Advances in Animal and Veterinary Sciences
Research Article
In vitro Inhibition Kinetics of Crocodylus mindorensis (Philippine crocodile) Serum against Human Immunodeficiency Virus Type I Reverse Transcriptase
Alfredo A. Hinay Jr, Lilen Dorothy C Sarol
Department of Microbiology, College of Public Health, University of the Philippines, Philippines.
Abstract | The study aimed to determine the inhibition kinetics of Crocodylus mindorensis (Philippine crocodile) serum against recombinant HIV-1 Reverse Transcriptase (RT) using Enzyme-linked Immunosorbent Assay. The study utilized an experimental design where different concentrations of Philippine crocodile serum were used to determine its quantitative inhibitory effect and mechanism of inhibition against the HIV-1 reverse transcriptase activity. A colorimetric enzyme immunoassay that incorporates digoxigenin and biotin-labeled dUTP onto DNA were used for the assay (Roche Version 13.0, 2010). In addition, the kinetic activity of HIV-1 Reverse transcriptase was studied to determine the mechanism of enzyme inhibition by Philippine crocodile serum. A minimum of 2 mL serum sample from each of the seven (7) purposively selected Crocodylus mendoremsis were collected at the Animal clinic of the Davao Crocodile Park facility. The results showed that the HIV-1 Reverse transcriptase activity was inhibited by high as 92.93±0.72% (CM-07) at 0.5 vol/vol % and as low as 4.66±20.76 at 4 vol/vol % (CM-04) of Philippine crocodile serum. The average half maximal inhibitory concentration (IC50) of the seven crocodile serum samples was 1.47% vol/vol.The 92.93±0.72% inhibition of 0.5% vol/vol of Philippine crocodile serum has almost the same inhibition as the positive control Nevirapine (92.64±0.20%). The inhibition kinetics of the Philippine crocodile suggests a potential novel HIV-1 Reverse Transcriptase inhibitor.
Keywords | HIV-1, Reverse transcriptase inhibitors, Philippine crocodile, Competitive inhibitors, Inhibition kinetics
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 03, 2018; Accepted | May 09, 2018; Published | May 31, 2018
*Correspondence | Alfredo A Hinay Jr, Department of Microbiology, College of Public Health, University of the Philippines, Philippines; Email: redhinayjr@yahoo.com
Citation | Hinay AA, Sarol LDC (2018). In vitro inhibition kinetics of crocodylus mindorensis (philippine crocodile) serum against human immunodeficiency virus type i reverse transcriptase. Adv. Anim. Vet. Sci. 6(5): 213-218.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.5.213.218
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Hinay and Sarol. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The HIV epidemic is one of the biggest challenges in public health. In 2014, approximately 36.9 million people were living with HIV. Moreover, nearly 8 million AIDS-related deaths occurred since 2000. In South and South East Asia, where 30% of the global population is found, it was estimated that 4 million cases of HIV with 240,000 deaths have occurred in 2015 (UNAIDS, 2015).
In the 2013 UNAIDS Asian and Pacific report, the Philippines was identified as one of the seven (7) Asian countries with an increased incidence rate (>25%) together with Armenia, Bangladesh, Georgia, Kazakhstan, Kyrgyzstan, and Tajikistan. As of March 2016, the Department of Health (DOH) AIDS Registry in the Philippines has reported 32,647 people living with HIV/AIDS. In addition, 736 cases of HIV-1 seropositive individuals was confirmed by the STD/AIDS Cooperative Central Laboratory (SACCL). Compared to the same period last year, an increase of 10% in the number of cases was noted. To date, only 13,387 people living with HIV are under Anti-Retroviral Therapy (ART) or have access to the antiretroviral drugs through the 22 treatment hubs in the country (Department of Health, 2016).
Highly-Active Antiretroviral Therapy (HAART) is the recommended treatment and management strategy for HIV infection. Anti-HIV-1 drug combinations inhibit viral replication, allowing infected individuals to live longer and healthier lives (U.S. Department of Health and Human Services, 2012). Nucleoside and non-nucleoside reverse transcriptase, fusion, integrase and protease inhibitors are classes of synthetic-derived anti-HIV medications approved by the U.S. Food and Drug Administration (FDA) for the treatment of HIV-1/AIDS (Asres et al., 2001; Vermani and Garg, 2002). Although the existing antiretroviral drugs are very significant in improving the quality and extending the lives of HIV/AIDS individuals, the drugs still have many limitations including the development of resistance, toxicity due to drug interactions and their limited availability. These limitations continue to open new opportunities in the use of ethnomedicine for the management of HIV/AIDS (Merchant, 2005).
