Advances in Animal and Veterinary Sciences
Case Report
Canine Parvovirus (CPV) Infection in a Great Dane Puppy: A Case Report
Raslan Ain-Fatin1, Saulol Hamid Nur-Fazila1*, Abd Rahaman Yasmin2, Abd Rahaman Sifa-Shaida2, Wan Noor Ayuni2, Zakaria Muhamad-Alif2
1Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; 2Department of Veterinary Laboratory Diagnostics, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
Abstract | Canine parvovirus (CPV) infection is a major disease affecting young pups with high contagiousness and mortality worldwide. Unvaccinated pups between 6 weeks to 6 months are most susceptible to CPV infection. It manifests into enteritis and myocarditis forms. Classical signs of enteritis form include acute onset of vomiting, haemorrhagic diarrhoea, and pyrexia. A case of unvaccinated 3-month-old female Great Dane puppy was presented with complaints of hematemesis, hematochezia and shooting diarrhoea. A tentative diagnosis of CPV was made. It was humanely euthanised due to chronic body weight loss and poor response to treatments. Post-mortem examinations revealed congested lung with frothy exudate, whitish plaques on heart coronary groove, thickened intestinal mucosa, and generalised reddening in the liver and kidneys. Microscopic changes revealed interstitial pneumonia with edema, lymphocytic myocarditis, viral enteritis with villous atrophy, and liver congestion. A faecal samples for polymerase chain reaction (PCR) method revealed positive result for parvovirus. Based on pathological and PCR findings, it was definitively diagnosed with CPV infection. The chance of its survival without aggressive treatment remains low. Treatment and management are still limited to supportive care without existing agent-specific treatment. Therefore, CPV infection should be controlled and prevented by providing vaccination to the pups from the age of 6 weeks.
Keywords | Canine, Histopathology, Parvovirus, PCR, Post-mortem
Received | October 07, 2019; Accepted | February 17, 2020; Published | August 10, 2020
*Correspondence | Saulol Hamid Nur-Fazila, Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; Email: nurfazila@upm.edu.my
Citation | Ain-Fatin R, Nur-Fazila SH, Yasmin AR, Sifa-Shaida AH, Ayuni WN, Muhamad-Alif Z (2020). Canine parvovirus (CPV) infection in a great dane puppy: a case report. Adv. Anim. Vet. Sci. 8(10): 1075-1078.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.10.1075.1078
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ain-Fatin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Canine parvovirus, also known as canine panleukopenia is classified under the Protoparvovirus genus and Parvoviridae family (Cotmore et al., 2019). Canine parvovirus is denoted as CPV, and the virus is separated into two antigenic forms known as canine parvovirus type 2 (CPV-2) and canine parvovirus type 2a (CPV-2a) (Parrish, 1990). The first virus detected, CPV-1 or the Minute Virus of Canines was believed to be non-pathogenic (Binn et al., 1970). However, CPV-2 is discovered as the second type parvovirus infection. It is recognised as a pathogenic form that can causes a disease associated with high contagiousness and mortality that primarily affects juvenile pups who are unvaccinated or having incomplete vaccination regimes between the ages of 6 weeks to 6 months (Hoskins, 1997; Kahn and Line, 2010; Prittie, 2004). The incubation period is around 3 to 7 days before the clinical symptoms appear (MacCartney et al., 1984). Infections caused by CPV-2 manifested in two forms which are enteritis and myocarditis (Goddard and Leisewitz, 2010). The enteritis form causes various symptoms such as vomiting, pyrexia, dehydration, and haemorrhagic diarrhoea, whereas the myocarditis form leads to myocarditis in utero or after birth, showing symptoms before the age of 3-month-old. Predisposing factors to parvovirus infection include intestinal parasites, inadequate protective immunity, sanitary compromises, overcrowding, and stressful conditions (Binn et al., 1970). Viral replication takes place in rapidly dividing cells such as precursor cells in the bone marrow, intestinal crypt epithelial cells, and myocardiocytes (Goddard and Leisewitz, 2010; MacCartney et al., 1984; McCaw and Hoskins, 2006).
CASE REPORT
History
A 3-month-old, female Great Dane puppy with no history of vaccination status, was presented to University Veterinary Hospital (UVH), Universiti Putra Malaysia (UPM) with complaints of hematemesis, hematochezia and shooting diarrhoea by the owner. Upon presentation, the patient was emaciated, depressed with 7% dehydration, and generally in very poor body condition. A tentative diagnosis of CPV was deduced based on the symptoms shown and the history given by the owner. The initial intended plans were to perform fluid therapy and diagnostic tests for complete blood count and serum biochemistry, bacterial culture of faecal sample and PCR analysis. However, the decision to humanely euthanise the dog by anaesthetic drug overdose was finally decided as agreed by the owner due to the patient’s deteriorating condition, severe body weight loss and poor response to treatments.
