Advances in Animal and Veterinary Sciences
Research Article
Toxicopathological Changes in Internal Organs of Albino Mice after Treatment with Sumithrin
Salema Lafta Hassan
Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Baghdad Iraq.
Abstract | The objective of the present study was to evaluate the toxic effect of sumithrin on the histological state of internal organ and body weight change of mice. Forty adult Swiss Albino mice at the age of two months were divided into four groups. The1st group(G1) was administrated orally with ( 60 mg / kg b.w) of sumithrin daily for 6 weeks, 2nd group(G2) was administrated sumithrin as G1 group and at the same time fed on pellet contains (200 mg/ kg pellet) of beta-glucan for 6 weeks. 3rd group (G3)was fed with beta-glucan as 2nd group. 4rd group (G4) was considered as control negative.At end of the experiments (6 weeks), all animals were sacrificed and Specimens were taken from internal organs like liver, kidney, spleen, heart and brain. The tissues were kept in 10% formaldehyde solution, for fixation, and then processed routinely by using the histokinete. Tissue sections were embedded in paraffin blocks, and sectioned by microtome and stained with hematoxylin and eosin stain, then examined by using light microscope. The pathological lesions showed that the animals exposed to toxic dose of sumithrin was characterized by inflammatory reaction, hemorrhage, congested blood vessels, necrosis, fibrosis and multiple granuloma lesions in internal organs and proliferation Astrocyte and gliosis in the brain while less lesions were recorded in the groups treated with beta-glucan showed improvement against toxic effect of sumithrin. As well as sumithrin causes dacrease body weight.
Keywords | Sumithrin, Beta-glucan, Histopathological changes, Internal organs, Albino mice
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 30, 2017; Accepted | March 20, 2017; Published | April 17, 2017
*Correspondence | Salema Lafta Hassan, Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Baghdad Iraq; Email: salema.l@covm.uobaghdad.edu.iq
Citation | Hassan SL (2017). Toxicopathological changes in internal organs of albino mice after treatment with sumithrin. Adv. Anim. Vet. Sci. 5(4): 167-173.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.4.167.173
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Hassan SL. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Sumithrinis a wide spread insecticide all over the world that called Phenothrin, and d-phenothrin (Calderon-Segura et al., 2012) is a synthetic pyrethroid that kills adult fleas and ticks. It has also been used to kill head lice in humansAs well as used as a component of aerosol insecticides for domestic use. sumithrin is often used with methoprene, an insect growth regulator that interrupts the insect’s biological life cycle by killing the eggs.Phenothrin has increased risk of liver cancer in rats and mice in long term exposure. (Calderon-Segura et al., 2012) It is capable of killing mosquitoes, although remains poisonous to cats and dogs, with seizures and deaths being reported due to poisoning. Sumithrin links with breast cancer; the link made by sumithrin’s effect on increasing the expression of a gene responsible for mammary tissue proliferation (Robinson, 2001). D-Phenothrin is registered for use in commercial and industrial settings, medical institutions, and other settings including aircraft, railroad cars, ships, trailers, homes, animal kennels, gardens, greenhouses, and pet products (RED, 2008) Uses for individual products containing d-phenothrin vary widely. Pyrethroids, including d-phenothrin, are lipophilic. Following absorption from inhalation or ingestion, the chemical is distributed throughout the body primarily to fatty tissues.administered a single oral dose of 200 mg/kg d-phenothrin to rats and it was rapidly absorbed in the gastrointestinal tract and distributed to body tissues and organs. Peak concentrations occurred at three hours after dosing, and occurred in the liver, kidneys, and blood (Soderlund et al., 2002). In target Organisms Sumithrin kills insects by direct contact and ingestion (Tomlin, 2006) that affects the insect’s central and peripheral nervous system. Following exposure to sumithrinvoltage-gated sodium channels are kept open for prolonged periods of time, causing repetitive nerve discharge and increased excitation (Costa, 2008). In Non-target Organisms sumithrin and other pyrethroid insecticides are generally lower in toxicity to mammals than insects because they interfere with sodium channels more strongly at lower temperatures. The body temperature of insects and other invertebrates is approximately 10 degrees below that of mammals. Higher mammalian body temperatures also contribute to the increased metabolic degradation of pyrethroids (Song, 1996).
