Advances in Animal and Veterinary Sciences
Review Article
Sperm Sexing and its Utility in Commercial Cattle Production: A Review
Ashaq Manzoor1, Raoof Ahmad Patoo*, Touseef Akram, Anees Ahmad Shah, Tahir Nazir
1Division of Livestock Production Management, Faculty of Veterinary Science and Animal Husbandry, Shuhama SKUAST-K 190006, Jammu and Kashmir, India.
Abstract | Selection of offspring of a desired sex is one of the determining factors to increase the genetic progress and farmer´s profitability in either beef or dairy cattle. In fact, the sex-sorted semen technique has been applied worldwide combined with artificial insemination (AI) upon estrus detection. Sex-sorted semen is not genetically engineered or modified. It is a natural product based on the principle of difference in DNA content between X and Y spermatozoa where X spermatozoa contains more DNA. Sperm sexing through use of flowcytometry ensures promising results in the production of calves of desired sex, replacement and extension of herd quickly, selective culling, reducing dystokia by preventing production of male calves, production of superior bulls, lowering the cost of progeny testing programs and embryo transfer and enhances the value of genetic markers. Despite significant advances in sex-sorting semen using flow cytometry, lower fertility, conception rate and embryo transfer rate occurs when compared to the rates obtained with non sex-sorted semen. The aim of this review is to elucidate an understanding regarding the utility of sex-sorted semen.
Keywords | Sexed semen, Flow cytometer, Performance, Fertility, Limitation
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 22, 2017; Accepted | July 01, 2017; Published | July 25, 2017
*Correspondence | Raoof Ahmad Patoo, Division of Livestock Production Management, Faculty of Veterinary Science and Animal Husbandry, Shuhama SKUAST-K 190006, Jammu and Kashmir, India; Email: raoofpattoo@gmail.com.
Citation | Manzoor A, Patoo RA, Akram T, Shah AA, Nazir T (2017). Sperm sexing and its utility in commercial cattle production: A review. Adv. Anim. Vet. Sci. 5(7): 293-298.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.7.293.298
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Ashaq Manzoor et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Conventionally, random coupling of gametes during fertilization determines the sex and expected sex ratio is likely to be approximate 1:1 in the offsprings. By application of sexed semen, female calves are ensured in about 90% of the cases in contrast to the 49% average frequency obtained with conventional semen (DeJarnette et al., 2009; Norman et al., 2010).Use of sexed bovine frozen semen gives new opportunities to all sectors of artificial insemination industry: cross-breeding, inbreeding, progeny testing, embryo transfer, genetic markers and genome selection (Galli and Balduzzi, 2009). Sex sorting of sperm cells by flow cytometry is an established method that has been commercially used in cattle (Rath et al., 2013). This technology is an important tool for the dairy and beef industry, leading to greater supply of replacement heifers and the consequent hastening genetic gain (De Vries et al., 2008; Chebel et al., 2010). This technology has improved by increasing sorting rates and conception rates over the past decade (Schenk et al., 2009). Successful use of sexed semen requires excellent management of cattle, careful handling of semen and use of a skilled inseminator (Seidel, 2007). Advances in semen sex sorting have enabled incorporation of this technology into commercial operations (Norman et al., 2010).
Benefits of Sex-Sorted Semen
Methods of Sperm Sexing
There are several methods of sperm sexing and important amongst them are listed as:
1) Identification of H-Y antigen (Eichwald and Silmer, 1955)
2) Albumin gradient (Ericsson et al., 1973)
3) Free-flow electrophoresis (Kaneko et al., 1984)
4) Detection of sex specific proteins (Blencher et al., 1999)
5) Centrifugal counter current distribution (Ollero et al., 2000)
6) Volumetric differences (Van Munster, 2002)
7) Percoll density gradient
8) Flow- cytometry
Gledhill et al. (1976) first attempted to separate X and Y sperm by analytical flow cytometry. First successful separation of sperms was made in mammals (Pinkel et al., 1982). Sex sorting process by flow cytometry is the most efficient method to separate X from Y spermatozoa in a large scale (Rath et al., 2013; Seidel, 2014).
