Introduction
Nosocomial infections are hospital acquired infections which may be localized or systemic form acquired by the patient who was admitted for reasons other than infection, because of the existence of an infectious agent or its toxin which was not present or incubating at the time of hospital admittance (Horan et al., 2008). Furthermore they are the high-flying reasons for the failure of advanced health treatment (Mielke, 2010). Generally Nosocomial infections are frequent impediment encountered by the hospitalized human beings, and nosocomial bloodstream infections are the eighth leading cause of death in the United States (Burke, 2003). About 5–10% of patients acquire infections from human hospitals and approximately 90,000 deaths per year due to nosocomial infections (Burke, 2003). The risk factors in veterinary hospitals are comparable to those in human hospitals. Prevalence studies have shown that 4-9% patients endure from a nosocomial infection (Mielke, 2010). The occurrence of nosocomial infections in veterinary hospitals has not been well established and in nascent stage (Boerlin et al., 2001; Johnson, 2002; Morley, 2004; Smith, 2004; Traub-Dargatz et al., 2004; Morley and Weese, 2008), even though the nosocomial infections are of immense significance in the field of veterinary medicine since quite a lot of nosocomial outbreaks of different etiologies in veterinary hospitals has been documented (Castor et al., 1989; Madewell et al., 1995; Hartmann et al., 1996; Konkle et al., 1997; Tillotson et al., 1997; Seguin et al., 1999; Schott et al., 2001; Weese and Armstrong, 2003; Cherry et al., 2004; Wright et al., 2005; Weese et al., 2006a; Dallap et al., 2010; Goehring et al., 2010; Steneroden et al., 2010). Six of those outbreaks had evidence of zoonotic infection (Konkle et al., 1997; Seguin et al., 1999; Schott et al., 2001; Cherry et al., 2004; Wright et al., 2005; Weese et al., 2006a).
History
In the middle ages, the hospitals were established to treat pitiable plague victims and to till date the hospitals and health care personnel are serving the society with great dedication and hospitals has become more obligatory in the healthy living of both human and animals. In 1869, Sir James Young Simpson did an initial signature in the field of hospital epidemiology by his study with more than 4000 amputees in Scotland and England where he found the mortality rate was higher in patients who remained in the hospital for post-operative care. He used the term “hospitalism” to express the risk linked to hospital care. After many years of dedicated works by people like Oliver Wendell Holmes, Ignaz Philipp Semmelweis, Louis Pasteur, Joseph Lister, and Robert Koch, to illuminate the risk factors connected with hospital-related infections (Semmelweis, 1848; Major, 1954; Eickhoff, 1981) and introduction of rectification guidelines like hand washing and sterilization of instruments used for surgery reduced the propagation of disease and death (Major, 1954). The “tide of complacency” in the history of control of nosocomial infection is after the discovery of antibiotics, since the understanding and research of nosocomial infections was not highly developed in the time of world wars (Eickhoff, 1981). In 19th century after the commencement of advanced research in medicine and surgery which revolutionised the healthcare facilities and interventions but also unearthed the unintended harm to patients i.e. Nosocomial infections through health care settings.
Epidemiology and Risk Factors
Nowadays, some of the important factors which have been often associated with the nosocomial infections in human health care centres like employing invasive devices (e.g., intravenous and urinary catheters), lengthened hospitalization of decisively ill patients, complex medical treatment and surgical procedures, and the extensive exploitation of antibiotics (Boerlin et al., 2001; Johnson, 2002) are increasing in veterinary hospitals. Intensive care Patients are at 5 to 10 times higher risk of developing a nosocomial infection than normal patients (Weber et al., 1999). The threat of developing infection for hospitalized patients are due to extrinsic risk factors, such as the use of invasive devices and intrinsic risk factors, like underlying ailment during hospitalization (National Nosocomial Infections Surveillance System, 1991; Emori and Gaynes, 1993). Nosocomial infections are either site specific (e.g., surgical site infection) or unit specific (e.g., intensive care unit). The possible source for nosocomial agents are the patient’s own flora, health personnel, instruments and equipment of hospital (Hardy et al., 2006; Marshal et al., 2009) (Figure 1). The amplifying risks which favours the transmission of antibiotic resistant pathogens and other nosocomial infections in veterinary hospitals are lack of hygiene, employing invasive devices, prolonged treatment, longer visits by health care worker and caseloads (D’Agata et al., 2012), delayed and breaks in treatment (D’Agata et al., 2008), lengthy hospital stay (D’Agata et al., 2007) and dependence on antimicrobials (Johnson, 2002; Morley et al., 2005), administration of antiulcer drugs (Ruple-Czerniak et al., 2013).
In one individual-based model study to scrutinize the effect of movement of dogs to various locations in a veterinary teaching hospitals, it was found out that nosocomial infection transmission is greater in diagnostic rooms, intensive care units and housing wards than operation theatre and lobby and it also revealed that transmission following the contact with healthcare personnel was more common (Suthar et al., 2014). Veterinary hospitals are the major source and accountable for increasing prevalence of a MDR E. coli in horses (Ahmed et al., 2012). Veterinary personnel and veterinary hospital environments are allegedly most important key factors in risk of gaining antibiotic resistant pathogens by hospitalized dogs (Hamilton et al., 2013; Heller et al., 2010; KuKanich et al., 2012).
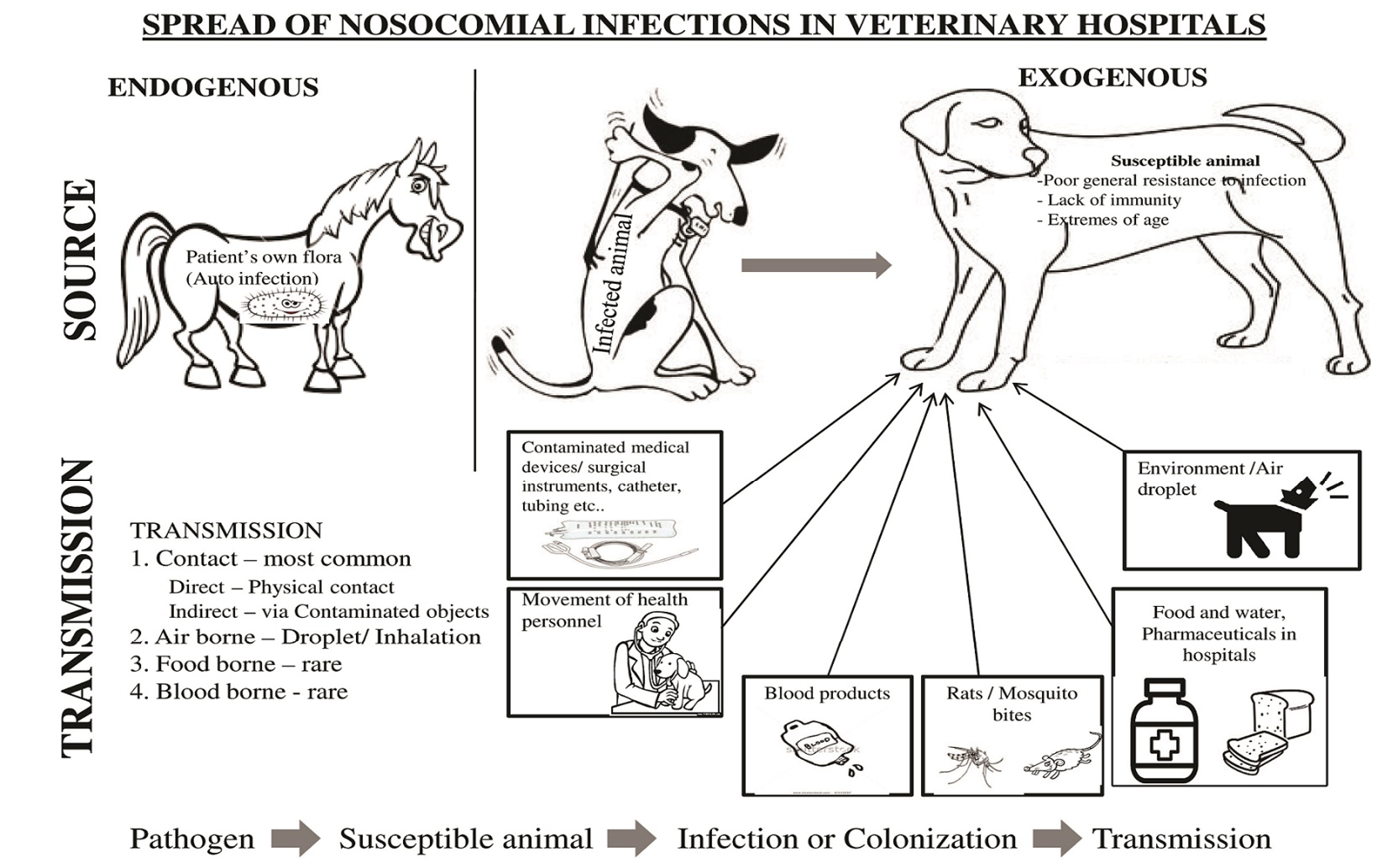
Figure 1: Spread of nosocomial infections in veterinary hospitals
Impacts of Veterinary hospital associated infections
Nosocomial infections are the growing cause of morbidity and mortality in both human and veterinary medicine (Weese, 2008a; Weese, 2008b; Owens et al., 2008; Faires et al., 2010). Implication of multiple drug resistant bacterias in nosocomial infections are the main concern because of high morbidity, high cost and duration of treatment and more challenging to eliminate (Weese, 2008a; Owens et al., 2008; Faires et al., 2010) and there is potential zoonotic risk (Suthar et al., 2014). The food animals with resistant antimicrobial bacteria pose a serious public health risk (Howard et al., 2003), but the pet animals transmit the resistant bacteria directly to human population by acting as a reservoir (Guardabassi et al., 2004; Lloyd, 2007; Murphy et al., 2009; Song et al., 2013). MDR in companion animals encompass stern public health impacts (Abraham et al., 2014). Undue exploitation of antibiotics and horizontal gene transfer may lead to increased possession of antibiotic resistance, thereby escorting to longer period of antibiotic treatment and treatment failure (Levin et al., 2006; Bootsma et al., 2012). The occupational risk to the veterinary health personnel and staff are other concern (Jordan et al., 2011). There will be huge economic loss due to this outbreaks and it may tarnish the reputation of the concerned veterinary hospital with reduced client confidence and increased panic.