Gislaine et al. (2012) reported insecticide such as permethrin caused severe alterations in the liver cells, reducing the size of the nuclei and causing hydropic degeneration of the hepatocytes, in addition to stimulating the proliferation of Kupffer cells, altered the amount of proteins, polysaccharides, lipids, and vacuoles in the cytoplasm of the hepatocytes and congested the hepatic capillaries. As for the spleen of the treated mice, no alterations were observed in the morphology in relation to the control group, what would suggest that the spleen would continue performing its functions, without suffering morphological alterations even in the presence of the toxic agent.
Chronic administration of DZN in mice at doses of 300 mg/kg food for aperiod of 45 days induced histopathological changes in spleen, thymus and lymph nodes (Handy et al., 2002). As well as (Daniela et al., 2013) repeated exposure, at subclinical doses, to organophosphorus MLT and DZN causes some, histopathological in rats (vasodilatation, microvacuoles, and granular dystrophy) in the liver, with macrovacuolar steatosis.
Β-Glucans are heterogeneous non starch polysaccharides of glucose polymer, the activity of β-glucan depends on the molecular structure, size, branching frequency, structural modification, confirmation, and solubility. It appears that the most active forms of β-glucans contain β-(1-3) (1-6) linkages (Brown and Gordon, 2003).
It was shown that yeast beta glucan increased the macrophage activity of guinea pigs. It was also shown that superoxide activity was increased. Super oxide dismutase (SOD) is one of the basic antioxidant enzymes we have that fight free radicals. SOD falls as we age and free radicals become muchmore effective and harmful. They said, “Macrophages from guinea pigstreated with glucans exerted toproduce superoxide.” Impressive (Mason, 2011).
The aims of the present study are determine the effects of sumithrin on internal organs of sumithrin on mice.
MATERIALS AND METHODS
Experimental Design
Forty albino mice randomly divided equallyinto 4 groups, each group contains (10) animals as following: G1 was administrated orally with ( 60 mg / kg b.w) of sumithrin daily for 6 weeks, G2 group was administrated sumithrin as G1 group and at the same time fed on pellet contains (200 mg/ kg pellet) of beta-glucan for 6 weeks. G3 group was fed with beta-glucan as 2nd group. G4group was considered as negative control group.
Treatment
Sumithrin is present in the form of powder.60 mg dissolved in 0.5cc of distal water and given to one mice by fine plastic stomach tube given to G1,G2.
Preparation Of Beta-Glucan Diet
Commercial assorted pellets were blended by food blender to which 200 mg of beta-glucan was added to each kilogram of grinded pellets mixed well and converted into the original pellets that left for drying (Salwa, 2012).
Parameter
A-Clinical signs and symptoms: Clinical signs were closely observed and continuously recorded along the period of experiment which is in 6 weeks, also any change in activity or behavior was noted.
B-Body weight changes: The result showed that there was a highly significant (p<0.01) decrease in the body weight of mice of (18.43± (G3) Compared With (G1, G2), (18.43±, 22.58± ), And Control group (21.532± ), respectively (Table 1).
Table 1: changes in the body weight after adminstration of sumithrin to mice.
|
weight |
G1 |
G2 |
G3 |
G4 |
|
B.Wt.(g) |
18.43± D |
22.58± B |
25.45± A |
21.532± C |
Means with different capital letters means significant (P≤0.05) different among groups. G1 was administrated orally with sumithrin. G2 group was administrated sumithrin as G1 group and at the same time feeding on pellet contains of beta-glucan. G3group was feeding with beta-glucan. G4group was considers as control negative group.
C-Macroscopic And Microscopic Examination:At the end of the experiments (6 weeks), all animals were sacrificed and Specimens were taken from internal organs like liver, kidney, spleen, heart and brain. The tissues were kept in 10% formaldehyde solution, for fixation, and then processed routinely by using the histokinete. Tissue sections were embedded in paraffin blocks, and sectioned by microtome and stained with hematoxylin and eosin stain, then examined by using light microscope (Luna, 1968).
