Advances in Animal and Veterinary Sciences
Research Article
The Influence of Brucella Abortus Strain RB51 Vaccine Which is Given to the Mice (Mus musculus) and Infected by Local Isolat Brucella Suis for the Figures of Hepatic Fibrosis of the Mice (Mus musculus)
Putri Ekha Puspitoyani, Emy Koestanti Sabdoningrum*, Didik Handijatno
Faculty of Veterinary, Universitas Airlangga, Surabaya, Indonesia 60115.
Abstract | Brucellosis in hog is caused by a bactery named Brucella Suis. Vaccine is used as a prevention and elimination act for Brucellosis. However, Brucellosis vaccine is not yet available, instead Brucella abortus strain RB51 is used as a vaccine for cattles. On the other hand, the used of RB51 vaccine is still a controversy since there are various result on the related research that have been done. This study was designed to analyze the changes in histopathologic figures of hepatic mice that had been vaccinated against Brucella abortus strain RB51 and infected by Brucella suis. This study used samples of 18 males (Mus musculus) mice aged 8 weeks which weight about 20-30 g and divided into 3 treatment groups, P0 +, P0-, and P. Degree of liver damage as a result of treatment performed on hepatic scores using modification method Knodell (2007) who got the presentation of the scoring result from each treatment. The results of this study shows that the Brucella abortus strain RB51 vaccine did not decrease the number of hepatic necrosis cells of mice (Mus musculus) that had been infected by Brucella suis and Brucella abortus strain RB51 vaccine did not decrease the occurrence of hepatic fibrosis of mice (Mus musculus) that have been infected by Brucella suis. Brucella Abortus strain RB51 vaccine that was given to the mice which had been infected by Local Isofat Brucella suis did not cause any significant changes of the mice histopathological figures of hepatic.
Keywords | Brucellosis, Swine, Vaccine RB51, Hepar, Histopathological
Received | October 13, 2019; Accepted | January 10, 2020; Published | February 20, 2020
*Correspondence | Emy Koestanti Sabdoningrum, Faculty of Veterinary, Jalan Mulyorejo, Universitas Airlangga, Surabaya, Indonesia, 60115; Email: emykoestantisabdoningrumunair@yahoo.com
Citation | Puspitoyani PE, Sabdoningrum EK, Handijatno D (2020). The Influence of Brucella Abortus Strain RB51 Vaccine Which is Given to the Mice (Mus musculus) and Infected by Local Isolat Brucella Suis for the Figures of Hepatic Fibrosis of the Mice (Mus musculus). Adv. Anim. Vet. Sci. 8(2): 208-212.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.2.208.212
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Puspitoyani et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Brucella Suis bacteria is the cause of Brucellosis in hogs. Brucellosis can contaminate through direct contact with infected hog. The heir of a normal hog mother and infected male hog will inherit the disease (Sihombing, 2006). The heir could be infected in the process of birth or breastfeed if the mother is also infected by the disease (Alton et al., 1991). This disease has been a problem in many countries since it gives impact on the financial loss and veterinary health. The financial loss that is caused by the disease are the death of fetus, barren hog, weak or dead offspring. According to Corbel (1997), Brucellosis has a zoonosis character so that it can infect human. Infected people usually are those who have a job that enable them to have direct contact with infected hogs such as farmer, breeder, vet and butcher (Lindstrom, 2003).
Brucellosis which penetrates into the body will be through phagocytosis process which occurs because leukocytes polymorphonuclear. Brucellae usually survives and breeds in this cells since the cell can prevent the bactericidal myeloperoxidase-peroxide-halide system to be occurred by releasing guanosine and adenine. In the beginning of infection, macrofag is relatively ineffective in killing the intracell of brucella bacteries. The spreading of the bacteria is systematically unclear on how it is distributed, whether it is in neutrophyl and macrofag or blood circulation outside the cell. However, microorganism can spread widely from regional lymphoid tissue according to the first place where it is first penetrates. Furthermore, the mtcroorganism is located inside the target organ such as lymph nodes, spleen, liver, spinal cord and reproduction organ (Enright, 1990). Clinical symptoms on the infected hog are mostly found on adult hog such as abortion, infertility, orchitic, and post paralysis (Deyoe, 1981).