MRSA
With all the evidence Staphylococcus aureus is an important pathogen of both human and animal causing meek skin infections to grave bacteraemia. Generally almost all S. aureus strains are resistant to beta-lactam antibiotics since it is producing pencillinases. Owing to this resistance, meticillin which is resistant to penicillinases were extensively used to treat S. aureus Infections. In late 1950s the meticillin use was initiated in human medicine to treat resistant S. aureus infections. Meticillin resistance was accounted in the early 1961 (Jevons, 1961) and the resistance development was demonstrated (Barber, 1961). In 1970s MRSA emerged as a staid public health issue in US hospitals (Panlilio et al., 1992). In 1990s MRSA expanded itself as a solemn nosocomial infection throughout the world (Ayliffe, 1997). Eventhough MRSA was first emerged as a human pathogen, nowadays MRSA reports in animals are gradually increasing mainly in equine and companion animals. It is known that S.aureus is one of the chief cause for mastitis and the extensive use of intramammary antibiotics in cattle, so it is not unanticipated that, MRSA was isolated from milk of a mastitic animal, which is first among animals (Devriese et al., 1972). After this MRSA has been isolated from various species like dogs (Pak et al., 1999), cats (Scott et al., 1988), sheep (Goni et al., 2004), horses (Seguin et al., 1999), pigs (Voss et al., 2005) and chickens (Lee, 2003). Methicillin-resistant Staphylococcus aureus (MRSA) is an important concern nowadays in pet animals for being a significant cause in nosocomial infections (Weese, 2008b; Faires et al., 2010; Weese et al., 2010) and also a threat of zoonoses to veterinary health care personnel (Loeffler et al., 2010; Burstiner et al., 2010) and horses may act as a reservoir of MRSA and serve as a source of infection to humans (Weese et al., 2005a). Interestingly, various studies reported that MRSA isolated from companion animals like dogs and cats are found indistinguishable from MRSA isolated from human associated infections (Baptiste et al., 2005, Loeffler et al., 2005; O’Mahony et al., 2005; Middleton et al., 2005; Rich et al., 2005; Hanselman et al., 2006; Malik et al., 2006). The association was found out by various phenotypic typing and molecular typing methods like PFGE (O’Mahony et al., 2005), SCCmec, MLST (Enright et al., 2000), spa typing (Moodley et al., 2006). The data obtained through all these studies sturdily suggesting the bidirectional transmission of MRSA between companion animals and human (Leonard et al., 2006). Whereas with the assistance of epidemiological typing it is proved that strains of MRSA isolated from horses and equine associated personnel are different (Baptiste et al., 2005; O’Mahony et al., 2005; Weese et al., 2005a; Cuny et al., 2006). Outbreaks linking both horses and humans in veterinary hospitals were reported from the United States (Seguin et al., 1999), Canada (Weese et al., 2005a), Ireland (O’Mahony et al., 2005), Austria (Cuny et al., 2006, 2008), the Netherlands (van Duijkeren et al., 2010) Switzerland (Sieber et al., 2011) and Israel (Schwaber et al., 2013). The typing studies revealed that the MRSA isolated from pigs (Voss et al., 2005; Van Dijke et al., 2006) and cattle (Kwon et al., 2005) are distinct from MRSA of human lineage. Numerous studies have acknowledged the increasing MRSA infections in companion animals are coupled with post-surgical infections and wounds (Rich et al., 2004; Leonard et al., 2006) and indwelling devices and suture materials (Leonard et al., 2006). Smith et al. (1989) accounted 38% isolation rate of bacteria in dogs from the orthopaedic implant fixation site soon after the removal following the union of the closed fracture (Smith et al., 1989). The primary route of MRSA infection transmission in veterinary hospitals is through hands of healthcare personnel (Leonard et al., 2006). As far as public health is concerned many years ago likelihood of companion animals to act as the source for the zoonotic infections of Staphylococci is suggested (Mann, 1959) and many reports have offered the suggestion that animals may act as reservoirs of MRSA infections to human. In one case a cat was involved as a MRSA source for nurses in geriatric care unit (Scott et al., 1988). In another case of two nurses, a dog was considered as a reservoir for the treatment failure for MRSA and reinfection (Cefai et al., 1994). Another interesting case of recurrent MRSA infection in a diabetic patient and his wife, the source was found out to be a dog after the sampling of dog’s nares which is colonized with identical PFGE type of MRSA from the couple and the infection was halted in the couple when the dog was treated completely for MRSA (Manian, 2003). Some reports implies that swine farmers are at risk of S.aureus and MRSA colonization (Armand-Lefevre et al., 2005; Voss et al., 2005). An attending pet surgeon who was an asymptomatic carrier of MRSA was found to be associated with postoperative wound infection of MRSA in five dogs (Leonard et al., 2006). In a survey conducted in a referral hospital for small animals from the nasal and oral mucosae of hospital staff and animals, MRSA was isolated from four dogs and 14 staff (Loeffler et al., 2005). The veterinary personnel working in veterinary hospitals are at greater risk of MRSA colonization and hence MRSA colonization is regarded as an occupational risk (Hanselman et al., 2006; Anderson et al., 2008; van Duijkeren et al., 2010; Jordan et al., 2011). MRSA colonized personnel plays a vital task in the MRSA introduction and transmission in veterinary health care settings (van Duijkeren et al., 2010; Sieber et al., 2011) and besides this they spread the infection to other persons (Anderson et al., 2008). An outbreak occurred at teaching veterinary hospital in Israel by an uncommon MRSA strain displayed the bidirectional spread between animal and humans (Schwaber et al., 2013). From animal health relevance methicillin-resistant Staphylococcus pseudintermedius (MRSP) is often associated with pyoderma and surgical site infections (Weese, 2008b) has swiftly emerging as an opportunistic nosocomial infectious agent (Laarhoven et al., 2011). MRSP can be detected in apparently normal animals (Murphy et al., 2009) and in veterinary hospital environment (Nienhoff et al., 2011). A large outbreak was recorded in Finnish veterinary teaching hospital caused by MRSP (Gronthal et al., 2014).
