Advances in Animal and Veterinary Sciences
Review Article
Bird Glutathione S-transferases: Endogenous and Exogenous Toxic Insults
Sreenivasulu Dasari*, Muni Swamy Ganjayi, Balaji Meriga
Department of Biochemistry, Sri Venkateswara University, Tirupati 517502, Andhra Pradesh, India.
Abstract | All organisms are producing non-nutritional chemical agents during normal cellular metabolism which commonly termed as endogenous toxic species. In addition to that the living beings are exposing to un-useful foreign chemical species which commonly termed as xenobiotics. Consequently that the organisms have evolved mechanisms to minimize the effects oxidative metabolism that is the production of reactive oxygen species (ROS), and have developed effective antioxidant defence system to mitigate harmful toxic chemicals. Because of highly mobile nature birds are the victims of exposure to wide spectrum of environmental pollutants. Hence, they have developed more efficient antioxidant enzymatic system to detoxify both endogenous and exogenous (xenobiotic) toxic agents for their survival. That the antioxidant enzymatic system which has been divided into three phases. Glutathione S-transferases (GSTs) are belongs to phase II defence enzymes which play major rolein both endogenous and exogenous toxic agents detoxification. GSTs are act as the biomarkers in birds which are either living in polluted ecosystem or exposed to xenobiotics. That the gradual loading of the environment with toxic chemicals which leads to considerable attention on xenobiotic metabolism in wild animals and birds. The screening of GSTs in birds can provide the information about the metabolism related specific mechanisms of chemical toxicity. That the single amino acid change in GST can alter the catalytic property of enzyme which leads to inactivation of detoxification capacity. The goal of this review is to provide the better information about GSTs role in detoxification of endogenous and exogenous toxic agents in birds.
Keywords | Bird, Glutathione S-tranferases, Biomarker, Detoxification, Toxic agents
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 24, 2017; Accepted | July 19, 2017; Published | September 11, 2017
*Correspondence | Sreenivasulu Dasari, Department of Biochemistry, Sri Venkateswara University Tirupati, 517502, Andhra Pradesh, India; Email: dasarisreenivaasulu@gmail.com
Citation | Dasari S, Ganjayi MS, Meriga B (2017). Bird glutathione s-transferases: endogenous and exogenous toxic insults. Adv. Anim. Vet. Sci. 5(9): 388-394.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.9.388.394
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Dasari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
That the xenobiotic metabolism is the biochemical modification by living organisms through the action of specialized enzymatic system. At the time of xenobiotic metabolism lipophilic chemical compounds are converts into hydrophilic compounds which are ready to excrete. That the xenobiotic metabolism which is divided as three phases. The glutathione S-transferases (GST) are most important xenobiotic metabolizing enzymes in phase II defence enzymes. Glutathione S-transferases (GSTs) (EC. 2.5.2.18) are belongs to a family of multifunctional enzymes which conjugate electrophilic intermediates with the endogenous tripeptide glutathione (GSH) (Hayes et al., 2005).Based on both functional and sequence similarities that the cytosolic GSTs are classified, which composed of two sub units (dimers) within the same class (Wilce and Parker, 1994). There are cytosolic, mitochondrial and membrane associated GSTs, but detoxification is the key function of cytosolic GSTs (Hayes et al., 2005). Mammalian cytosolic GSTs are extensively studied (Frova, 2006). GSTs play a key role in cellular detoxification, protection of macromolecules from reactive electrophiles, environmental carcinogens, reactive oxygen species and chemotherapeutic agents (Strange et al., 2000), which catalyse the nucleophilic addition of glutathione to several xenobiotics that including phase I electrophilic and carcinogenic metabolites (Senhaji et al., 2015; Nebert and Vasilou, 2004; Hayes and Strange, 2000). The GSTs can serve as peroxidases, isomerases and thiol transferases (Board et al., 2000).