Prevention and elimination acts for Brucellosis in Indonesia have been carried out since awhile, but there is still some region which are not fully free from Brucellosis. Moreover, Brucellosis has been igonered since the clinical symptoms often invisible (Pawar et al., 2012). Brucellosis treatment is not recommended because it is high in cost and needs long period of time. The most effective way to prevent brucellosis is vaccination (Adjid, 2014). Vaccination is the most applied to prevent the disease in the endemic area and it is less in cost. Many countries have developed prevention acts to eliminate cattle diseases. These programs can minimalize finance loss due to abortion, infertility and weakly born cattle (Olsen and Stoffregen, 2005). The used of vaccine to prevent brucellosis in hog is the same vaccine which is used for cattle. The vaccine for hog is not yet available so Brucella abortus strain RB51 for cattle is used instead. however, the used of this vaccine is still a controversy since the result of the research in related studies show different result (Stoffregen et al., 2006).
Liver is an important organ since it receives 80% blood supply from vena porta to be detoxificated in it. It produces enzymes which have ability to bio transform in various endogen and exogen substance that will be eliminated from the body. The process of biotransformation can activate some substance to be more toxic which can cause wound in the liver (Carlton, 1995). It can cause liver damage although liver can regenerate its cell quickly in order to function properly. According to that, this study aims to observe the histopathologic figures of hepatic on mice which had been vaccinated with Brucella abortus strain RB51 and infected with Brucella Suis.
Materials and Methods
Animal
The sample animal that were used for this study was from Faculty of Pharmacy Universities Airlangga Surabaya Indonesia. Brucella Suis from Karanganyar animal farm which was isolated and identified in Association of Veterinary Wates Indonesia. Brucella Abortus was received from U.S. Vet license number 188 Professional Biological Company USA. This research was conducted for two months, started from July 2016 until September 2016. Food supply for the mice were PAR-LI complete food for hen age 18 month, Brucella jelly as the media, Brucella Broth, clear water, PBS, pure water, cotton, Ketamin, Alcohol 70%, NaCl physiologic, formalin 10% PAS buffer, Xylol, and paraffin. This research was conducted in Bacteriology and Mycology Laboratorium of Faculty of Veterinary Universities Airlangga, Surabaya, Indonesia.
Experimental design
This research is an experimental research and the research design which was used in the current research was Complete Randomized Design. This research includes 3 treatments (t=3) dan 6 repetition (n=6), so it needed 18 male mice (Mus Musculus). The number of repetition was decided according to t (n-1) ≥15, t is treatment and n is repetition (Kusriningrum, 2008). The samples were 18 male mice (Mus Musculus) age 8 weeks which weight 20-30 gram in healthy condition. Federer’s formula was used to decide the number of sample. Based on the formula, 6 mice was needed to be through 3 treatment. In brief, this research needed 18 mice.
Measurement of feeding behaviour
The mice was raised and adapted for 1 week inside the cage. It was given food in a form of pellet and clear water in ad libitum way. After it was adapted for a week, the mice was mixed and categorized into 3 group which are treated differently, there are:
- a. Group P0-: 6 mice as negative controller and it was only injected with 0.1 mL NaCl intramuscularly, on 2nd, 4th, 6th and 8th week.
- b. Group P0+: 6 mice as positive controller, which were injected with 0.1 mL NaCl on 2nd, 4th and 6th week then on 8th week it was infected with Brucella Suis (1x108 cell/mL) intramuscularly.
- c. Group P: 6 mice were vaccinated with 0.1 mL Brucella abortus strain RB51 vaccine intramuscularly on 2nd week, first booster was given on 4th week, second boster on 6th week and were infected with Brucella Suis 1x108 cell/mL intramuscularly.