Clostridium difficile
Following the discovery of C. difficile in 1935, it was thought to be the normal faecal flora of the newborns rather than pathogenic (Hall and O’Toole, 1935). But the preamble of broad spectrum antibiotics prompted the emergence of pseudomembranous colitis and it is now considered one of the serious cause of nosocomial infections since it is emerging in the human community and food animals (Rupnik et al., 2009). Clostridium difficile is a gram positive, spore forming anaerobic bacterium. C. difficile spores are comparatively resistant to common disinfectants making C. difficile a lasting environmental contaminant. Many studies have reported a strong association between nosocomial C. difficile infection and antibiotic therapy in horses (Madewell et al., 1995; Ruby et al., 2009; Barr et al., 2013). In a Prospective study conducted in horses pre-treated with penicillin followed by experimental infection resulted in increased isolation of C. difficile from faecal samples, revealing the role of antimicrobials as a risk factor (Gustafsson et al., 2004). Prevalence and distribution of C. difficile was studied in dogs and cats visiting Veterinary Hospitals and animal shelters (Clooten et al., 2008; Schneeberg et al., 2012). A study conducted in an animal shelter in Germany, reported C. difficile prevalence rate of 5.5% and 3.5% in dogs and cats respectively further the study acknowledged that dogs and cats kept in animal shelters act as a reservoir for C. difficile PCR ribotypes that can infect human (Schneeberg et al., 2012). An another prospective study conducted in a veterinary teaching hospital reported that C. difficile could be isolated from 18% from dogs and cats and the study concluded that antibiotic administration prior to admission and administration of immunosuppressive drugs during hospitalization were risk factors for the nosocomial colonization (Clooten et al., 2008). C. difficile Ribotype 001, which is very common in hospitalized patients in Ontario was also frequently encountered among dogs in Ontario (Weese et al., 2010). A high environmental load of C. difficile is present in veterinary hospitals (Weese et al., 2000). An environmental survey conducted in a teaching veterinary hospital for the presence of C. difficile documented an isolation rate of 6.3%. The places where C. difficile was isolated are places of high animal traffic and rough floor (spores persist in cracks) with more likelihood of faecal contamination (Weese et al., 2000). Similar type of ribotype was reported from various species like in piglets (Keessen et al., 2013), calves (Costa et al., 2011), humans (Debast et al., 2009) and other food animals (Indra et al., 2009) and also in meat implicated in outbreaks (Rodriguez-Palacios et al., 2009). This paved way for making an assumption that C. difficile transmission from animals to human is likely to occur. An Austrian study concluded that animal reservoirs can be a possible source for human CDI infection through the food animals (Indra et al., 2009). However frank zoonosis was not established, owing to the ubiquitous nature of the organism it may act as common source for both animals and human beings.
Multidrug-Resistant Escherichia coi and other Enterobacteriaceae
Multidrug-resistant nosocomial infections is one of the big challenges in tertiary-care veterinary hospitals and have turned out to be endemic in the veterinary hospital environment (Sanchez et al., 2002). Managing E. coli infections in veterinary hospitals has become more challenging task due to the emergence of multiple-antimicrobial-resistant E. coli in food animals and pet animals (Bischoff et al., 2001; Wagner et al., 2014; Zhao et al., 2001). The growing predominance of infections with organisms generating broad spectrum b-lactamses such as the ESBLs (mainly the CTX-M type), AmpC and carbapenemase enzymes are threatening the future of the b-lactam drug. Since b-lactams are inevitable to veterinary practice and the outlook of losing these drugs, warrants the necessity for defining the epidemiology of ESBL and carbapenemase producers in companion animals (Rubin et al., 2014). In the ICU of University of Georgia Veterinary Teaching Hospital, E.coli resistant to 12 antibiotics could be isolated from two dogs housed together, of which one dog died due to sudden septic shock. Followed by this incident 21 hospital-acquired E. coli infections were reviewed and found out that the isolates had similar antibiotic resistance profiles (Sanchez et al., 2002). Mostly E. coli isolates of nosocomial events were found to be resistant to cephalosporins, beta lactams and beta lactamase inhibitor clavlanic acid and this attributes to a common ampC class of cephamycinases (Oteo et al., 2010). Especially, ampC-like gene, blaCMY2 was noticed in ceftriaxone-resistant E. coli isolates of animal origin (Zhao et al., 2001). Carriage of ESBL and AmpC-producing E. coli has been acknowledged in many species (Bortolaia et al., 2011). ESBL E. coli (O’Keefe et al., 2010) and AmpC producing E. coli (Oteo et al., 2010) are the emerging problems. ESBL E. coli are linked to range of clinical diseases like urinary tract infections, neonatal septicaemia and wound infections (Pitout, 2010; Ewers et al., 2014) and reported from canine clinical isolates (Sanchez et al., 2002; Shaheen et al., 2011). ESBL-producing uropathogenic Escherichia coli (CTX-M-15) were isolated from 5% dogs and 3.3% cats in Switzerland (Huber et al., 2013). A survey carried out in Netherlands in companion animals and horses demonstrated a 2% prevalence of ESBL and AmpC-producing isolates. The same study identified, ESBL producing S. enterica, Proteus miriabilis and Enterobacter cloaceae from urinary, wound, respiratory, abdominal and bone infections (Dierikx et al., 2012). In Germany from six nosocomially infected dogs, OXA-48 type Carbopenamases producing E. coli and K. pneumonia were isolated (Stolle et al., 2013) and in France CTX-M-15 generating K. pneumoniae have been detected from urinary tract infections of dogs and cats (Haenni et al., 2012). Clinical samples from urinary, wound infections etc. from Germany and European countries were investigated for ESBL producing K. pneumoniae from horses and companion animals. MLST and PFGE were performed for comparing with human isolates. About 75.8% of the strains possess ST15-CTX-M-15, which is a clonal group that emerged in humans in recent times and this strain shared PFGE clusters with human isolates, signifying the dissemination of this clonal group between human and animal populations (Ewers et al., 2014).There are reports of ESBL in other Enterobacteriaceae members like Enterobacter sp., K. pneumoniae, Citrobacter sp. and Salmonella enterica serovar Newport (Haenni et al., 2012; Ma et al., 2009). A recent study conducted in Netherlands reported a high prevalence of faecal carriage of Enterobacteriaceae resistant to third-generation cephalosporins in cats and dogs (Hordijk et al., 2013). Various risk-based case control studies reported that hospitalization is a major risk associated with the dogs to become multi-drug resistant (MDR) E. coli rectal carriers (Gibson et al., 2011; Hamilton et al., 2013). Enterobacter cloacae, also a nosocomial agent and particularly the multidrug resistant strains, are concerning (Wilberger et al., 2012).
Acinetobacter baumannii
Acinetobacter spp. is environmental bacteria and normal flora of skin and mucous membrane in humans and animals and often associated with opportunistic infections in animals (Clemetson and Ward, 1990). Acinetobacter baumannii has emerged over the last decade as a cause of nosocomial infections and the pathogenicity is mainly due to multidrug resistance and biofilm formation. Its ability to acquire the resistance easily making it as one of the ominous agent to the existing antibiotic era (Kempf and Rolain, 2012). Outbreaks have been recorded in hospitalized animals (Francey et al., 2000) and associated with indwelling vascular catheter infection in horses (Vaneechoutte et al., 2000). A. baumannii have been isolated from the clinical samples of companion animals (Francey et al., 2000; Zordan et al., 2011). It is evident that companion animals can be reservoirs of antimicrobial resistant bacteria and the role of pets in the dissemination of antimicrobial resistance is well understood (Guardabassi et al., 2004). The spread of multiresistant A. baumannii from a companion animal clinic to a horse clinic was recorded and the reason behind the transmission was believed to be the hands of staff personnel and students working simultaneously in both the clinics (Boerlin et al., 2001). Majority of the Acinetobacter baumannii isolates belong to ST25, where ST is an emergent clonal lineage which includes Carbapenemases including oxacillinases (OXA-58 and OXA-72), metallo-ß-lactamases (NDM-1) (Zarrilli et al., 2013) and recently for the first time OXA-23 mediated Carbapenem resistance in sequence type 2 multidrug-resistant Acinetobacter baumannii was recorded from a cat with urinary tract infection. A recent study demonstrated increased resistance of A. baumannii towards dessication and its biofilm forming ability on abiotic surfaces which facilitates its persistence in hospital environment (Giannouli et al. 2013), one more study revealed the isolation of carbapenem-resistant A. baumannii from hospital sewges in Beijing (Zhang et al., 2013). A multicentre cross-sectional study conducted to detect the carriage of Acinetobacter baumannii in pets revealed a carriage prevalence of 6.5% and nine carriers were identified from four veterinary clinics. Hospitalization and recent antibiotic therapy was notably associated with the carriage of A. baumannii (Belmonte et al., 2014).