Materials and Methods
Specimen Collection
A minimum of 2 mL serum sample from each of the seven (7) purposively selected male Crocodylus mindorensis were collected at the Animal Clinic of the Davao Crocodile Park facility. The serum was separated from archived whole blood sample collected by a veterinarian as part of the health check of the crocodile. Specifically 5 mL of whole blood was allowed to clot at room temperature for approximately three (3) hours. The serum was separated by centrifugation at 3,000 x g for 15 minutes and frozen at -20°C until ready for use.
HIV-1 RT Inhibition Assay
The colorimetric reverse transcriptase immunoassay kit (Roche, Applied Science) was utilized to measure the inhibitory activity of Crocodylus mindorensis (Philippine crocodile) serum. The recombinant HIV-1 Reverse transcriptase contained in the kit was prepared using autoclaved redistilled water into 10mU/uL final concentration. After the preparation, the crocodile serum (4%, 2%, 1%, and 0.5%) was added to the mixture and incubated for 1 hour at 370C. Nevirapine used as the positive control was prepared the same way as the crocodile serum. Both test serum sample and positive control were run in triplicates. Thereafter, the mixtures were dispensed into the microplate wells and incubated for another hour at 370C. The assay well was washed 5 times with the washing buffer contained in the kit using an automated ELISA microplate well washer. Anti-digoxigenin-peroxidase working solution was then added to the microplate and the step followed by 1-hour incubation at 370C. The microplate was again washed 5 times, after which ABTS substrate solution was added into each well. The absorbance of the product was measured at 405nm using an enzyme-linked Immunosorbent assay reader.
Reverse Transcriptase Inhibitor Kinetics Assay
The inhibition mechanism of Crocodylus mindorensis (Philippine crocodile) serum was determined through calculation of the Reverse transcriptase Theoretical Maximum velocity (Vmax), Kinetic activator constant (Km) and Initial Velocity (Vi) in the presence and absence of the Crocodylus mindorensis (Philippine crocodile) serum. To determine the values for these parameters, the same standard colorimetric assay procedure for the determination of retroviral reverse transcriptase described earlier was carried-out. However, the concentrations of the substrates were varied to 10, 5, 2.5, 1.125 and 0.625 uM. Moreover, incubation periods of 30, 52, 80, 105 and 130 minutes were adopted for the experiment.
The resulting average change in optical density with respect to the substrate and incubation period was plotted and regarded as the Vi of the enzymatic reaction. Using the Michaelis–Menten equation below,
Vi = (Vmax × S) / (KM + S)
where Vi=initial rate; Vmax = maximum rate; KM = Michaelis constant, and S = substrate concentration, Vmax was determined. The resulting figures were graphed and translated into a Lineweaver-Burke plot where the linear-regression pattern of each concentration of the inhibitor determined the inhibitory activity of the Crocodylus mindorensis (Philippine crocodile), expressed either as competitive, uncompetitive or non-competitive inhibition.
Data Analysis
The results of anti-HIV-1 reverse transcriptase activity were expressed as means ±SD in triplicates. The Percent (%) Inhibition, Theoretical Max Velocity (Vmax), Kinetic activator constant (Km), and Inhibition constant (Ki) were calculated using the Microsoft Excel programme and GraphPad software.
Ethical Considerations
No ethical concerns were involved in the study since there were no human subjects involved and specimens used were already archived samples which form part of the health check-up of the animals.
Results
Inhibition of HIV-1 Reverse Transcriptase
Philippine crocodile serum samples from seven (7) purposively selected male crocodiles collected by a veterinarian during a health check and which were archived for at least one week were utilized for the inhibition assay. Inhibition of HIV-1 Reverse Transcriptase was tested using the ELISA method with the non-nucleoside, non-competitive inhibitor nevirapine as a positive control. The different concentrations of each crocodile serum were 4% vol/vol, 2% vol/vol, 1% vol/vol and 0.5% vol/vol prepared in redistilled water. For the quantification of the effect of Reverse Transcriptase inhibitors, a colorimetric recombinant reverse transcriptase assay was used. The activity of Reverse Transcriptase inhibitors was calculated as percent inhibition compared to a sample that does not contain an inhibitor.