Kumar et al. (1980) said that the relative specific activities of GSTs in various tissues of pigeon are as follows: kidney > liver > testes > brain > lung > heart. Quail liver cytosolic GSTs were purified by using S-hexylglutathione affinity column in tandem by a glutathione (GSH) affinity column and designated as QL 1, QL 2, QL 3a, QL 3b and QL 4 (Dai et al., 1996). Based on immunochemical studies, it was reported that the QL 1 and QL 2 are similar to the rat mu (μ) class GSTs, QL 3a and QL 3b are related to the rat and mouse alpha (α) class GSTs (Dai et al., 1996). N-terminal sequence of QL 2 is identical to the chicken mu (μ) class GST i.e CL 2 (Dai et al., 1996). The two initial valine residues which are on the N-terminus are unique character of bird mu (μ) class GSTs when compared the mammals (Dai et al., 1996).
Chang et al. (1990) was purified CL1, CL2, CL3, CL4 and CL5 GSTs from chicken liver by using S-hexylglutaathione and glutathione (GSH) combination affinity column. Chang et al. (1992), Liu et al. (1993), alpha (α) class GST, Liu and Tam (1991) mu (μ) class GST, Hsiao et al. (1995) theta (θ) class GST and Thomson et al. (1998) sigma (σ) class GST cDNAs isolated by both cloning experiments and Expressed Sequence Tag (EST) database search from domestic chicken. It was reported that the CL1 (θ GST) and CL5 (σ GST) are not detected chick liver purified fraction by glutathione (GSH) affinity column (Hsieh et al., 1999). Chicken GSTs protein structure and DNA sequence are elucidated (Chang et al., 1990; Liu and Tam, 1991; Liu et al., 1993). Chick embryo brain GSTs were purified by using glutathione CL-agarose affinity column and designated them as CBI and CBII (Dasari et al., 2016). Based on biochemical characterization, CBI and CBII GSTs which related alpha (α) and mu (μ) class GSTs (Dasari et al., 2016).
Kim et al. (2011) was cloned six alpha (α) class GST subunits of turkey were cloned and expressed in E. coli. It was reported that the molecular weights of expressed tGSTA subunits were similar to the alpha (α) class GSTs (Kim et al., 2010). GSTs are ubiquitous multifunctional enzymes and they play an important role in detoxification of pesticides (Ezemonye and Tongo, 2010); carcinogens like mycotoxin aflatoxin B1(AFB1) (Eaton and Bammler, 1999). GST has catalyses the addition of glutathione (GSH) to endogenous xenobiotics (Satheesh et al., 2010).
Environmental Toxic Agents and Oxidative Stress
That the environment is whether intentionally or unintentionally loading with foreign chemical compounds (xenobitics) which released by industries. But thousands of organic pollutants have been produced which released into environment from twentieth century (Helm et al., 2011). Many of such chemical agents are extremely stable, which hazard to the living organisms. That the stable organic pollutants which accumulate in all ecosystems and they are transported by air, water and migratory species and deposited in too distance from the place of their production (Choi and Wania, 2011).
That the oxidative stress can be characterized by either an oxidative burst or rapid and transient production of high levels of reactive oxygen species (ROS). That the oxidative burst can be inducing either directly by various pollutants or indirectly by their metabolism (Droege, 2002). Several mechanisms are there that the formation of oxidative stress by xenobiotics. That the free radicals which can readily react with biomolecules.The cells have developed protection mechanism against oxidative stress, due to the inefficiency of protection mechanism, oxidative stress can damage biomolecules (Zhang et al., 2004). That the biochemical markers which can provide information about the health status of an organism and this can be used as early warning signals of particular stress (Korte et al., 2000).