Table 1: Difference between X and Y spermatozoa
|
Parameter |
Difference |
|
DNA content |
Less in Y sperm |
|
Size of X sperm |
Larger |
|
Motility of Y sperm |
Faster |
|
Surface charge of X sperm |
Negative |
|
Presence of cell surface antigen H-Y antigen |
Y sperm |
Mechanism of Flow Cytometry
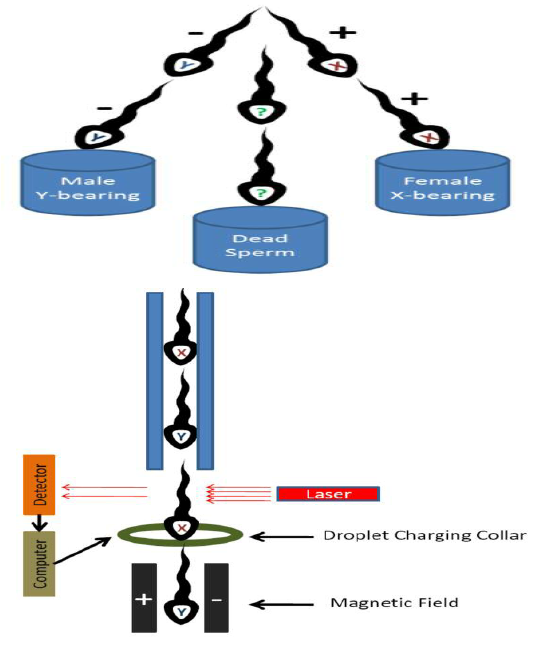
Principle of this method is based on the fact that X-bearing (female) sperm contain 3.8 percent more DNA than Y-bearing (male) sperm (Johnson, 2000). Before sorting, the sperm cells are stained with a fluorescent dye (Hoechst -33342 (a DNA binding fluorochrome [2-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5-bis-1H-benzimadazole-trihydrochloride) and then passed through the flow cytometer as droplets containing the single sperm cell. X-bearing sperm shine brighter than the Y-bearing sperm when illuminated with laser for fluoresence. A positive or negative charge is then applied to the droplets. Charged (+/-) droplets are deflected in opposite directions and uncharged droplets pass straight through. Uncharged droplets may contain multiple sperm, damaged material, or cells that were not aligned in proper direction (Figure 1).
Effect of Sexed Semen on Performance of Cattle Fertility and Conception
Fertility rate with sexed semen was lower than conventional semen (Norman et al., 2010; Healy et al., 2013; Karakaya et al, 2014). Sexed sperms of bull had 75-80% of the fertility of conventional non-sexed, frozen-thawed semen (Schenk et al.,2009). Conception and pregnancy rates with sexed sperms had reduced whether artificially inseminated fresh (sheep (Grossfeld et al., 2005)) or after freezing and thawing (cattle (Frijters et al., 2009). Conception rate or risks on an average were 21% for the 2 × 106 sexed sperm artificial inseminations (AIs) and 46% for the conventional control 15 × 106 sperm AIs (Andersson et al., 2006). (Djedovic et al., 2016) reported 55% conception rates for conventional and 44% for sexed semen. Healy et al. (2013) reported 52% pregnancy rates to sexed semen while 58% to conventional semen. There occurred reduction of 4% to 38% in pregnancy rates in heifers, and 33% in postpartum cows on using sexed semen (Rhinehart et al., 2011). Pregnancy/AI of females inseminated with sex-sorted semen may be influenced by their reduced lifespan in the uterus (Maxwell et al., 2004), reduced number of sorted sperm per straw (Schenk et al., 2009; De Jarnette et al., 2011) and bull related fertility problems (Frijters et al., 2009; De Jarnette et al., 2011; Sales et al., 2011).In contrast, conception rate of 69.7% (30/43) for sexed semen and 66.5% (1545/2325) for unsexed semen following AI was reported in China (Lu et al., 2010).Marginal increases infertility had been achieved (in some sires) by increasing the total number of bull spermatozoa inseminated to 3.5 or 5 million (De Jarnette et al.,2008; De Jarnette et al.,2010).However, Campanile et al. (2011) reported that use of sexed semen in buffalo heifers gave satisfactory and similar pregnancy rates when compared with conventional non sexed semen. Deposition of sexed semen into the body of the uterus, however, increased pregnancy rates significantly (Campanile et al., 2011). Heifers and postpartum cows showed no difference in fertility when inseminated with sexed semen (Rhinehart et al., 2010). However, Healy et al. (2013) reported that pregnancy rates are considerably less in dairy cows than in dairy heifers with sexed semen and is not recommended for use in lactating cows or super-ovulated females (De Graaf et al., 2014). Careful development of sorting media in heifers and use of non-frozensorted semen in lactating cows and sound animal management could solve the fertility issue (Janett et al., 2005; De Graaf et al., 2014).
Type of Breed and Service Number
Jersey heifers had lower fertility (52.2%) than Holstein heifers (56.3%) with sexed semen. Conception risk by service number decreases from 53% for first services to 33% for seventh services (Kuhn et al., 2006).Conception risks from first to seventh service decreased from 56% to 24% (Michael, 2008).