Production of brucella suis bacteria suspension
Brucella suis local isolate was taken from hog farm in Karanganyar, Central Java which was isolated and identified in Research Center of Veterinary Science Wates Yogyakarta. The isolat was propagated in Brucella broth and incubated on 37-degree Celsius for 72 hours. Suspension was made with concentration 1.0x108 cell/mL according to Mc. Farland no. 1 standardization. After that, the mice were killed in occipital dislocation way. The, it was operated, and the hepaic was taken to be preserved in a plastic pot which contained formalin buffer 10%, was given label and it is made to histopathic blood smear.
Production of hepatic histopathic supply
The histopathic blood smear was produced in Pathology Laboratorium of Department of Pathology, Faculty of Veterinary Universitas Airlangga Surabaya using HE (Hematoxycilin Eosin) coloring. The organs were taken to find out the alteration of histopathic which happened on 10th week after the mice were vaccinated with Brucella abortus strain RB51 and infected with Brucella Suis.
The observation of hepatic histopathology alteration
The variables which were observed in this research was Hepatic Histology which included Necrosis, the cell damage which is marked with picnosis (hypercromatic core and shrink), Karyorexis (broken core), and Cariolysis (disappeared core). There is also fibrosis which is marked with the formed of connective tissue which is dominated with MN cell. The observation was centered in the lobules around central vein of hepatic or porta area (Kiernan triangle). Scoring was given to 5 field of view for each of the blood smear. The scoring used the method of Knodell Modification Scoring (1981) (Knodell et al., 1981).
Statistical analysis
Kruskall Wallis test was used as the data analysis which was continued with statistical analysis using SPSS (Statistical Program and Service Solution) rev. 23 for windows.
Result
Necrosis
The number of necrosis on the mice hepatic group P0- (negative controller and injected with 0.1 mL NaCl intramuscularly, on 2nd, 4th, 6th and 8th week), group P0+ (positive controller and injected with 0.1 mL NaCl on 2nd, 4th and 6th week then on 8th week it was infected with Brucella Suis (1x108 cell/mL) intramuscularly) and group P (the treatment of the mice were vaccinated with 0.1 mL Brucella abortus strain RB51 vaccine intramuscularly on 2nd week, first booster was given on 4th week, second boster on 6th week and were infected with Brucella Suis 1x108 cell/mL intramuscularly) shows that there is not noticeable differences (p<0.05), (Table 1 and Figure 1).
Table 1: The influence of Brucella abortus strain RB51 vaccine which was given to the mice (Mus Musculus) that was infected by Brucella Suis Local Isolate on the Mice Hepatic Necrosis.
| No. | Treatment | The number of Necrosis |
| 1. |
P0- |
2,13a±0,81 |
| 2. |
P0+ |
2,13a±0,61 |
| 3. | P |
2,46a±1,07 |
Note: Different Superscript in the column which shows noticeable differences (p<0.005).
Fibrosis
The number of the fibrosis in the hepatic mice which divided into 3 groups consists of group P0- P0- (negative controller and injected with 0.1 mL NaCl intramuscularly, on 2nd, 4th, 6th and 8th week), group P0+ (positive controller and injected with 0.1 mL NaCl on 2nd, 4th and 6th week then on 8th week it was infected with Brucella Suis (1x108 cell/mL) intramuscularly) and group P (the treatment of the mice were vaccinated with 0.1 mL Brucella abortus strain RB51 vaccine intramuscularly on 2nd week, first booster was given on 4th week, second boster on 6th week and were infected with Brucella Suis 1x108 cell/mL intramuscularly) shows that there is not noticeable differences (p<0.05) (Table 2 and Figure 2).
Table 2: The influence of Brucella abortus strain RB51 vaccine which was given to the mice (Mus Musculus) that was infected by Brucella Suis Local Isolate on the Mice (Mus musculus) Hepatic Fibrosis.
| No. | Treatment | The number of necrosis |
| 1. |
P0- |
0,33α ±0,23 |
| 2. |
P0+ |
0,26α ±0,12 |
| 3. | P |
0,17α ±0,19 |
Note: Different Superscript in the column which shows noticeable differences (p<0.05).