Multidrug-Resistant Enterococci
Enterococci are saprophytic, Gram-positive, facultative anaerobes inhabitating the intestinal tract of humans and animals as a commensal. But now Enterococci has been emerged as an important nosocomial agent , particularly multiple drug resistance species of Enterococci (Poeta et al., 2006) and the development of multiple drug resistance among Enterococci species rose as a significant public health issue due to abuse and over exploitation of antibiotics in human and veterinary practices. It can also thrive well in dry hospital surfaces, medical instruments and resistant to heat, alcohol and chlorine (Fisher et al., 2009; Giraffa, 2002). In Europe Enterococci are ranked second most hazardous pathogen among intensive care unit-acquired bloodstream infections (Bohme et al., 2012). In the USA around 12% of the nosocomial infections are due to Enterococcus species. E. faecalis is the predominant species responsible for clinical infection whereas E. faecium claims the higher antibiotic resistance (Giraffa, 2002). Globally multiresistant E. faecium causing increasing number of hospital associated infections (Willems et al., 2012) and embodies upto to one third of Enterococcal infections (Willems and VanSchaik, 2009). In 1972, Vancomycin was first used and first vancomycin-resistant Enterococci (VRE) were documented 15 years later and NNIS accounted increase of 7.6% in Vancomycin resistant Enterococci between 1989 and 1993 (Metan et al., 2005). In a study conducted in Korea to investigate the possibility of MDR Enterococcus cross transmission, Enterococcus species was isolated from dogs, dog owners, veterinary personnel and five veterinary hospital environment and the study demonstrated a high prevalence of 62.5% of MDR E. faecalis and 75% of MDR E. faecium (Chung et al., 2014). In a study conducted in veterinary teaching hospital in Switzerland, two multiresistant E. faecium isolates with similar antibiotic resistant and PFGE profiles was recovered from two different cats with less than one month interval suggesting the persistence in hospital environment and nosocomial transmission (Boerlin et al., 2001). In the early 1980’s the emergence of Hospital associated Ampicillin resistant Enterococci (ARE) in USA preceded rise of vancomycin resistance in enterococci, which happend in the 1990s, is the reason why virtually all VRE of nosocomial infections in humans are also ampicillin resistant (Grayson et al., 1991) but ARE associated with human infections remain vancomycin susceptible (Tremblay et al., 2013). The hospital associated ARE have been recovered from dogs suffering from urinary tract infections in US (Simjee et al., 2002), Denmark (Damborg et al., 2009), Korea (Kwon et al., 2012) and also from the faeces of dogs departing the intensive care unit of an American veterinary medicine teaching hospital (Ghosh et al., 2011). In a study conducted in veterinary teaching hospital of Canada with the objective of characterizing the ARE strains of dogs and human revealed the cross-transmission between humans and dogs and further supports the significance of antibiotic stewardship to avoid zoonotic spread of canine ARE (Tremblay et al., 2013).
Salmonella
Salmonella enterica was found out to be a most common agent associated with nosocomial outbreaks in veterinary teaching hospitals through a survey conducted by the biosecurity experts (Benedict et al., 2008). Many outbreaks of nosocomial salmonellosis have been reported from different large animal hospitals especially among horses (Steneroden et al., 2010; Tillotson et al., 1997; Dallap et al., 2010; Schott et al., 2001). In horses Salmonella spp. can cause enterocolitis but the organism also present in the absence of the disease making the horse, a transient, sub-clinical shedders of Salmonella (Jones, 2008). Studies have reported 1% to 5% prevalence of the sub-clinical shedding of Salmonella by horses entering veterinary clinics (Roberts and O’Boyle, 1981). Persistence of Salmonella in the hospital environment has been often reported to be associated with nosocomial outbreaks (Steneroden et al., 2010; Schott et al., 2001) and environmental contamination with S. enterica in veterinary hospitals are identified as a hazard related to the nosocomial infections (Pandya et al., 2009; Ewart et al., 2001). In fact, many nosocomial outbreaks recorded in equine veterinary hospitals have been associated with multi drug resistant strains (Table 1).
MDR Salmonella Typhimurium have been reported from companion animals (Cherry et al., 2004; Low et al., 1996; Wall et al., 1996). In an outbreak occurred in a small animal hospital of US, two cats, one dog, 3 pet owners and two technicians were affected from a same strain of Salmonella enterica serovar Typhi-murium (Cherry et al., 2004). A report documented multi-drug resistant Salmonella Typhimurium in four veterinary health care centres described that Pet care centres can act as a foci of Salmonella transmission between animals and humans, if necessary precautions are not followed (Wright et al., 2005).
Table 1: MDR Salmonella strains of nosocomial outbreaks in horses
|
Serial no.
|
MDR Salmonella strains of nosocomial outbreaks - Horses
|
References
|
|
1
|
S. Infantis
|
Tillotson et al., 1997; Dunowska et al., 2007
|
|
2
|
S. Anatum
|
Hartmann, 1995; Hartmann et al., 1996; Castor et al., 1989
|
|
3
|
S. Heidelberg
|
Amavisit et al., 2001
|
|
4
|
S Agona
|
Donahue et al., 1986; Castor et al., 1989
|
|
5
|
S Krefeld
|
Ikeda et al., 1985
|
|
6
|
S Saintpaul
|
Ikeda et al., 1985; Hird et al., 1986
|
|
7
|
S Newport
|
Castor et al., 1989; Dallap et al., 2010
|
|
8
|
S. Oranienburg
|
Kevin et al., 2014
|
Others
Nosocomial transmission of Cryptosporidium was documented in a veterinary hospital through an outbreak involving multiple species, which originated from an infected dairy calf (Konkle et al., 1997). Biofilms are group of adherent microorganisms encased in a self-produced extracellular polymeric substance or slime, formed in both biotic and abiotic surfaces. They are often associated with a number of persistent infections that poorly respond to antibiotics (Hall-Stoodley, 2004). Nosocomial spread of Mycobacterium bovis between cats was recorded in a veterinary clinic in Ireland (Murray et al., 2014). In Hospital settings biofilm formation in indwelling medical devices like i.v catheter can harbour numerous potentially infectious pathogens resulting in elevated morbidity, mortality and propagation of antimicrobial resistance. The main pathogen for i.v catheter, urethral catheter and endotracheal tubes are coagulase negative Staphylococci, E. coli and enteric gram negative bacilli respectively. The other common pathogens forming biofilms are S. aureus, K. pneumonia, P. mirabilis, E. faecalis, P. aeruginosa, Streptococcus spp, Candida spp (Lynch and Robertson, 2008). The nosocomial outbreak of canine parainfluenza infection was recorded in a veterinary referral hospital among dogs (Weese and Stull, 2013).
Surveillance in veterinary hospitals
Even though loads of recommendations for prevention and control of nosocomial infection is available out of the lesson learnt from previous experiences (Hartmann et al., 1996; Schott et al., 2001; Wright et al., 2005; Dallap et al., 2010; Goehring et al., 2010; Steneroden et al., 2010). Presently there is no recognized or published principles or standards for surveillance and control of infection in veterinary hospitals, which makes the system weak to determine rates and fraction of nosocomial and preventable infections respectively (Morley, 2002, 2004; Benedict et al., 2008; Morley and Weese, 2008).
The surveillance is broadly classified into Hospital wide surveillance and Targeted surveillance. The Hospital wide surveillance are very expensive, time consuming, laborious and tend to detect more infections which cannot be prevented (Pottinger et al., 1997). A study conducted for understanding the trends in nosocomial infections in University of Virginia Hospital for the period of eight years revealed that nosocomial infection rates are 4 times greater in intensive care units than in general wards (Landry et al., 1982), and during the study period there were seven infectious disease outbreaks and interestingly all the seven were in critical care units. One more study conducted on the same hospital exposed that the competence of surveillance systems for spotting nosocomial infection was maximum when it is focused in intensive care units rather than hospital wide surveillance (Wenzel et al., 1981). An additional study performed in German hospital exclusively in an intensive care unit revealed that only a minimum difference in the sensitivity and specificity when there is a targeted approach when compared to entire hospital wide surveillance (Dettenkofer et al., 2001). By another study researcher found that narrowing the population under surveillance, like targeting the hospitalized patients with increased risk of developing nosocomial infections may increase the sensitivity and reduce the labour, money and time consumption (Brusaferro et al., 2006). Targeted surveillance is efficient and less time, money and labour consuming than hospital wide surveillance which requires more cost and effort for data collection (Morley, 2004). The only drawback of this approach is missing out of nosocomial infections in general ward and other area patients.