Results And Discussion
Clinical Signs And Symptoms
All treated animals were depress and showed decrease appetite for food consumption during the study period The loss of body weight and anorexia were due to pathological lesions which lead to indigestion of stomach and malabsorption of intestine (Summaedaey, 1989).while the control group showed normal consumption.
Histopathological Examinations
Histopathological changes of animals treated with sumithrinat 6 weeks post treatment.
Liver: multiple small granulomatous lesions consisting from aggregation of lymphocytes and macrophages scattered in the liver parenchyma. As well as proliferation of kupffers cells.atrophy of hepatic cord with severe dilated of sinusoids with inflammatory cells in their lumen.
Kidney: shows granulomatous lesion consisting from aggregation macrophages and lymphocytes in interstitial tissue (Figure 1). Furthermore, desquamation of the epithelial lining cells of renal tubules and congestion of blood vessels and severe acute cellular degeneration characterized by hyaline degeneration.
Spleen: The spleen showed inflammatory cells infiltration in the red pulp (Figure 2). With depletion of white pulp as well as an increase in thickness of capsular region.
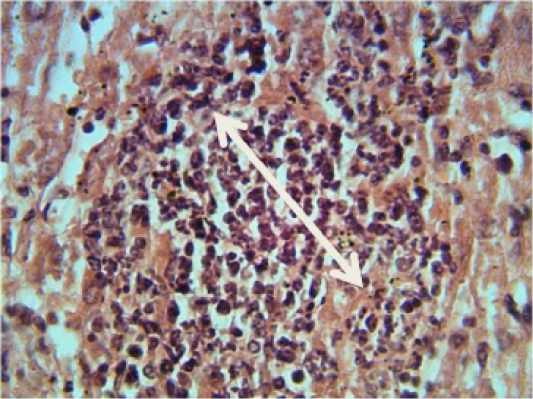
Figure 1: Histopathological section in the liver of animal at 6 weeks post treatment with sumithrin shows granulomatous lesion consisting from aggregation of neutrophil macrophages and lymphocytes in the liver paranchyma white arrow (H and E stain 40X)
Lung: Thickening of interalveolar septa due to mononuclear cells infiltration with areas of emphysema especially at 6 weeks periods (Figure 3).
Brain: there is multiple areas of encephalomalacia (Figure 4). Proliferation of astrocytes and gliosis. As well as Slight congestion, perivascular and perineuronal edema.
Intestine: Erosion of epithelial lining cells, in addition to neutrophils aggregation in the lamina properia of the villi with goblet cell hypertrophy and hyperplasia in other section mucus and neutrophils were seen in the lumen of the intestine.