Figure 1 shows the inhibitory activity of the seven (7) coded Philippine crocodile serum samples (CM-01 to CM-07). Reverse Transcriptase activity at 4% vol/vol concentration inhibited 35±48%, 5±21%, 52±9%, 5±5%, 17±28%, 37±4%, and 13±0.0% compared to 0.5% vol/vol concentration which inhibited Reverse Transcriptase activity of 77±10%, 80±10%, 90±10%, 82±10%, 79±0.26%, 87±1% and 93±0.72% respectively. The positive control nevirapine at 1,250 ug/mL concentration inhibited 92.64% of HIV-1 Reverse Transcriptase activity. The crocodile serum CM-07 showed the highest % inhibitory activity which was comparable to the % inhibition of the positive control, nevirapine. The variability between the inhibition activities observed among the seven serum samples could be attributed to the different physico-chemical properties of a single compound substance such as synthetic and commercialized drugs (nevirapine) versus mixed compounds seen in crocodile serum.
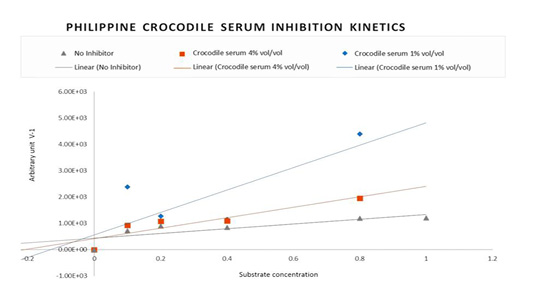
Figure 1: Percent (%) Inhibition and Logarithmic calculation of Half Maximal Inhibitory concentration of the Philippine crocodile serum.
Half Maximal Inhibitory Concentration
The values generated were used to determine the logarithmic equation through Microsoft Excel application to compute for the half maximal inhibitory concentration of the Philippine crocodile sera. Using the linear equation defining the linear regression model, the theoretical half maximal concentration of the seven (7) Philippine crocodile serum samples varies from 1.29 to 1.70% vol/vol with the average of 1.47% vol/vol.
Inhibition Kinetics of HIV-1 Reverse Transcriptase
The kinetic activity of HIV-1 Reverse transcriptase was studied to determine the mechanism of enzyme inhibition by Philippine crocodile serum. To determine the values of the Reverse transcriptase Theoretical Maximum velocity (Vmax), Kinetic activator constant (Km) and Initial Velocity (Vi) in the presence and absence of the Crocodylus mindorensis (Philippine crocodile) serum samples, the concentrations of the substrate were varied into 10, 5, 2.5, 1.125 and 0.625 uM and the incubation period into 30, 52, 80, 105 and 130 minutes. To produce a plot for the linear equivalent Lineweaver-Burke, two inhibitor concentrations were used; the highest concentration (4% vol/vol) and the mid-lowest concentration (1% % vol/vol). Nevirapine was not run as it is a known non-competitive inhibitor.
The average change in absorbance in five incubation periods was determined to generate the Initial velocity. The initial velocity of HIV-1 Reverse transcriptase is equivalent to the inverse average change of absorbance readings in different substrate concentrations and incubation times. Graphpad Prism 7.0 generated the changes in HIV-1 RT activity reflected as initial velocity with respect to substrate concentrations used to measure the Michaelis-Menten coefficient (Km) and the Maximum Velocity of Enzyme Activity. As calculated the Vmax is 0.00138, 0.001032 and 0.0007224 with Km of 0.5964, 0.4389 and 0.7665 respectively.
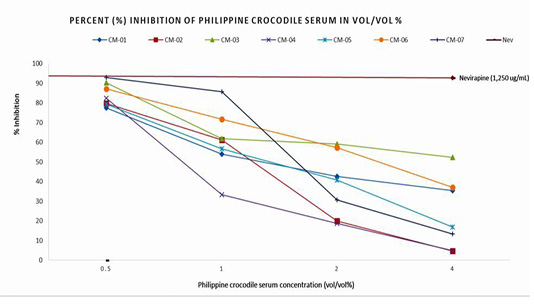
Figure 2: Lineweaver-Burke Plot: Non-competitive Inhibition kinetic pattern of HIV-1 Reverse Transcriptase with Philippine crocodile serum.
Reverse transcriptase Theoretical Maximum velocity (Vmax), Kinetic activator constant (Km) and Initial Velocity (Vi) in the presence and absence of the Crocodylus mindorensis (Philippine crocodile) serum were also used to plot for the linear equivalent Lineweaver-Burke. Figure 2 shows the Inhibition Kinetics of Philippine crocodile serum CM-07. The Lineweaver-Burk double reciprocal plot for this set of data shows a series of lines converging on the same point on the X (1/S) axis where the Km is unchanged, but Vmax is reduced. The figure describes the activity of the Philippine crocodile serum to be Non-competitive.