Different methods of Targeted surveillance
Device associated infection surveillance
Specific etiological agent surveillance
Syndromie based surveillance
Environmental based surveillance
Laboratory based surveillance
Device associated infection surveillance
Most of the device associated infections that had been published are restricted to dogs with urinary catheter (Wise et al., 1990). A prospective study done with 18 male cats to evaluate the incidence of catheter-associated urinary tract infections by collecting urine samples three times, i.e. straight away after catheterization, 24 hours after and before removal. The bacterial culture of the urine samples detected that six cats (33.3%) developed bacteriuria, 5 cats with Escherichia coli, Staphylococcus species, one cat with Streptococcus bovis and one more cat developed fungal infection (Hugonnard et al., 2013). A prospective study conducted by Smarick and others in 2004 reported 10.3% of healthy catheterized dogs of both sex developed urinary tract infection. Furthermore this study revealed that female dogs are more susceptible to develop the urinary tract infection (Smarick et al., 2004). A randomized clinical trial was performed with dogs underwent intervertebral disk correction surgery. Based on sex, the dogs were stratified and assigned randomly with three different urinary bladder managing methods, viz. manual expulsion, intermittent catheterization or catheter insitu. The proportion of urinary tract infection was higher in dogs with indwelling catheter and 32% showed noticeable growth of minimum one bacterial species (Bubenik and Hosgood, 2008). The other device always associated with nosocomial infection in animal is intravenous catheter (Marsh-Ng et al., 2007) and it is due to the contamination of the catheter (Johnson, 2002). Number of studies have been conducted to estimate the prevalence of contamination of intravenous catheter in veterinary hospitals. In a study conducted for one year period at intensive care unit Ontario Veterinary College, they collected all the intravenous catheters from dogs and cats and cultured and found out the bacterial contamination rate of 10.7% (Mathews et al., 1996). With another study the bacterial contamination rate of intravenous catheter from 100 dogs was found out to be 22% and the bacteria isolated were often found to be of gastrointestinal and environmental origin (Lobetti et al., 2002). Other studies reported 24.5% (Marsh-Ng et al., 2007) and 23.2% (Jones et al., 2009) of bacterial contamination rates due to intravenous catheters in dogs and cats.
Specific etiological agent surveillance
Some hospitals are performing surveillance based on specific etiological agents which are associated with nosocomial infections. In 2008, Benedict et al. (2008) conducted a study in US veterinary teaching hospitals and reported that 53% of hospitals are collecting samples for detecting the specific pathogen and it is their one of the element of infection control practices. One of the specific contagious pathogen often encountered in veterinary nosocomial outbreaks and also incorporated in hospital surveillance is Salmonella (Schott et al., 2001; Cherry et al., 2004; Benedict et al., 2008; Steneroden et al., 2010). Patient who are under greater risk of shedding Salmonella are included in the surveillance (Morley, 2002, 2004; Morley and Weese, 2008; Ekiri et al., 2010). The sampling plan includes faecal sampling at the time of admittance and then at regular intervals during the hospital stay (Morley, 2002; Ekiri et al., 2010). If Salmonella was not present at the time of admittance and detected during subsequent sampling, which of similar serotype and antibiogram pattern as other isolates in the patients, then the Salmonellosis is considered to be of nosocomial origin (Ekiri et al., 2010). A study conducted for Salmonella surveillance in 246 hospitalized colic horses reported positive result in 9% of the faecal sample (Kim et al., 2001). In Human medicine one of the most significant nosocomial pathogen is Methicillin-resistant Staphylococcus aureus (MRSA) and in all probability it is one of the emerging multiple drug resistant bacteria in veterinary health settings (Beard, 2010). In a survey conducted in seven teaching veterinary hospitals in the United States regarding the S.aureus isolated from hospitalized patients revealed that 14% of animals were infected with Methicillin-resistant Staphylococcus aureus and the highest prevalence was estimated in horses and dogs (Middleton et al., 2005). In an attempt to determine the prevalence rate of MRSA, an emerging equine pathogen, a screening program was organized, in which nasal swab was collected from all horses during admission, hospitalization and discharge. MRSA was isolated from 5.3% of horses and the incidence rate of overall nosocomial MRSA was found to be 23/1000 admissions (Weese et al., 2006b). A study conducted in a small animal hospital revealed MRSA prevalence of 9% in dogs (Loeffler et al., 2005). Various studies have also documented clear rise in the MRSA infections on surgical sites of companion animals (Boag et al., 2004; O’Mahony et al., 2005). A retrospective study with the faecal culture data conducted in a teaching veterinary hospital following an outbreak of salmonellosis with high percentage of cases within 24 hours of admission revealed the etiological agent was Salmonella enterica serovar Oranienburg. Severe scrutiny of their medical records exposed the concealed epidemiological association to the veterinary hospital following the index case (Cummings et al., 2014).
Syndrome based surveillance
Syndrome based surveillance means detecting simple adverse conditions (pyrexia of unknown origin) in hospitalized patients or distinct signs and symptoms of any infection (Mostashari and Hartman, 2003). This system can be used based on the monitoring of clinical datas (flu-like symptoms) and nonclinical datas (sale of over-the-counter drugs or school absenteeism) (Lombardo et al., 2003; Mostashari and Hartman, 2003). On an added advantage, to make an evidence-based veterinary practices, the Syndromic surveillance in veterinary hospitals can be used concurrently to collect information about prevalene, incidence and various risk factors prevailing in the animal population (Stone and Hautala, 2008). Various studies has been conducted with syndromic surveillance in veterinary hospitals (Nicholson et al., 2002; Eugster et al., 2004; Ahern et al., 2010; Ruple-Czerniak et al., 2014). A syndromic surveillance for evaluating the occurrence of healthcare-associated infections in Critical Care Units of Small Animal Referral Hospitals reported that 16.3% of dogs and 12% of cats had ≥1 nosocomial syndrome during hospitalization (Ruple-Czerniak et al., 2013). Another Syndromic surveillance used to estimate the rate of nosocomial infections in equine hospitals reported that 19.7% of the study population had at least one nosocomial event during hospital stay and the most commonly observed nosocomial syndromes were surgical site inflammation and intra-venous catheter site inflammation (Ruple-Czerniak et al., 2014). Though the specificity of this system is low in outbreak surveillance, the sensitivity is high by guaranteeing the pertinent markers of disease (Van Metre et al., 2009). Some of the relevant markers used in the surveillance are:
Acute respiratory tract disorders: evidence of coughing, abnormal lung sounds, dyspnoea or tachypnea, sneezing, nasal discharges.
Acute infectious gastrointestinal disorders: diarrhoea, vomiting, abdominal pain or discomfort.
Pyrexia of unknown origin: temperature more than 102.0ºF in horses or more than 102.5ºF in dogs and cats.
Septicemia: clinical or microbiological confirmation of septicemia
Surgical wound analysis: inflammation, infection or discharges.
Environmental based surveillance
In Environment based surveillance the hospital should submit the hospital environment sample for routine culture. Many studies has been published stating that hospital equipment and fomites harbouring potential nosocomial infectious agents and act as a reservoir such as sponge pots with benzalkonium chloride (Fox et al., 1981), stethoscopes (Fujita et al., 2013), thermometers (Van den Berg et al., 2000; Weese et al., 2000), Faucet handles and computer keyboards (Bures et al., 2000), endoscopes (Schelenz and French, 2000; Cowen, 2001), multiple-dose vials (Sabino and Weese, 2006), Cellular phones (Brady et al., 2007; Julian et al., 2012), white coats and surgical scrubs (Singh et al., 2013). Examination tables, floors and doors (van Balen et al., 2013). In 2004, Burgess et al., reported that the nosocomial infectious agents are often encountered in the environment whenever the nosocomial rates are increased (Burgess et al., 2004). And it has been reported that contamination of veterinary hospital environment was responsible for many nosocomial infection outbreaks (Castor et al., 1989; Hartmann et al., 1996; Tillotson et al., 1997; Schott et al., 2001; Weese and Armstrong, 2003; Wright et al., 2005; Dallap et al., 2010; Steneroden et al., 2010). The percentage recovery of nosocomial agents in environmental survey of hospitals is depicted in table 2.
Table 2: Percentage recovery of nosocomial agents in environmental survey of hospitals
|
S.no
|
Nosocomial agent
|
Percentage recovery in environmental survey of hospitals
|
Reference
|
|
1
|
Salmonella spp
|
2.1%
|
(Alinovi et al., 2003)
|
|
2
|
Salmonella spp
|
11.9%
|
(Burgess et al., 2004)
|
|
3
|
Clostridium difficile
|
6.3%
|
(Weese et al., 2000)
|
|
4
|
MRSA
|
9.6%
|
(Weese et al., 2004)
|
|
5
|
Staphylococci count
|
55.9% of sample contain ≥2.5 cfu/cm2
|
(Aksoy et al., 2010).
|
Laboratory based surveillance
The Surveillance system used in human health care centres includes laboratory diagnosis (Emori and Gaynes, 1993; Brossette et al., 2006). Many researchers in veterinary nosocomial infections also included laboratory diagnosis for confirmation (Biertuempfel et al., 1981; Mathews et al., 1996; Lobetti et al., 2002; Smarick et al., 2004; Marsh-Ng et al., 2007; Bubenik and Hosgood, 2008; Jones et al., 2009) and some researchers have not included laboratory confirmation (Nicholson, 2002; Eugster et al., 2004; Ahern et al., 2010). Committing laboratory confirmation is not cost effective and also more time consuming which prevents immediate action during outbreaks. In other way laboratory diagnosis can be used as a passive surveillance in regular basis. Passive surveillance means collecting data for other purposes, like samples which are regularly collected from patients can be tested for nosocomial infectious agents and Active surveillance means data collection for the specific purpose, and these are the types based on data collection (Morley, 2004). But the sensitivity of active surveillance is greater than the passive surveillance (Brachman, 1993).