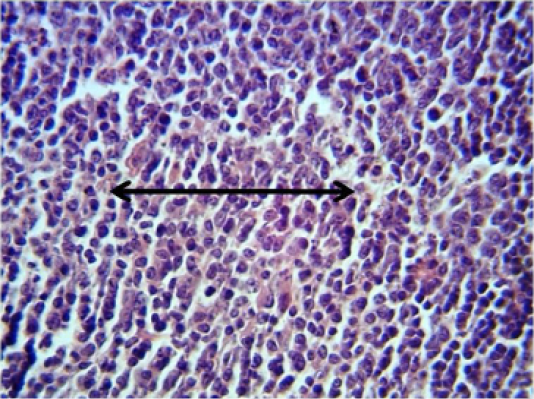
Figure 2: Histopathological section in the spleen of animal at 6 weeks post treatment with sumithrin shows inflammatory cells infiltration in the red pulp with depletion of white pulp black arrow (H and E stain 40X)
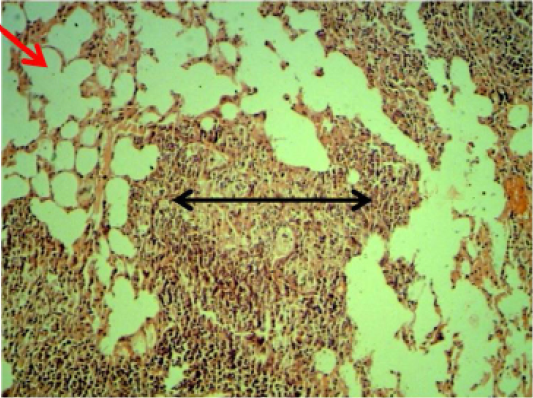
Figure 3: Histopathological section in the lung of animal at 6 weeks post treatment with sumithrin shows multiple abscess (dead and live neutrophils) black arrow with emphysema red arrow in the lung parenchyma with emphysema (H and E stain 10X)
Histopathological Changes Of Animal Treated With Sumithrin And Beta-Glucan
Liver: Small granulamatous lesions (Figure 5). Were the main lesions in the liver parenchyma, hyperplasia of kupfferscellsas
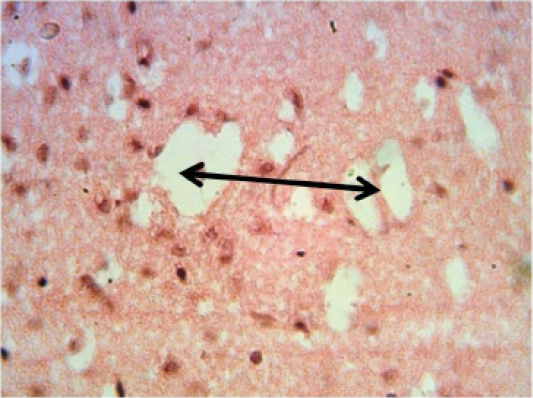
Figure 4: Histopathological section in the brain of animal at 6 weeks post treatment with sumithrin shows multiple irregular spaces in the brain parenchyma (spingioss) black arrow (H and E stain 40X)
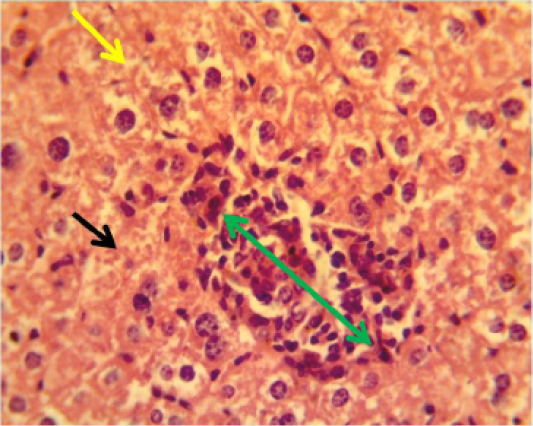
Figure 5: Histopathological section in the liver of animal feeding B-glucan at 6 weeks post-adminstration with sumithrinshows granulomatous lesion green arrow in the liver parenchyma with congested dilated sinusoids yellow arrow and vacuolar degeneration black arrow of hepatocytes (H and Estain 40X)
well asmononuclear cells aggregation in the portal area mainly around portal vein and bile duct.
Kidney: There vacuolar degeneration and enlargement of epithelial cells of renal tubules wth mononuclear cells infiltration around glomerula (Figure 6).
Spleen: The main lesion in the spleen was Moderate hyperplasia of white pulp.
Lung: Perivascular and peribronchiolar lymphocytic cuffings (aggregation of lymphocytes forming follicle like structures). Also hemorrhage in alveolar spaces and congested interalveolar capilleries blood vessels (Figure 7).
Brain: Focal gliosis slight edema and inflammatory cells in the lumen of congested blood vessels in pia matter (Figure 8).