Detoxification of Exogenous Hazardous Agents by Gst
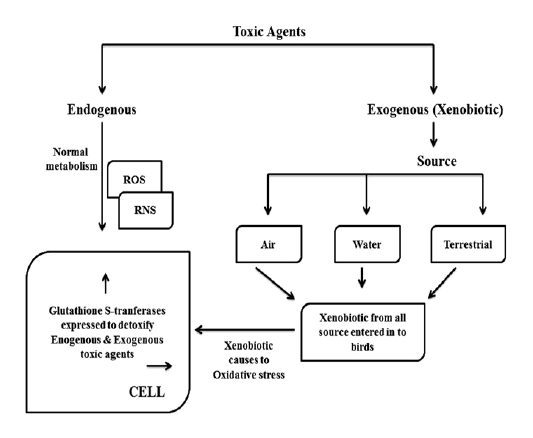
Glutathione S-transferases (GSTs) are detoxifying a large number of exogeneous toxic agents like carcinogens, drugs and environmental pollutants as shown in Figure 1. That the chemotherapeutic agents of cancer such as adriamycin, 1, 3-bis (2-chloroethyl)-1-nitrosourea (BCNU), busulfan, carmustine, chlorambucil, cis-platin, crotonyloxymethyl-2-cyclohexenone (COMC-6), melphalan, mitozantrone, thiotepa, cyclophosphamide and ethacrynic acid are detoxified by GSTs (Hamilton et al., 2003). Environmental chemicals and their metabolites like acrolein, atrazine, DDT, inorganic arsenic, lindane, Malathion, methyl parathion, muconaldehyde and tridiphane are detoxified by GST isoenzymes (Abel et al., 2004a, 2004b). A large number of epoxides like fosfomycin and those derived from environmental carcinogens, polycyclic aromatic hydrocarbons (PAHs) are detoxified by GST. Activated metabolites N-acetoxy-PhIP of heterocyclic amine, 2-amino-1-methyl-6-phennylimidazo [4, 5-b] pyridine (PhIP) which produced by cooking protein-rich food is also detoxified by cytosolic GST isoenzymes.
Activation of Xenobiotics by GST
The conjugation reaction catalysed by GST can form less reactive and readily excreted products. But in some cases that the glutathione (GSH) conjugate is more reactive than the parent compound like short chain alkyl halides that contain two functional groups and 1, 2-dihaloethanes, where the GSH conjugate rearranges to form an episulfonium intermediate which responsible for DNA modification (Guengerich et al., 2003). The conjugation of GSH with the solvent dichloromethane facilitate formation of the highly unstable Schloromethlglutathione which capable to modify DNA (Guengerich et al., 2003; Wheeler et al., 2001).
That the moderately toxic compounds like allyl-, benzyl-, phenethyl-isothiocyanates and sulforaphane are reversibly conjugated with GSH by GST to form thiocarbamates which spontaneously degrade to their isothiocyanates by releasing GSH. Again that the isothiocyanates may be taken up by the cell and re-conjugated with GSH and then form thiocarbamate and then revert to the isothiocyanate. Due to this cyclic process, intracellular GSH levels are decreased and facilitate the distribution of isothiocyanates entire the body. Such isothiocyanate either low GSH content or not conjugated with GSH, but rather are more likely to thiocarbalate proteins, which result in cell death (Xu and Thornalley, 2001).
Detoxification of Endogenous Hazardous Agents by Gst
The GST isoenzymes are exhibits moderate role in lipid peroxidation in biological membranes. The GSTs exhibit non selenium glutathione peroxidase (GPx) activity with 1-palmitoyl-2-(13-hydroperoxy-cic-9, trans-11-octadecadienoyl)-L-3-phosphadylcholine, phospatidylcholine hydroperoxide and redusing lipid hydroperoxides which are in membranes (Yang et al., 2002; Li et al., 2005; Prabhu et al., 2004). The transferases can reduce cholesteryl hydroperoxides (Hamdy et al., 2003), fatty acid hydroperoxides, (S)-9-hydroproxy-10, 12-octodecadieonic acid and (S)-13-hydroperoxy-9, 11-octadecadieonic acid (Liang et al., 2004). That the lipid peroxidation end products like 2-alkenals acrolein, crotonaldedyde and 4-hydroxy-2-alkenals are conjugate with GSH by GSTs (Liang et al., 2004). GSTs catalyze the GSH conjugation with cholesterol-5, 6-oxide, epoxyeicosatrienoic acid and 9, 10-epoxystearic acid, which indicating its role in cellular protection against oxidative stress harmful electrophiles (Hayes et al., 2005).