Methods in Data Collection and Comparsion
The data collection may be either Prospective or Retrospective. Data collection by monitoring the patient during hospitalization is Prospective and data collection by reviewing the hospital records after the patient has been discharged is Retrospective (Abrutyn and Talbot, 1987). Eventhough the Prospective data collection is gold standard there is a possibility of missing out of nosocomial infections after the patient is discharged (Freeman and McGowan, 1981) however it is wise to follow prospective surveillance during outbreaks of nosocomial infections (Wenzel et al., 1976). But retrospective method is used in hospital readmissions for infections (Pottinger et al., 1997). Lot of studies has been conducted to illustrate the magnitude of nosocomial infections occurring after discharge from hospitals especially post-surgical infections (Brown et al., 1987; Delgado-Rodriguez et al., 2001; Eugster et al., 2004). There are two methods in prospective data collection i.e patient-based collection methods by nurse or physician while attending the patient (Abrutyn and Talbot, 1987; Pottinger et al., 1997) and diagnostic laboratory-based data collection which is time consuming, lacking in clinical data and with more false positive and false nagatives (Abrutyn and Talbot, 1987).
Inter-hospital comparison of data of nosocomial infections will facilitate the effective surveillance and for this nosocomial infection rates should be determined in each hospital (Gaynes, 1997). But the crude rates cannot be compared since it varies with the patient number, hospital size, facilities, case load, underlying illness and severity of disease (Pottinger et al., 1997; Sax et al., 2002). For effective comparison it must be standardized like, which risk factors should be taken into account like length of hospital stay, exposure to invasive devices and intrinsic factors of patient (Pottinger et al., 1997).
Prevention and control of Nosocomial Infections: Best to be proactive rather than reactive
Hand hygiene is the easiest, cost effective and most underused measure (Sax et al., 2007). Hand washing with plain soaps failed to remove pathogens (Ehrenkranz et al., 1991) and also there are ironical reports stating that hand washing with plain soaps increases the bacterial counts on the skin (Larson et al., 1986; Winnefeld et al., 2000). Proper hand hygiene (Sax et al., 2007) and 70% alcohol gel based hand sanitizers should be placed throughout the hospitals (Kampf et al., 2002; Leonard et al., 2008). The alcohol based hand sanitizers have increased antimicrobial activity and reduces the cross contamination from taps and paper towel dispensers (Harrison et al., 2003). Hand washing should be done– i) before and after contact with a patient, ii) before and after contact with objects in the animals environment, iii) after contact with potentially infective biological specimen or discharges and iv) before and after wearing gloves is necessary (CCAR, 2008).
Preliminary screening of all patients for signs of contagious disease before admission to the hospital and separate isolation facility for the contagious disease infected animals (Traub-Dargatz et al., 2004).
If environmental contamination is suspected, regular monitoring and intervention is required with periodic spot checks of environment (Traub-Dargatz et al., 2004).
Veterinary health care personnel should take necessary occupational health measures like personal protective equipment, vaccination while handling infected materials like discharges, blood and sharps (Mielke, 2010).
The outerwear should be changed frequently since gross contamination does not need for pathogen to be present and attached laundry services may be provided.
Cover skin lesions and open wounds. Strict asepsis in surgery with autoclaved surgical instruments and thoroughly cleaned endotracheal tubes (CCAR, 2008).
Removal of implants and appropriate antibiotic treatment based on antibiogram (Leonard et al., 2006).
Animals with suspected clinical signs of any infectious diseases should not be admitted for any elective procedures (CCAR, 2008).
Animals belonging to hospital workers should be screened (Leonard et al., 2008)
Animals with multi-drug resistant infections should be handled with great precautions since the body sites like nose, rectum could be colonized with those pathogens (CCAR, 2008).
Ensure appropriate handling of sterile medical devices particularly indwelling devices like urinary catheter and drugs for parental administration. Invasive procedures should only be performed if mandatory.
Regular collection of Laboratory samples as a part of comprehensive monitoring programme. For example, Faeces can be collected for culture to detect salmonella from all the animals admitted for colic. If a particular nosocomial agent has been a problem in the past, there should be a readymade programmed action plan (Traub-Dargatz et al, 2004).
Special precautions on re-dispensation of medical products by proper disinfection and sterilization and disinfection of hand touch surfaces. Touching clean items like telephone or microscope while handling potential infectious material like swabs should be avoided.
Ensure healthy patient environment and waste disposal (Mielke, 2010).
Monitoring of antibiotic drugs consumption and development of resistance. Ensure antibiotic stewardship.
Adequate education and training for staff on infection control and occupational health supervision and Education of veterinary graduates and personnel about nosocomial infections and the significance of infection control measures (Schwaber et al., 2013).
Client education particularly about zoonosis if the veterinarian has a reasonable suspicion of any zoonotic infectious disease.
The surveillance of nosocomial infections should be accomplished by creating nationwide reference data which will facilitate the quick reporting about infection clusters, outbreak and thereby makes evidence based preventive and control measures (Mielke, 2010).
Conclusions
Nosocomial infections are preventable yet it is more neglected area of veterinary practice since it warrants some added tasks which do not fetch money. Unfortunately it will influence the success of the treatment and care provided so it cannot be ignored any longer. The mandate elements for providing patient safety and to prevent nosocomial infections are clean hospital environment, clean equipment and clean procedures or practices. Further in broader sense proactive policies, health worker education, safe use of medicines and surveillance system can strengthen patient safety, curtail dissemination of antibiotic resistance and zoonotic diseases.
Reference
Abraham S, Wong HS, Turnidge J, Johnson JR, Trott DJ (2014). Carbapenemase-producing bacteria in companion animals: a public health concern on the horizon. J. Antimicrobial. Chemo. 69(5): 1155-1157. http://dx.doi.org/10.1093/jac/dkt518
Abrutyn E, Talbot, GH (1987). Surveillance strategies: a primer. Infect. Control. 8(11): 459-464.
Ahern BJ, Richardson DW, Boston RC, Schaer TP (2010). Orthopedic infections in equine long bone fractures and arthrodeses treated by internal fixation: 192 cases (1990-2006). Vet. Surg. 39(5): 588-593. http://dx.doi.org/10.1111/j.1532-950X.2010.00705.x
Ahmed MO, Williams NJ, Clegg PD, van Velkinburgh JC, Baptiste KE (2012). Analysis of risk factors associated with antibiotic-resistant Escherichia coli. Microb. Drug Resist. 18 (2): 161–168. http://dx.doi.org/10.1089/mdr.2011.0213
Aksoy E, Boag A, Brodbelt D, Grierson J (2010). Evaluation of surface contamination with Staphylococci in a veterinary hospital using a quantitative microbiological method. J. Small Anim. Pract. 51(11): 574-580. http://dx.doi.org/10.1111/j.1748-5827.2010.00994.x
Alinovi CA, Ward MP, Couëtil LL, Wu CC (2003). Detection of Salmonella organisms and assessment of a protocol for removal of contamination in horse stalls at a veterinary teaching hospital. J. Am. Vet. Med. Assoc. 223(11): 1640-1644. http://dx.doi.org/10.2460/javma.2003.223.1640
Amavisit P, Markham PF, Lightfoot D, Whithear KG, Browning GF (2001). Molecular epidemiology of Salmonella Heidelberg in an equine hospital. Vet. Microbiol. 80(1): 85–98. http://dx.doi.org/10.1016/S0378-1135(00)00373-4
Anderson MEC, Lefebvre SL, Weese JS (2008). Evaluation of prevalence and risk factors for methicillin-resistant S. aureus colonization in veterinary personnel attending an international equine veterinary conference. Vet. Microbiol. 129 (3-4): 410–417. http://dx.doi.org/10.1016/j.vetmic.2007.11.031
Armand-Lefevre L, Ruimy R, Andremont A (2005). Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human control and pigs. Emerg. Infect. Dis. 11(5): 711–714. http://dx.doi.org/10.3201/eid1105.040866
Ayliffe GA (1997). The progressive intercontinental spread of methicillinresistant Staphylococcus aureus. Clin. Infect. Dis. 24 (Suppl.1): S74–S79. http://dx.doi.org/10.1093/clinids/24.Supplement_1.S74
Baptiste KE, Williams K, Williams NJ, Wattret A, Clegg PD, Dawson S, Corkill JE, O’Neill T, Hart CA (2005). Methicillin resistant Staphylococci in companion animals. Emerg. Infect. Dis. 11(12): 1942–1944. http://dx.doi.org/10.3201/eid1112.050241
Barber, (1961). Methicillin-resistant Staphylococci. J. Clin. Pathol. 14(4): 385–393.