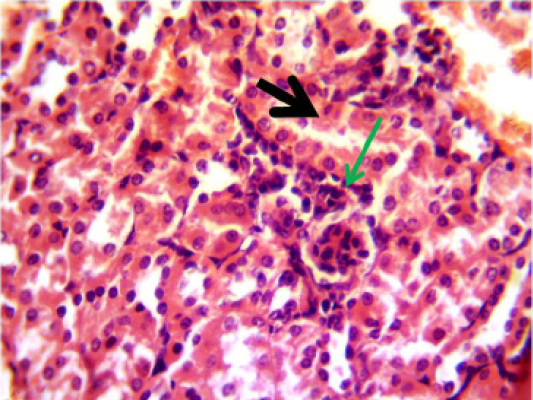
Figure 6: Histopathological section in the kidney of animal feeding B-glucan at 6 weeks post-adminstration with sumithrin shows vacuolar degeneration black arrow and enlargement of epithelial cells of renal tubules wth mononuclear cells infiltration around glomerula green arrow (H and Estain 40X)
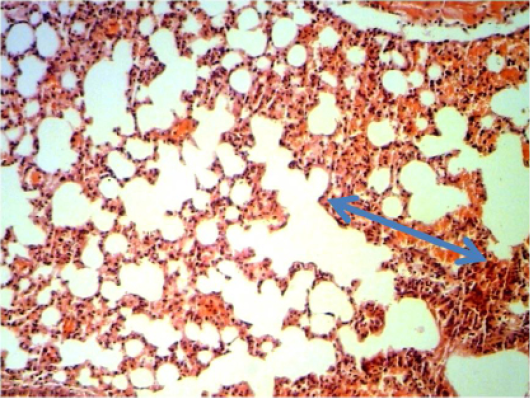
Figure 7: Histopathological section in the lung of animal feeding B-glucan at 6 weeks post-adminstration with sumithrinshows hemorrhage in alveolar spaces and congested inter alveolarcapileries blood vessels blue arrow (H and E stain 40X)
Intestine: Hyperplasia of goblet cells and marked lymphoid tissue hyperplasia were seen between the mucosal glands and mucin in the lumen of the intestine.
Histopathological Change Of Animals Treated With Beta-Glucan
Liver: mononuclear cells aggregation in portal area around proliferation of bile duct and portal blood vessels (Figure 9). As well as in one side of dilated central veins with proliferation of kupffer cells (Figure 10).
Spleen: moderate hyperplasia of white pulp (Figure 11).
Kidney: mononuclear cells aggregation around glomerula and between renal tubules (Figure 12).
Lung: Perivascular and peribronchiolar lymphocytic cuffings (aggregation of lymphocytes forming follicle like structures).
Intestine: No clear lesion in the brain.
Brain: No clear lesion in the brain.
Control group: There was no significant lesion.
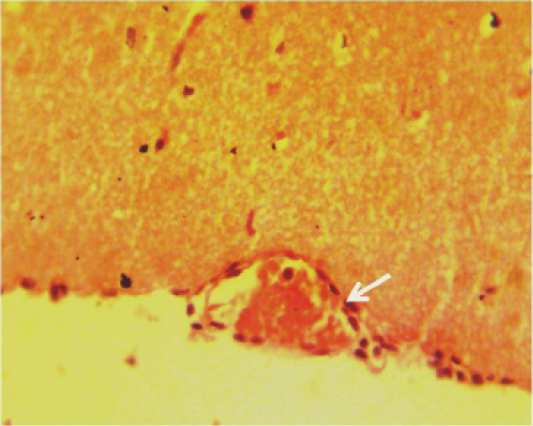
Figure 8: Histopathological section in the brain of animal feeding B-glucan at 6 weeks post-adminstration with sumithrin shows inflammatory cells in the lumen of congested blood vessels in pia matter white arrow (H and Estain 40X)
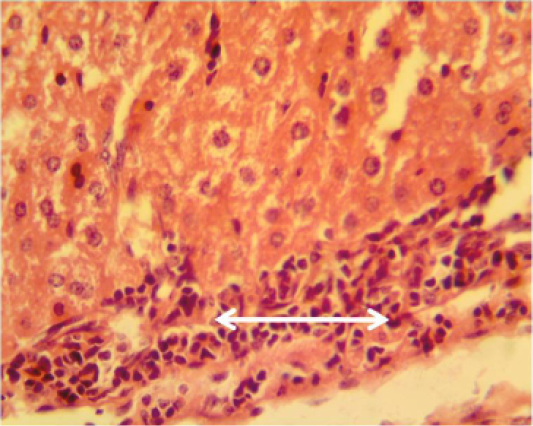
Figure 9: Histopathological section in the liver of animal feeding B-glucan shows mononuclear cells aggregation in portal area around proliferation of bile duct and portal blood vessels white arrow (H and E stain 40X)
The result of the body weight showed significant increase in (G3, G2), suggested that due to viscosity of beta-glucan these results consistent with (Kerckhoffs et al., 2003) reported both beta-glucan from oats (Manthey et al., 1999) and mushrooms (Guo et al., 2004) can slow down the digestion transit time in the lower part of the small intestine and in the large intestine, therefore delaying nutrient absorption. As well as B-glycan diet had an effect on body weight gain prevention (Noh et al., 2011).