Birds in Polluted Environments
As shown in Figure 1, birds are victimizing to toxic agents by air, water and territory. It was reported that the effects of indirect pollution may be very common in nature than the direct pollution and which may affect even at lower contaminant levels (Eeva et al., 2003). In addition to normal metabolism during the processing of many toxic compounds produce several reactive oxygen species and they may increase the oxidative stress in species which are live in polluted environments (Valko et al., 2005). Great tits are showed lower breeding performance with poor quality diet in polluted environment which suggests the importance of secondary environmental changes including food quality (Eeva et al., 2005; 2009). That the individuals which are living in polluted environments can exposed to a mixture of pollutants at sub lethal concentrations which shows synergistic, antagonistic effects. Based on oxidative potential and reactivity with biomolecules the hydroxyl radicals are the most important in biological and toxicological terms (Ercal et al., 2001). Little attention was paid on the function of antioxidant enzymatic system and it’s levels in different bird species as an indicators of oxidative stress in polluted environments (Berglund et al., 2007; Norte et al., 2009; Hegseth et al., 2011). It was reported that the oxidative stress may be a physiological challenge and which is a factor potentially affecting bird migration strategies, but it is not yet recognized (Jenni-Eiermann et al., 2014).
Defensive System in Birds
Especially in birds that the detoxification efficiency is depend on with diet composition (Ronis and Walker, 1989; Fossi et al., 1995) and sometimes it is associated with the metabolic rate (Ronis and Walker, 1989). Low rates of mitochondrial oxygen radical production and high blood glucose levels, which are the unique molecular mechanisms in birds than the other vertebrates and due to these unique character birds can easily defend free radicals and oxidative stress (Pamplona and Costantini, 2011). Recognizable attention is increasing that the balancing investment in antioxidant defences to sustain to oxidative damage has possibly influence life history traits (Monaghan et al., 2009). It is suggests that the up regulation of antioxidant system in free flying migrants which adapt their antioxidant system to the sustainability to extraordinary exercise at very high metabolic rate (Jenni-Eiermann et al., 2014).
Gst is a Biomarker in Birds
GSTs are useful as biomarker in ecological risk assessment of pesticide contaminated environment (Ezeji et al., 2012). It was reported that the presence of glutathione S-trnsferases (GSTs) activity in wild birds (Witt and Snell, 1968). Mukthar and Bresnick (1976) said that the GST is an inducible enzyme. It have been identified that the species differences in conjugation reactions, some birds use ornithine instead of glycine for conjugation with xenobiotics (Baldwin et al., 1960), it may be due to difference in evolutionary scale of avian species. That the significant level of GST activity in hepatic and extra hepatic tissues of pigeons are similar to the tissues of rodent. Pigeon hepatic GSTs were inducted with 3-methylcholanthrene (3-MC) (Kumar et al., 1980). It was reported that the various biomarkers are measure different aspects of redox balance, which suggests the careful planning is need at the level of designing certain study set up (Horak and Cohan, 2010). Glutathione related enzymes like GPx and GST are most prominent biomarkers in birds (Isaksson, 2010) and elevation of those enzyme activities was reported in white stork (Ciconia ciconia) (Kaminski et al., 2009), the pied flycatcher (Berglund et al., 2007), the great tit (Isaksson et al., 2005; Norte et al., 2010). It was reported that the GST activity induction is an evolutionary response of cells to protect against metabolites and degenerative disorders which related to oxidative stress (Hayes et al., 2005; Raza, 2011). In Avian species, tissue distribution of GST is not similar to mammals; high renal specific activity is the most distinctive feature of Avian GST (Maurice et al., 1991). As shown in Figure 1, GST is expressed in response to both endogenous and exogenous toxic agents.
Usually, as shown in Figure 1, in normal metabolism toxic agents like reactive oxygen species (ROS) and reactive nitrogen species (RNS) are formed in cellular system. In addition to that, environments are loading with toxic chemical agents in the form of pesticides, insecticides, agriculture related chemical formulas, industrial effluents and motor vehicle pollutants etc. Because of highly mobile nature, birds are victimizing to these exogenous (xenobiotic) toxic agents by air, water and terrestrial. When the toxic agents enter into cellular system, they cause to oxidative stress in addition to deleterious effect of them. Glutathione S-transferse (GST) is expressed more and more to detoxify both endogenous and exogenous toxic insults.