Barr BS, Waldridge BM, Morresey PR, Reed SM, Clark C, Belgrave R, Donecker JM, Weigel DJ (2013). Antimicrobial-associated diarrhoea in three equine referral practices. Equine. Vet. J. 45(2): 154–158. http://dx.doi.org/10.1111/j.2042-3306.2012.00595.x
Beard LA (2010). Multiple drug resistant bacteria in equine medicine: an emerging problem. Equine Vet. Educ. 22(6): 287–289. http://dx.doi.org/10.1111/j.2042-3292.2010.00067.x
Belmonte O, Pailhories H, Kempf M, Gaultier MP, Lemarie C, Ramont C, Joly Guillou ML, Eveillard M (2014). High prevalence of closely-related Acinetobacter baumannii in pets according to a multicentre study in veterinary clinics, Reunion Island. Vet. Microbiol. 170(3-4): 446–450. http://dx.doi.org/10.1016/j.vetmic.2014.01.042
Benedict KM, Morley PS, Van Metre DC (2008). Characteristics of biosecurity and infection control programs at veterinary teaching hospitals. J Am Vet Med Assoc. 233(5): 767–773. http://dx.doi.org/10.2460/javma.233.5.767
Bohme H, Konigsmark C, Klare I, Zischka M, Werner G (2012). Cross-transmission rates of enterococcal isolates among newborns in a neonatal intensive care unit. Pediatr. Reports. 4(1): e15. http://dx.doi.org/10.4081/pr.2012.e15
Bischoff KM, White DG, McDermott PF, Zhao S, Gaines S, Maurer JJ, Nisbet DJ (2001). Characterization of chloramphenicol resistance in beta-hemolytic Escherichia coli associated with diarrhea in neonatal swine. J. Clin. Microbiol. 40(2): 389–394. http://dx.doi.org/10.1128/JCM.40.2.389-394.2002
Biertuempfel, P. H., Ling, G. V., and Ling, G. A. (1981). Urinary tract infection resulting from catheterization in healthy adult dogs. J Am Vet Med Assoc. 178(9): 989–991.
Boag A, Loeffler A, Lloyd DH (2004). Methicillin-resistant Staphylococcus aureus in small animal practice. Vet. Rec. 154: 366–371.
Boerlin P, Eugster S, Gaschen F, Straub R, Schwalder P (2001). Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet. Microbiol. 82(4): 347-359. http://dx.doi.org/10.1016/S0378-1135(01)00396-0
Bootsma MC, van der Horst MA, Guryeva T, ter Kuile BH, Diekmann O (2012). Modeling non-inherited antibiotic resistance. Bull. Math. Biol. 74(8): 1691–1705. http://dx.doi.org/10.1007/s11538-012-9731-3
Bortolaia V, Larsen J, Damborg P, Guardabassi L (2011). Potential pathogenicity and host range of extended-spectrum b-lactamase producing Escherichia coli isolates from healthy poultry. Appl. Environ. Microbiol. 77(16): 5830–5833. http://dx.doi.org/10.1128/AEM.02890-10
Brachman PS (1993). Nosocomial infections surveillance. Infect. Control Hosp. Epidemiol. 14(4): 194–196. http://dx.doi.org/10.2307/30149727
Brady RR, Fraser SF, Dunlop MG, Brown SP, Gibb AP (2007). Bacterial contamination of mobile communication devices in the operative environment. J. Hosp. Infect. 66(4): 397-398. http://dx.doi.org/10.1016/j.jhin.2007.04.015
Brossette SE, Hacek DM, Gavin PJ, Kamdar MA, Gadbois KD, Fisher AG, Peterson LR (2006). A laboratory-based, hospital-wide, electronic marker for nosocomial infection. American J. Clin. Pathol. 125(1): 34-39. http://dx.doi.org/10.1309/502AUPR8VE67MBDE
Brown RB, Bradley S, Opitz E, Cipriani D, Pieczarka R, Sands M (1987). Surgical wound infections documented after hospital discharge. Am. J. Infect. Control. 15(2): 54-58. http://dx.doi.org/10.1016/0196-6553(87)90002-2
Brusaferro S, Regattin L, Faruzzo A, Grasso A, Basile M, Calligaris L, Scudeller L, Viale P (2006). Surveillance of hospital-acquired infections: a model for settings with resource constraints. Am. J. Infect. Control. 34(6): 362-366. http://dx.doi.org/10.1016/j.ajic.2006.03.002
Bubenik L, Hosgood G (2008). Urinary tract infection in dogs with thoracolumbar intervertebral disc herniation and urinary bladder dysfunction managed by manual expression, indwelling catheterization or intermittent catheterization. Vet. Surg. 37(8): 791-800. http://dx.doi.org/10.1111/j.1532-950X.2008.00452
Bures S, Fishbain JT, Uyehara CFT, Parker JM, Berg BW (2000). Computer keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. Am. J. Infect. Control. 28(6): 465-470. http://dx.doi.org/10.1067/mic.2000.107267
Burgess BA, Morley PS, Hyatt DR (2004). Environmental surveillance for Salmonella enteric in a veterinary teaching hospital. J. Am. Vet. Med. Assoc. 225(9): 1344-1348. http://dx.doi.org/10.2460/javma.2004.225.1344
Burke JP (2003). Infection control – a problem for patient safety. N. Engl. J. Med. 348(7): 651-656. http://dx.doi.org/10.1056/NEJMhpr020557
Burstiner LC, Faires M, Weese JS (2010). Methicillin-resistant Staphylococcus aureus colonization in personnel attending a veterinary surgery conference. Vet. Surg. 39(2): 150-157. http://dx.doi.org/10.1111/j.1532-950X.2009.00638.x
Castor ML, Wooley RE, Shotts EB, Brown J, Payeur JB (1989). Characteristics of Salmonella isolated from an outbreak of equine salmonellosis in a veterinary teaching hospital. J. Equine Vet. Sci. 9(5): 236-241. http://dx.doi.org/10.1016/S0737-0806(89)80078-4
CCAR. Infection prevention and control best practices for small animal veterinary clinics. Canadian Committee on Antibiotic Resistance (CCAR) 2008. p 1-71.
Cefai C, Ashurst S, Owens C (1994). Human carriage of methicillinresistant Staphylococcus aureus linked with pet dog. Lancet. 344(8921): 539–540. http://dx.doi.org/10.1016/S0140-6736(94)91926-7
Cherry B, Burns A, Johnson GS, Pfeiffer H, Dumas N, Barrett D, McDonough PL, Eidson M (2004). Salmonella typhimurium outbreak associated with veterinary clinic. Emerg. Infect. Dis. 10(12): 2249-2251. http://dx.doi.org/10.3201/eid1012.040714 PMid:15663875 PMCid:PMC3323366
Chung YS, Kwon KH, Shin S, Kim JH, Park YH, Yoon JW (2014). Characterization of Veterinary Hospital-Associated Isolates of Enterococcus Species in Korea. J. Microbiol. Biotechnol. 24(3): 386–393. http://dx.doi.org/10.4014/jmb.1310.10088 PMid:24296459
Clemetson LL, Ward AC (1990). Bacterial flora of the vagina and uterus of healthy cats. J. Am. Vet. Med. Assoc. 196(6): 902-906.
Clooten J, Kruth S, Arroyo L, Weese JS, (2008). Prevalence and risk factors for Clostridium difficile colonization in dogs and cats hospitalized in an intensive care unit. Vet. Microbiol. 129(1-2): 209–214. http://dx.doi.org/10.1016/j.vetmic.2007.11.013 PMid:18164560
Costa MC, Stampfli HR, Arroyo LG, Pearl DL, Weese JS (2011). Epidemiology of Clostridium difficile on a veal farm: prevalence, molecular characterization and tetracycline resistance. Vet. Microbiol. 152(3-4): 379-384. http://dx.doi.org/10.1016/j.vetmic.2011.05.014 PMid:21641131
Cowen AE (2001). The clinical risks of infection associated with endoscopy. Can. J. Gastroenterol. 15(5): 321-331.