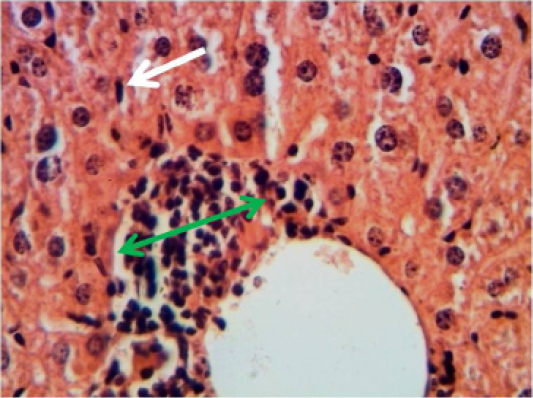
Figure 10: Histopathological section in the spleen of animal of feeding B-glucan shows moderate hyperplasia of white pulp red arrow (H and E stain 40X)
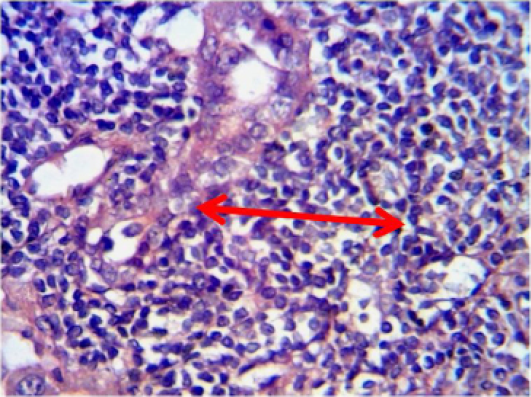
Figure 11: Histopathological section in the liver of of animal feeding B-glucanshows mononuclear cells aggregation in one side of dilated central veins green arrow with proliferation of kupffer cells white arrow )H and E stain 40X)
The decrease in the body weight in (G1), revealed that Sumithrin has effects on sex hormones, (mimic estrogens) in one of their biological activities, (Go et al., 1999) looked at the activity of a gene called pS2 because the expression of pS2 is activated by estrogens, and they found that in human cells sumithrin (as well as synthetic pyrethroid insecticide, fenvalerate) activates the expression in cells of pS2 like estrogens do.
The pathological lesions in examined organs in group treated with sumithrin may be attributed to oxidative stress is importantly involved in the pathophysiology of various liver lesion due to treatment with sumithin which led to Reactive oxygen species, such as superoxide, hydrogen peroxide and hydroxyl radical are released by neutrophils and have been shown to play an important role in inflammation and cell injury (Vladimirov, 2004).
