their basic components.Morphological improvement in both cellular immunity (DTH), immunity against Newcastle disease and the relative weight of fabricia at the high levels of the aqueous extract of thyme leaves may be due to the traits of the extracts which made it have a good and effective effect on the functions of the body. As well as contain compounds such as phenolic compounds (Thymol and carafacrol) in addition to contain vitamin E, which makes the level of antibodies high and therefore the immunity of the body of birds are highly resistant to pathogens (Tawfik, 2002, Najafi and Torki, 2010) noted that the use of medicinal plants, including thyme, may improve the immune system of broiler chicks due to the development of the ratio between T lymphocytes cells assistance and inhibitory, Enhancing the activity of natural killer cells or activating B lymphocytes on the production of antibodies and stimulating the bone marrow to produce white blood cells and increase the production of some staph lymphocytes (Cytokines) of lymphocytes because of the effect of active compounds and most important resin materials such as tannin and thymine.
Bloods Traits
The aqueous extract of thyme leaves is better than the blood traits of broiler chicks. The increase in red blood cell count may be due to the need of the body to meet the requirements of transporting food and oxygen to the body cells due to the increase in the metabolism of birds or it may be due to the effect of phenolic compounds which have a role in protecting blood cells from damage caused by oxidation (Burton and Guin, 1968). As well as Tannin also plays an active role in stimulating the production of white blood cells in the blood of broilers chicks (Youssef, et al., 2017). The significant increase in Hematocrit and hemoglobin concentration was due to increased red blood cell count. Al-Jashaamy (2011) noted that the significant increase in red blood cell count has led to a significant increase in all the volume of blood cells and concentration of hemoglobin.The results indicate that the concentration of blood glucose has increased significantly in the high levels of the aqueous extract of thyme leaves may be due to the effect of the corridors of thyme similar to Glucocorticoids, causing an increase in the level of glucose in blood plasma of birds resulting from the formation of glucose from non-carbohydrate sources through the process of gluconeogenesis (Fallah and Mirzaei, 2016). It is also noted that the concentration of total protein has increased in the treatment of the aqueous extract of thyme leaves compared to the control treatment, may be because the containment of thyme leaves on both lyciccerin and klcselratik acid, which have a role similar to steroid hormones, which increase the composition of proteins as well as reduce the degradation, The growth of muscles and bones in addition to their significant role in increasing metabolic rate (Kuhn, 1993). The protein concentration in blood plasma is due to increased metabolism of protein in blood plasma. Where plasma proteins, especially albumin, carry carbohydrates, fatty acids, vitamins, certain mineral elements and certain hormones such as thyroxine (Varley et al., 1980). As increased levels of thyme will work to reduce cholesterol in plasma blood, Gumus et al. (2017) noted that thyme leaves have a role in lowering levels of cholesterol and triglycerides in blood plasma due to increased metabolism, which is mainly reflected by increased thyroid activity and increased secretion of thyroxine.
ACKNOWLEDGMENTS
I give my great thanks to the Deanship of the college of Agriculture - Muthanna University and the station of research and agricultural experiments to facilitate procedures for the scientific research of birds and feed. As well as the use of the poultry hall in this research.
COnflict of interest
This research is a personal non-profit work and there is no conflict of interest.
Authors Contribution
Jassim Kassim Menati is responsible for animal work and samples collection. Nihad Abdul-Lateef Ali and Hassan SaadAbidelhuseen are responsible for data analysis, writing correction and proof reading.
REFERENCES