The initial velocity of HIV-1 Reverse transcriptase is equivalent to the inverse average change of absorbance readings in different substrate concentrations and incubation times. As a result, the inverse average change of absorbance was used to plot the Lineweaver-Burke to determine the inhibition pattern.
Discussion
In this study, the potential of the Philippine crocodile serum as inhibitor against HIV-1 Reverse Transcriptase was determined. The findings demonstrated a significant anti-HIV-1 Reverse Transcriptase activity. Consistently shown among all samples tested was the inversely proportional pattern of inhibition to the crocodile serum concentration. Remarkably, at 0.5% vol/vol concentration, the seven Philippine crocodile serum samples demonstrated an average of 84.19% inhibitory activity. In particular, crocodile serum CM-07 produced 92.93% reduction in the enzyme activity which is comparable to nevirapine, the known therapeutic drug used as positive control in the study. Variations in the inhibition against HIV-1 Reverse Transcriptase across the seven (7) Philippine crocodile serum samples may be due to the uncontrolled factors in the study such as age, length of stay in the crocodile facility and total protein concentration (Coppo et al., 2006). The following factors mentioned may have influenced the crocodile serum’s component that was attributable to the enzyme inhibition. Therefore, controlling these possible factors may optimize the inhibitory activity of the Philippine crocodile serum and lead to achieve optimal percent inhibition.
While the result showed a significant reduction in the reverse transcriptase activity, the decreased absorbance values in the ELISA reading cannot readily be ascribed to the presence of inhibitors in the Philippine crocodile serum. Other components found in the crude serum preparation may have been the contributory factors in the apparent inhibitory activity observed. The following mechanisms may be considered as possible explanations in the inverse pattern of inhibition of the crocodile serum.
First, there may be the presence of enzymes in the serum that may degrade the nucleotides incorporated in the ELISA kit. The building blocks of nucleic acids, nucleotides, are composed of a nitrogenous base, sugar and a phosphate group such as deoxythymidine triphosphate (dTTP) and deoxyuridine triphosphate (dUTP). The dNTPs are the building blocks for DNA synthesis. RNAse, an enzyme with endonuclease activity and isolated as endopolynucleotidase in reptile’s blood, may hydrolyze the pyrimidine of RNA such as cytosine, thymine, and uracil (Russell, 2001). In the study, the ELISA kit template utilized nucleotides such as digoxigenin- labelled deoxyuridine triphosphate (DIG-dUTP), biotin- labelled deoxyuridine triphosphate (biotin-dUTP), and dTTPs as raw materials in the synthesis of cDNA. Upon addition of the crocodile serum that may contain RNAse, pyrimidine might have been degraded, causing a decrease in the synthesis of cDNA due to the inability of the primer DNA to elongate in the absence of the dNTPs. Another possibility would be the cleavage of newly HIV-1 Reverse Transcriptase-synthesized cDNA by DNAse. Reptile’s blood has been noted to contain, in addition to RNAse, DNAse enzyme which also has an endonuclease activity. Although HIV-1 Reverse Transcriptase may successfully act on the primer and produce cDNAs, the resulting product would not be able to bind to the peroxidase-labelled anti-DIG antibody if cleaved by DNAse. In this condition, the detection of the reaction will be impossible. At a higher serum concentration, all the above interactions would have a higher likelihood of occurring. Moreover, in the above scenarios, it is important to note that the endonuclease activity of DNAse and RNAse are directed either to the pyrimidine or to the cDNA itself and no actual HIV-1 Reverse Transcriptase inhibition occurred.
A second reason that can be considered to explain the result is the hindrance effect theory. This hypothesis is based on the crude characteristic of the Philippine crocodile serum. According to Huchzermeyer (2003), the Philippine saltwater crocodile (Crocodylus porosus), a close family member of the Philippine freshwater crocodile (Crocodylus mindorensis), contains a large Serum Total Protein of 4.1 to 7.0 g/dl. Part of this is Albumin (1.4 to 2.3 g/dl) and crocodile serum immunoglobulin specifically IgG (3.4 g/dl) (Huchzermeyer, 2003). These large proteins may block the streptawell in the ELISA kit. This simply means that the HIV-1 Reverse Transcriptase may be successful in synthesizing cDNA, but with the presence of large proteins that may block the streptawell, the newly HIV-1 Reverse Transcriptase-synthesized cDNA labelled with biotin may not be able to bind and might be removed during washing. In effect, upon addition of peroxidase-labelled anti-DIG antibody there would be no possible interaction resulting to decreased absorbance reading.