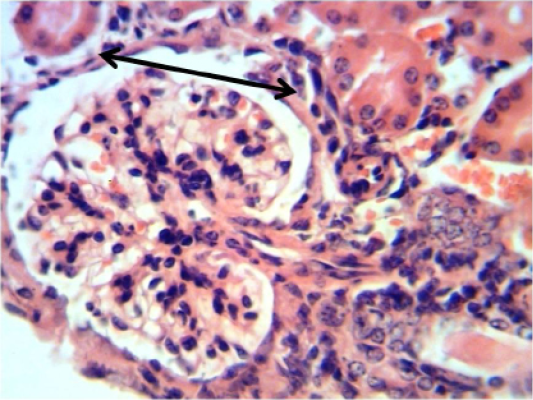
Figure 12: Histopathological section in the kidney of of animal feeding B-glucan shows increase thickness of glomerular wall due to fibrosis and mononuclear cell infiltration black arrow (H and E stain 40X)
The hepatocyte’s proteins, lipids and DNA are among the cellular structures to be affected primarily by ROS and reactive nitrogen species (RNS). This process disrupts at cellular and molecular level the structure-function relationship on liver cells at different sites (Arauz et al., 2016). The same lesions were recorded by (Gislaine et al., 2012), the liver cells of mice treated with Permethrin caused severe alterations in the liver cells, reducing the size of the nuclei and causing hydropic degeneration of the hepatocytes, in addition to stimulating the proliferation of Kupffer cells, altered the amount of proteins, polysaccharides, lipids, and vacuoles in the cytoplasm of the hepatocytes and congested the hepatic capillaries.
The main pathological lesions of the spleen depletion of white pulp may be due to high susceptibility of T lymphocytes to apoptosis induced by (IC) toxic effects (Gekle and Silbernagl, 1996) that agree with (Taylor et al., 2007) who stated that deltamethrin was able to cause thymus atrophy and apoptosis in the thymus of treated animals via the alteration of Ca/CaM-dependent protein kinase-phosphatase cascade.
The other pathological lesions Hemorrhage in the liver and spleen parenchyma attributed to toxic effects of sumithrin on endothelial cells that lead to rupture of blood vessels, that agree with (Szretter et al., 2007) who found that pesticides such as (IC) can induce rupture in the wall of central arteries of the spleen.
The pathological lesions in the kidney may be associated with increased levels of ROS/RNS and decreased antioxidant levels (Arauz et al., 2016) that agree with (Alwan, 1996) who found exposure to Ochratoxin A (OTA) leads to an impairment of postproximal nephron function, with subsequent disturbance of cellular acid-base homeostasis.
The histopathological lesions in the internal organs of animals at β-glucan were characterized by moderate lesions due to β-glucans stimulates production of both proinflammatory and anti-inflammatory cytokines, the cellular pathway involved in induction of TNF-α (a Sykin dependent pathway), differs from that for induction of IL-6 and IL-10 (a Skyin--dependent pathway). β-glucan fragments released during infection contributes to the activation of host defense (Alwan, 1996) as well as β-glucan enhance innate immune response and provided partially protective immune responses against sumithrin effect on organs by significant increase of IL-1 and IFN- by DC initiates and enhances both innate and acquired immunity (Robinson, 2008).
Proliferation of kupffer cells and hyperplasia of lymphoid tissue in the current study may be due to stimulated immune response (Sharon, 2013). Also small granulomatous lesions in the liver was due to β-glucan which stimulation phagocytic cells specially macrophages which destroyed and remove the foreign body and necrosis at the site internal organs in which activated macrophages remove them via granulomatous reaction that agree with (Adams, 1976) who considered the granulomatous response as a stronger host defense mechanism against foreign body.
The pathological lesions showed that the animals fed Beta glucan are influenced by several factors that agree with (Sandeep et al., 2011) who stated that beta glucan is a polysaccharide (insoluble long-chain carbohydrate) may stimulate the immune system by increasing chemicals, which prevent infections.
Also (Hong et al., 1950) referred that this mechanism of action is effective against cancers when used in combination with specific monoclonal antibodies that activate or cause the complement to be bound to the tumor.
According to above observations, we concluded that the sumithrin have toxic effect on internal organs in mice and beta glucan have modulate the effect of the sumithrin, while the activated immune response may prevent the toxic effect of sumithrin.
Authors Contribution
Conceived designed and Performed the experiments: Salema Lafta Hassan. Analyzed the data: Contributed reagents/materials/analysis tools and wrote the paper: Salema Lafta Hassan.
References