Age of First Parturition
Reduction in conception risks with sexed semen will result in extended breeding period and consequently in a greater age of first parturition. Use of sexed semen generally increases the cost of raising the heifer and a delayed entry into the lactating herd (Kohlman et al., 2008).
Still Birth and Dystocia
Stillbirth frequency was reduced by sexed semen use for cows (Norman et al., 2010). Use of sexed semen reduced the losses from dystocia in heifers (Fetrow et al., 2007). Dystocia and stillbirth were more frequent for heifers (6.0 and 10.4%, respectively, for conventional semen; 4.3 and 11.3%, respectively, for sexed semen) than for cows (2.5 and 3.6%, respectively, for conventional semen; 0.9 and 2.7%, respectively, for sexed semen). Difficult births declined by 28% for heifers and 64% for cows with sexed semen use. Stillbirths were more prevalent for twin births except for sexed semen bred heifer (Norman et al., 2010).
Performance of Calves
Calves produced with non-sexed and sexed semen differed significantly in viability (P < 0.001) and calves from sexed semen had a lower calf vigor score (Djedovic et al., 2015).However, Tubman et al. (2004) found no difference in calf vigor, birth weight, calf health, weaning weights, or mortality before weaning. Healy et al. (2013) also reported that calves produced from gender selected semen are normal with growth rates comparable to their herd mates that are products of conventional semen.
Economic Aspect
Sexed semen is more expensive and less available than conventional semen. Average premium is approximately $30 per straw compared to conventional semen (Fetrow et al., 2007). However, Olynk and Wolf (2007) reported higher profit with sexed semen as compared to conventional semen strategies in dairy heifers.
Impact on Reproductive Technologies
Using sexed semen in super-ovulated cows resulted in 20% to 35% reduction in the number of transferable embryos when compared with unsexed semen (Hayakawa et al., 2009; Larson et al., 2010). Percentage of transferable embryos on using sexed and conventional semen were18.6 and 43.5 (Schenk et al., 2006), 53.4 and 68.1 (Hayakawa et al., 2009), 70.3 and 75.0 (Peippo et al, 2009), 39.5 and 60.5 (Larson et al., 2010), respectively. Most of this reduction is due to an increased number of unfertilized ova. Pregnancy rates after transfer are similar among embryos produced with sexed or unsorted semen (Schenk et al., 2006; Hayakawa et al., 2009). In Vitro Fertilization (IVF) requires only 600-1500 sorted sperm to fertilize an oocyte (Xu et al., 2009). This greatly increases the potential sexed offspring from a sire. Pregnancy rates from IVF cultured embryo fertilized with sexed semen range from 30% to 50% (Pontes et al., 2010).
Limitation of Sex-Sorted Semen
Strategies Employed to Improve Fertility in Sex-Sorted Semen
During sorting process physical trauma, exposure to laser light and dye damages the sperms (Garner, 2006). Sorting process damages the acrosomal membranes and results in decreased motility (Carvalho et al., 2010). Use of pulse lasers and reduced sorting pressure decreases damage to sperm and increases fertility (Schenk et al., 2009). Implementation of semen preservation protocols (Sexcess®) during and after sorting may result in increased pregnancy rates (Rath et al., 2009). Sexed semen should be used in highly fertile herds (having AI pregnancy rates with conventional semen ≥ 60%) and in healthy cycling females bearing good body condition score. Semen should be carefully handled and thawed, and inseminated by experienced AI technicians at estrous time.
CONCLUSIONS
Flow cytometry is the most reliable method for spermsexing. There is lower fertility, conception and pregnancy rates with sexed semen. Jersey heifers have lower fertility than Holstein heifers with sexed semen. Conception rate with sexed semen decreases with progress of service number. Use of sexed semen reduced the losses from dystokia in heifers and stillbirth frequency in cows and greatly increases the potential sexed offspring from a sire. However, it results in reduction in the number of transferable embryos.Use of pulse lasers and reduced sorting pressure increases fertility in sexed semen. Sexed semen should be used in highly fertile herd and in healthy cycling females bearing good body condition score.
Acknowledgements
Authors acknowledge the Vice Chancellor of SKUAST-Kashmir for providing the faculty for compiling the manuscript and colleagues of FVSc and AH, Shuhama for providing references and correction of manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest for the contents in the manuscript.
AUTHORS’ CONTRIBUTION
The Authors worked cooperatively during the collection of information related to this review paper.
REFERENCES