Post-Mortem Examination
At necropsy, upon incision of the thoracic cavity, approximately 50 ml of serosanguinous fluid and frothy exudates were observed at the thoracic inlet, airways, and bronchi (Figure 1A), as indicative of pulmonary oedema. In lung parenchyma, about ten (10) round nodules sized 2 to 3 cm in length, and width containing abundant of caseous exudate and pus were seen (Figure 1B). Furthermore, there were presence of multiple irregular whitish plaques on the capsular surface of the heart coronary groove as shown in Figure 1C. Other than empty gastrointestinal content, gross lesions of the intestines showed thicken and redden mucosa (Figure 1D). Widespread reddening of the liver and kidneys observed indicating generalised congestion of both organs (Figure 1E, 1F).
Histopathological Findings
Tissue samples of lung, heart, intestine, and liver were fixed in 10% neutral buffered formalin. Paraffin-embedded sections were stained with hematoxylin and eosin (H&E) for histomorphological examination by using a light microscope (Nikon Eclipse TI). Histologically, lung tissues revealed thickened alveolar walls with inflammatory cells infiltration as indicative of interstitial pneumonia with pulmonary edema and congestion (Figure 2A). In the heart, lymphocytic myocarditis observed with the recruitment of macrophages admixed with neutrophils along cardiac myocytes (Figure 2B). Microscopic changes in the intestinal tissues revealed viral enteritis with intestinal villous atrophy (Figure 2C), and the liver section showed hepatic congestion with engorged vascular and sinusoids dilatation (Figure 2D).
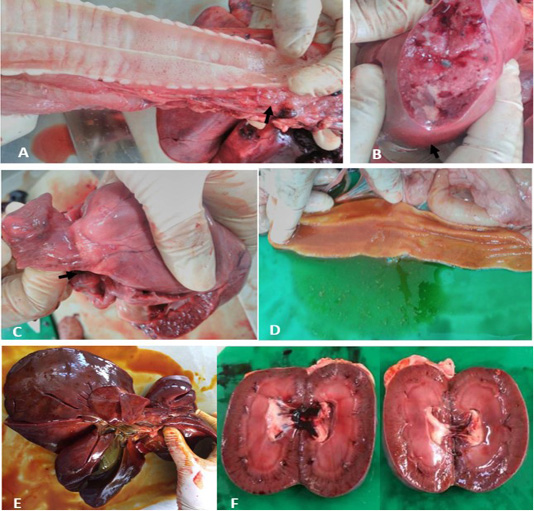
Figure 1: Gross lesions at necropsy. A) Trachea. Frothy exudate in thoracic inlet. B) Lung. Patchy round nodules containing caseous exudate. C) Heart. Whitish spots on the coronary groove of the heart (arrow). D) Intestine. Thicken and redden intestinal mucosa. E) Liver. Enlarged, reddened and firm consistency. F) Kidneys. Generalised reddening of both kidneys.
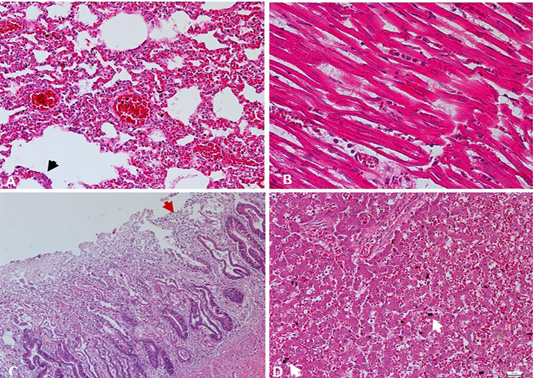
Figure 2: A) Lung. Interstitial pneumonia and pulmonary congestion, including dilatation of capillaries in the alveolar walls filled with red blood cells and thickened alveolar walls with inflammatory cells. Cuboidal hyperplasia of type II pneumocytes in the alveoli was also seen (arrow). H&E. 200X. B) Heart. Lymphocytic myocarditis, degenerated myofibrils with infiltration of macrophages admixed with neutrophils in between cardiac myocytes. H&E. 400X. C) Intestine. Enteritis, degeneration and necrosis of the intestinal villi with mononuclear cells infiltration among enterocytes, with shortened villi (arrow). H&E. 100X. D) Liver. Hepatic congestion, including marked vascular congestion, dilatation of the sinusoids, presence of organizing thrombus and degenerating hepatocytes contains cytoplasmic lipid droplets, with evidence of hemosiderin-laden macrophages (arrow). H&E. 200X.