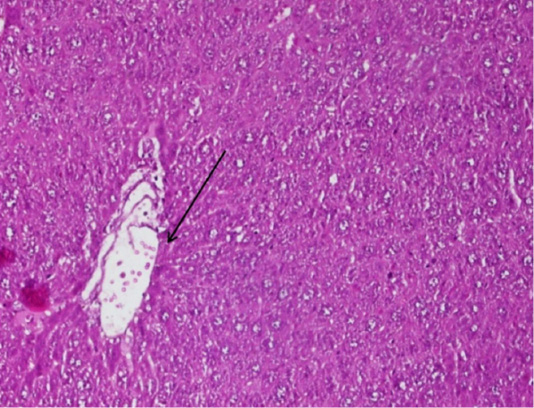
Figure 2: The arrow shows the figure of fibrosis tissue in the blood smear P of the mice (Mus Musculus) hepatic, the supply used Hematoxylin Eosin (HE) 200x magnification.
Discussion
Brucella abortus strain RB51 vaccine which was injected should be safe for the body and did not damage the organ. The safety of the vaccine could be observed from the liver histopathology blood smear because liver is an organ which could detect toxicity in the body. Blood circulation of the body must be through the toxic neutralization in liver (Guyton and Hall, 2006). The use of vaccine has risks such as virulen residual, toxicity, allergic, immunodeficiency disease from the host, neurological complication and harmful effect on fetus (Tizard, 2004). The result of statistical analysis did not show any noticeable differences (p<0.05) between P0+, P0- and P which meant that the distribution of the Brucella abortus strain RB51 vaccine which was infected with Brucella Suis Local Isolate did not cause any significant changes in the figure of hepatic histopathology of mice (Mus Musculus).
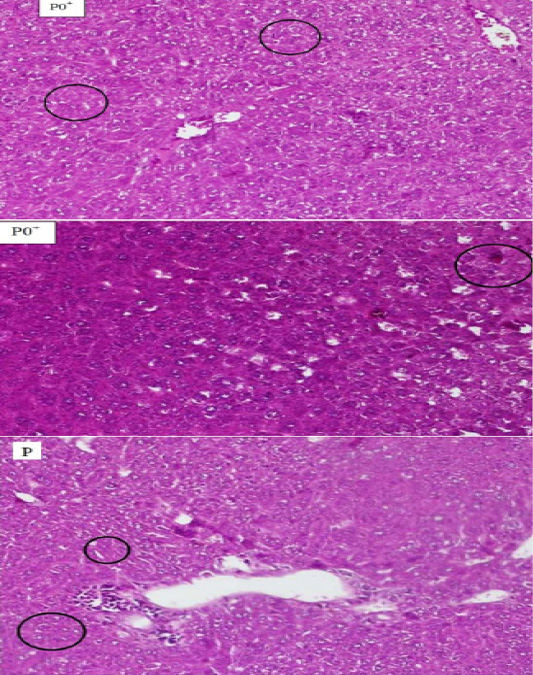
Figure 1: The comparison between hepatocyte Histopathology of mice (Mus Musculus) which experienced necrosis on the blood smears P0+, P0- and P. The circle shows the cell that experienced necrosis. The supply used Hematoxylin Eosin (HE) 200x magnification.
Brucella abortus strain RB51 vaccine has been permitted to be traded by Indonesian Ministry of Agriculture on 2004. Brucella Abortus strain RB51 is a rough load of virulen Brucella abortus train 2308 (S2308) and it is made with repeated passase. Brucella Abortus strain RB51 do not have O antigen series (Cheville, 2006). Besides that, Brucella Abortus strain RB51 is resistant towards rifampicin. Rifampicin is the most effective antibiotic to treat brucellosis (Eker et al., 2011; Gulsun et al., 2011)
Necrosis
In this research, the briedging of necrosis on P0+ P0-, and P did not occur, yet light and moderate necrosis were happened. Necrosis in this research was shown in group P0+ and P. the result of the statistical analysis on the necrosis scoring is marked with the increasing of the necrosis based on the treatment and control which is found that there are significant changes in P0-, P0+ and P. In the P0-, there were a light necrosis and karyorexis. In the blood smear P0+ and P were found necrosis around hepatic tubulus and there is also Karyolysis occurred in the cell core on the portal area.