Susceptibility of Birds
Poultry birds (domestic fowls) are victimizing to pesticide poisoning due to their tendency to eat pesticide contaminated food and other items in the environment (Ezeji et al., 2011). Domestic turkeys (Meleagris gallopavo) are one of the most susceptible species known to AFB1 (Klein et al., 2000; Rawal et al., 2010). The efficiency of GST conjugation is a principal ‘‘rate-limiting’’ determinant for AFB1 action in individuals and species (Ilic et al., 2010). Numerous studies have demonstrated that individual and species susceptibility to AFB1-induced toxicity and hepatocarcinogenicity is strongly associated with GST-mediated detoxification of AFBO (Monroe and Eaton, 1988). Acute and chronic toxic effects of AFB1, apparently because of the extremely high affinity toward AFBO exhibited by their hepatic Alpha-class A3 subunit (Buetler et al., 1992; Hayes et al., 1992).
Comparative toxicology studies have shown that wild turkeys are substantially more resistant to AFB1 compared to domestic turkeys (Quist et al., 2000). But GSTs of the domestic turkeys have little or no AFBO affinity activity with AFB1 (Klein et al., 2002; 2003). Wild and heritage turkeys might contain potentially useful GST alleles not found in domestic birds (Kim et al., 2013).
Bird Gsts Specific Activity and Mutations
In pigeon, GST activity with 1-chloro-2, 4-dinitobenezene (CDNB) is higher in 40 to 44 times in liver and kidney than with 1, 2-dichloro-4-nitrobenezene (DCNB) (Kumar et al., 1980). In Japanese quail, GST activity with CDNB and cumene hydroperoxide (CHP) elevated in kidney and liver than brain and lung (Dai et al., 1996). Dai et al. (1996) reported that both GST activity with multiple substrates and glutathione peroxidase (Gpx) activity with CHP are higher in kidney than in liver of Japanese quail and also Gpx activity is higher than GST activity in lung.All recombinant GSTA and GSTM of turkey have activity with prototype substrates (Kim et al., 2010, 2011, 2013). In turkeys, GST A1-2 and GST A1-3 shows the highest activity with CDNB, ethacrynic acid (ECA) and CHP but GST A1-1 shows highest activity with DCNB (Kim et al., 2011). Turkey hepatic cytosol GSTAs, shows catalytic activities with the GST substrates such as CDNB, DCNB, ECA and CHP, but with CDNB and CHP which appeared to be the much more (Kim et al., 2011). Turkey hepatic cytosol GSTA shows the least activity with 1, 2-dichloro-4-nitrobenzene (DCNB), which is a specific substrate of mu (µ) class GST (Vanhaecke et al., 2000).
Numerous studies have proved that the single amino acid site change can influence catalytic properties, substrate specificity and stereoselectivity of GSTs (Bammler et al., 1995). In chickens, Lys 15 and Ser 208 of GSTA 1 are responsible for high specific activity and selectivity with ethacrynic acid (ECA) (Liu et al., 1997). Kim et al. (2011) suggested that the site-directed mutagenesis with molecular modeling may be useful to elucidate the relative contributions of specific amino acid residues to the catalytic function of GSTAs of turkey.
Conclusion
That the endogenous and exogenous toxic agents which are not benefit to the body. In addition to that they injure to the body if they stayed long period in the living system. Therefore, they should be remove by set of metabolic pathways which mediated by antioxidant enzymatic system. Birds are frequently encountering environmental xenobiotic substances which have hazardous biological activity. GSTs are the versatile toxic agents metabolizing enzymes among antioxidant enzymatic system. In birds, GSTs are playing major role in detoxification of toxic agents and also act as biomarker to evaluate the health condition of birds. The single amino acid change can alter the GST antioxidant activity. It is concluding that the GST can detoxify toxic agents in birds and an amino acid can change GST activity which leads to susceptibility of birds to various environmental toxic agents and endogenous toxic species.
Acknowledgements
Authors are thankful to University Grants Commission, New Delhi, India (Grant No: 20601/UGC-I (2)/RGNF/2007) for providing Financial Assistance.
Conflict of interest
Authors do not have any potential conflict of interest.
AUTHORS Contribution
This review was written by DS, quality checked by GMS and technical guidance given by BM.
References