Polymerase Chain Reaction (Pcr) Method
PCR was conducted for targeting polymerase gene of CPV after DNA extraction using commercial extraction matrix (QIAamp) from faecal sample. Primer sequences used were CPV 555 F-5’ AGGAAGATATCCAGAAGGA 3’ (forward) and CPV 555 R-5’ GGTGCTAGTTGATATGTAATAAACA 3’ (reverse). Master Cycler Gradient Thermal Cycler (Eppendorf®, Germany) was used for DNA amplification. Amplified DNA fragments revealed positive for Parvovirus DNA as the band being expressed at the 600 base pair (bp) in size after electrophoresis in 1.5% agarose gel, consistent with canine parvovirus as demonstrated in Figure 3.
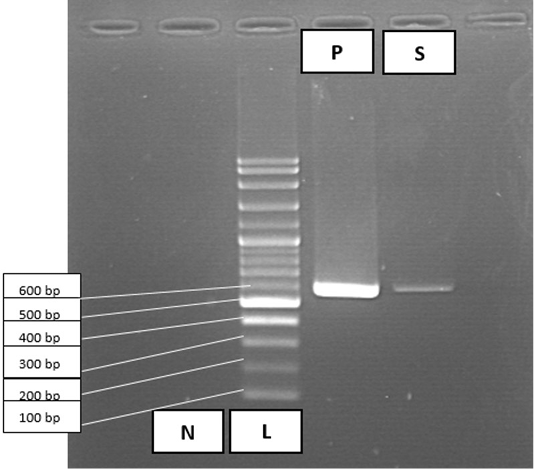
Figure 3: PCR amplification results for Canine Parvovirus in faecal sample. Lane 1, negative control (N); lane 2, 100 bp DNA ladder marker (L); lane 3, positive control (P); lane 4, faecal sample (S) positive for parvovirus at 600 bp.
DISCUSSION
Diagnostic tests should be carried out to obtain a definite diagnosis for parvovirus infection. Specific tests such as detecting viral antigen in faeces by ELISA, detecting viral particles from tissue and faecal material by electron microscopy and immunohistochemistry (IH) in the tissue also can be utilised to obtain a definitive diagnosis (Prittie, 2004). However, PCR-based methods are discussed to have higher sensitivity in detecting parvovirus as compared to traditional methods (Desario et al., 2005) used in this case to obtain a definitive diagnosis.
Canine parvoviral enteritis is continuously related to low survivability of 9.1% when no treatment was given but it was significantly increased up to 64% when treatment has opted (Otto et al., 1997). However, since there is no available agent-specific treatment to combat the infection, supportive care remains the best approach. Prevention by vaccination is effective in controlling the disease (Nandi et al., 2013). Vaccination of young pups is recommended in their first months of life during the ‘immunity gap’ between the ages of 7 to 12 weeks where the pups are vulnerable to infection due to a drop in maternal antibodies (Decaro and Buonavoglia, 2012; Siegl et al., 1985). A guide in preventing CPV infection is vaccinating the animals as early as 6 to 8-week-old and then repeatedly at intervals of 3 to 4 weeks before finishing around the age of 4-month-old or above (Day and Schultz, 2016; Jakel et al., 2012).
CONCLUSION
In conclusion, CPV is a highly contagious infection with high mortality that affects young pups. CPV can be diagnosed by PCR for a definitive diagnosis. Specific treatment for CPV remains obsolete. However, control and prevention by vaccination of pups from the age of 6 weeks would be the best option in countering the disease.
ACKNOWLEDGEMENTS
The authors thank Mr. Ghazali, Mrs. Jamilah, and the staff of Virology, Post-Mortem and Histopathology Laboratory, Faculty of Veterinary Medicine, UPM for technical assistance. This case report was supported by Universiti Putra Malaysia (UPM) as a grant provider (GP-IPM/2017/9526900).
conflict of interest
The authors declared that there is no conflict of interest.
AUTHORS CONTRIBUTION
RAF and SHNF were conducted the case and involved in the designing and commencement of the manuscript. ARY, AHSS, and WNA were involved in the PCR method. SHNF and ZMA were involved in necropsy, samples collection, and histological interpretations. All the authors have read and approved the final manuscript.
REFERENCES