Necrosis is uncontrollable death as the response of cell damage (Kemp et al., 2008). There are 2 main type of necrosis, first, coagulative necrosa (protein damage is more than enzyme damage). Microscopic figure of this type is the increasing of red color (eosinophilia) on the cytoplasm and the decreasing blue color (basophilia) in the nucleus. This type can be found in the organs which have more fat such as brain and it is usually followed with liquefactive necrosa. Second type is liquefactive necrosa which is the enzyme damage is more than protein damage. The cell will lose its shape, after that the macrofag will eat the death tissue. This type usually happens in the organs which have more fat and less protein such as brain and pancreas as an organ which has higher enzyme level. Fat necrosa is the change of adipose tissue because of the trauma or enzyme deficiency from pancreas. These reasons can cause the damage of fat and the excretion of fatty acid which is combined with calcium (chalky deposite) (Kemp et al., 2008).
The damage of the cells which is caused by chemical and other noxious stimuli can lead to a complex phenomenon of the cells death (Fawthrop et al., 1991). Necrosis which was occurred in this research was caused by the death of hepatic cell on the sample animals which was vaccinated with Brucella Abortus strain RB51 and infected by Brucella Suis local isolate that did not stimulate any changes. Then it caused the releasing of some enzymes such as ATPase, phosopholipase, protease and endonuclease. Some of those enzymes damage the membrane of the cells (phospholipase and protease), endonucleus cause the damage of main core chromatine. The damage of the membrane and the core can cause the death of the cell. Beside that, Lipoprotein from Brucella Abortus strain RB51 vaccine induce the secretion of TNFα and IL-6 which are the inflammatory mediator. TNFα also induce cell necrosis.
Fibrosis
According to statistical analysis, the scoring of fibrosis could be marked with the formed of scar tissue. The next development is in the form of nodules and hepatocytes which regenerated and become cirrhosis. Also, there was not any changes between P0-, P0+ and P Liver fibrosis is the response of scar healing towards repeated lesion. After the lesion of liver (e.g: hepatitis virus), parenchyma cells regenerates to replace necrotic cell or apoptotic and inflamed cell. This process relates to inflammation response and limited deposition of ECM. Inflammation cell such as lymphocytes or polymorphonuclear activates Hepatic Stellete Cells (HSCs) to secrete collagen. Active HSCs secretes inflamed chemokine, express cell adhesion molecules and set the activation of lymphocyte. Hepatocyte which damaged release ROS (reactive oxygen species) and fibrogenic mediator, also induce white blood cells for the inflamed cells. Therefore, a positive feedback loop exists in which inflamed and fibrogenic cells stimulate each other in amplifying fibrosis (Friedman, 2008).
Conclusions
Brucella abortus strain RB51 vaccine did not decrease the number of necrosis cell of mice (Mus musculus) hepatic which had been infected with Brucella suis. Besides that, Brucella abortus strain RB51 vaccine did not decrease the occurrences of fibrosis tissue in the mice (Mus Musculus) hepatic which had been infected with Brucella Suis.
Authors Contribution
Study design: Puspitoyani P.E and Sabdoningrum E.K. Data collection: Puspitoyani P.E, Sabdoningrum E.K, and Handijatno D. Data analysis: Puspitoyani P.E, Sabdoningrum E.K, and Handijatno D. Manuscript writing and revisions for important intellectual content: Puspitoyani P.E, Sabdoningrum E.K, and Handijatno D.
Conflict of Interest
The author reports no conflict of interest of this work.
References