To discount these possibilities and to establish the direct inhibitory activity of the Philippine crocodile serum against the reverse transcriptase enzyme, inhibition kinetics analysis was performed using the crocodile serum CM-07. As previously mentioned, CM-07 demonstrated the highest inhibitory activity of 92.93% at 0.5% vol/vol concentration. The Michaelis-Menten and double reciprocal Lineweaver-Burk analysis revealed that the crocodile serum inhibitors behaved in a non-competitive manner. This type of inhibition is mostly allosteric in nature, where the inhibitor binds to a site other than the active site of the enzyme to cause a conformational change in the enzyme structure, resulting in decreased affinity of the inhibitor for the active site. This strongly supports the initial result on the inhibitory activity shown by the crocodile serum. The decreased absorbance in the ELISA readings was not because of the possible mechanisms discussed earlier, but due to the presence of true serum crocodile inhibitors that bind directly to the HIV-1 Reverse Transcriptase allosteric site preventing the viral enzyme activity.
Surprisingly, an inverse relationship was observed between crocodile serum concentration and the anti-HIV-1 Reverse Transcriptase activity. This means that the inhibitory activity decreases as the Philippine crocodile serum concentration increases. This unexpected seemingly paradoxical result could be explained by the presence of an “inhibitor of the inhibitor” in the crocodile serum which may explain the result generated in the test. The best candidate inhibitor present in the crocodile serum with putative inhibition activity to HIV-1 Reverse Transcriptase is the Antimicrobial Peptides (AMPs) (Finger and Isberg, 2012; Andreu and Rivas, 1998). AMPs serve as precursor proteins for small active molecules which are necessary for immune defense. These proteins which consist of 45 amino acid residues require post-translational modification by proteolytic enzymes for activation. Importantly, AMPs prior to proteolysis may also serve as antagonists for pathogen-specific enzymes, in this case, HIV-1 Reverse Transcriptase10. This is supported by the Km value result of 0.4389 at 4% vol/vol. Since the lower the Km value is, the higher the affinity of the enzyme to the substrate, this may indicate that the increased affinity may be due to cleaved AMPs. Meanwhile, at 0.5% vol/vol the Km value is 0.7665. This is congruent with the principle that the higher the Km value is, the lower the affinity of the enzyme to the substrate. In effect, the decreased affinity indicates the binding of the putative inhibitor – AMPs to the enzyme’s allosteric site. As considered, the dilution of crocodile serum seems to be a crucial step that significantly affects the HIV-1 Reverse Transcriptase inhibition demonstrated by the Philippine crocodile serum.
Another significant finding is the average half maximal inhibitory concentration (IC50) of the seven crocodile sera at 1.47% vol/vol. The result was relatively high compared to Alligators serum 0.85% vol/vol6. The IC50 measures the effectiveness of the crocodile serum in inhibiting the HIV-1 Reverse Transcriptase activity. Specifically, the theoretical computation indicates how much concentration of crocodile serum is needed to inhibit the viral enzyme by 50%. The lower the IC50 of the Philippine crocodile serum, the lesser amount would be needed to achieve the desired inhibitory effect, with less likelihood to cause toxic effects in cell-culture based assay.
Reverse Transcriptase is an important viral enzyme in the HIV-1 replication. Inhibition of this enzyme impedes the viral replication in the host cell since succeeding steps would not occur as the single-stranded RNA needs to be transcribed first to its double-stranded form. The results of this study suggest the potential of the Philippine crocodile serum as an inhibitor of HIV-1 Reverse Transcriptase activity. The non-competitive inhibition kinetics of the Philippine crocodile serum suggests a potential novel Non-nucleoside HIV-1 Reverse Transcriptase inhibitor.
Acknowledgements
This work was supported by the Department of Science and Technology – Accelerated Science and Technology Human Resource Development Program (DOST-ASTHRDP).
Conflict of Interest
No conflicts of interests are declared by authors for the contents in this manuscript.
Authors Contribution
Alfredo A. Hinay Jr. designed and carried out the experiment and prepared the draft manuscript. provided guidance, technical support and edited the manuscript.
References