Cummings KJ, Rodriguez-Rivera LD, Katharyn JM, Karin H, Martin W, Patrick LM, Craig A, Lorin DW, Gillian AP (2014). Salmonella enterica Serovar Oranienburg Outbreak in a Veterinary Medical Teaching Hospital with Evidence of Nosocomial and On-Farm Transmission. Vector-Borne Zoonotic Dis. 14(7): 496-502.
Cuny C, Kuemmerle J, Stanek C, Willey B, Strommenger B, Witte W (2006). Emergence of MRSA infections in horses in a veterinary hospital: strain characterisation and comparison with MRSA from humans. Euro. Surveill. 11(1): 44–47. http://dx.doi.org/10.1016/j.jhin.2006.04.009 PMid:16835000
Cuny C, Strommenger B, Witte W, Stanek C (2008). Clusters of infections in horses with MRSA ST1, ST254, and ST398 in a veterinary hospital. Microb. Drug Resist. 14(4): 307-310. http://dx.doi.org/10.1089/mdr.2008.0845 PMid:19025385
D’Agata EM, Magal P, Olivier D, Ruan S, Webb GF (2007). Modeling antibiotic resistance in hospitals: the impact of minimizing treatment duration. J. Theor. Biol. 249(3): 487–499. http://dx.doi.org/10.1016/j.jtbi.2007.08.011 PMid:17905310 PMCid:PMC2432019
D’Agata EM, Dupont-Rouzeyrol M, Magal P, Olivier D, Ruan S (2008). The impact of different antibiotic regimens on the emergence of antimicrobial-resistant bacteria. PLoS One. 3: e4036. http://dx.doi.org/10.1371/journal.pone.0004036 PMid:19112501 PMCid:PMC2603320
D’Agata EM, Horn MA, Ruan S, Webb GF, Wares JR (2012). Efficacy of infection control interventions in reducing the spread of multidrug-resistant organisms in the hospital setting. PLoS One. 7: e30170. http://dx.doi.org/10.1371/journal.pone.0030170 PMid:22363420 PMCid:PMC3282714
Dallap Schaer BL, Aceto H, Rankin SC (2010). Outbreak of salmonellosis caused by Salmonella enterica serovar Newport MDR-AmpC in a large animal veterinary teaching hospital. J. Vet. Intern. Med. 24(5): 1138-1146. http://dx.doi.org/10.1111/j.1939-1676.2010.0546.x PMid:20584143
Damborg P, Top J, Hendrickx AP, Dawson S, Willems RJ, Guardabassi L (2009). Dogs are a reservoir of ampicillin-resistant Enterococcus faecium lineages associated with human infections. Appl. Environ. Microbiol. 75(8): 2360–2365. http://dx.doi.org/10.1128/AEM.02035-08 PMid:19233953 PMCid:PMC2675212
Debast SB, van Leengoed LA, Goorhuis A, Harmanus C, Kuijper EJ, Bergwerff AA (2009). Clostridium difficile PCR ribotype 078 toxinotype V found in diarrhoeal pigs identical to isolates from affected humans. Environ. Microbiol. 11(2): 505–511. http://dx.doi.org/10.1111/j.1462-2920.2008.01790.x PMid:19196280
Delgado-Rodriguez M, Gómez-Ortega A, Sillero-Arenas M, Llorca J (2001). Epidemiology of surgical-site infections diagnosed after hospital discharge: a prospective cohort study. Infect. Control Hosp. Epidemiol. 22(1): 24–30. http://dx.doi.org/10.1086/501820 PMid:11198018
Dettenkofer M, Ebner W, Els T, Babikir R, Lücking C, Pelz K, Daschner F (2001). Surveillance of nosocomial infections in a neurology intensive care unit. J. Neurol. 248(11): 959–964. http://dx.doi.org/10.1007/s004150170048 PMid:11757959
Devriese LA, Vandamme LR, Fameree L (1972). Methicillin (cloxacillin)-resistant Staphylococcus aureus strains isolated from bovine mastitis cases. Zentralblatt fur Veterinarmedizin B. 19(7): 598–605. http://dx.doi.org/10.1111/j.1439-0450.1972.tb00439.x PMid:4486473
Dierikx CM, van Duijkeren E, Schoormans AH, van Essen-Zandbergen, A, Veldman K, Kant A, Huijsdens XW, van der Zwaluw K, Wagenaar JA, Mevius DJ (2012). Occurrence and characteristics of extended-spectrum-beta-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 67(6): 1368–1374. http://dx.doi.org/10.1093/jac/dks049 PMid:22382469
Donahue JM (1986). Emergence of antibiotic-resistant Salmonella agona in horses in Kentucky. J. Am. Vet. Med. Assoc. 188(6): 592–594.
Dunowska M, Morley PS, TraubDargatz JL, Davis MA, Patterson G, Frye JG, Hyatt DR, Dargatz DA (2007). Comparsion of Salmonella enteric Serotype Infantis isolates from a Veterinary Teaching Hospital. J. Appl. Microbiol. 102(6): 1527-1536. http://dx.doi.org/10.1111/j.1365-2672.2006.03198.x PMid:17578417
Ehrenkranz NJ, Alfonso BC (1991). Failure of bland soap handwash to prevent hand transfer of patient bacteria to urethral catheters. Infect. Control Hosp. Epidemiol. 12(11): 654–662. http://dx.doi.org/10.1086/646261 http://dx.doi.org/10.2307/30146898 PMid:1753080
Eickhoff TC (1981). Nosocomial infections – A 1980 view: progress, priorities and prognosis. Am. J. Med. 70(2): 381–388. http://dx.doi.org/10.1016/0002-9343(81)90776-2
Ekiri AB, Morton AJ, Long MT, MacKay RJ, Hernandez JA (2010). Review of the epidemiology and infection control aspects of nosocomial Salmonella infections in hospitalised horses. Equine Vet. Educ. 22(12): 631–641. http://dx.doi.org/10.1111/j.2042-3292.2010.00144.x
Emori TG, Gaynes RP (1993). An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6(4): 428–442. PMid:8269394 PMCid:PMC358296
Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG (2000). Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of S. aureus. J. Clin. Microbiol. 38(3): 1008–1015. PMid:10698988 PMCid:PMC86325
Eugster S, Schawalder P, Gaschen F, Boerlin P (2004). A prospective study of postoperative surgical site infections in dogs and cats. Vet. Surg. 33(5): 542–550. http://dx.doi.org/10.1111/j.1532-950X.2004.04076.x PMid:15362994
Ewart SL, Schott HC, Robison RL, Dwyer RM, Eberhart SW Walker RD (2001). Identification of sources of Salmonella organisms in a veterinary teaching hospital and evaluation of the effects of disinfectants on detection of Salmonella organisms on surface materials. J. Am. Vet. Med. Assoc. 218(7): 1145–1151. http://dx.doi.org/10.2460/javma.2001.218.1145 PMid:11318367
Ewers C, Stamm I, Pfeifer Y, Wieler LH, Kopp PA, Schønning K (2014). Clonal spread of highly successful ST15-CTX-M-15Klebsiella pneumoniae in companion animals and horses. J. Antimicrobial. Chemo. 69(10): 2676–2680. http://dx.doi.org/10.1093/jac/dku217 PMid:24974381
Faires MC, Traverse M, Tater KC, Pearl DL, Weese JS (2010). Methicillin-resistant and -susceptible Staphylococcus aureus infections in dogs. Emerg. Infect. Dis. 16(5): 69–75. http://dx.doi.org/10.3201/eid1601.081758 PMid:20031045 PMCid:PMC2874348
Fisher K, Phillips C (2009). The ecology, epidemiology and virulence of Enterococcus. Microbiology. 155(Pt 6): 1749–1757. http://dx.doi.org/10.1099/mic.0.026385-0 PMid:19383684
Fox JG, Beaucage CM, Folta CA, Thornton GW (1981). Nosocomial transmission of Serratia marcescens in a veterinary hospital due to contamination by benzalkonium chloride. J. Clin. Microbiol. 14(2): 157–160. PMid:7024303 PMCid:PMC271926
Francey T, Gaschen F, Nicolet J, Burnens AP (2000). The role of Acinetobacter baumannii as a nosocomial pathogen for dogs and cats in an intensive care unit. J. Vet. Intern. Med. 14(2): 177–183. http://dx.doi.org/10.1111/j.1939-1676.2000.tb02233.x
Freeman J, McGowan JE (1981). Methodologic issues in hospital epidemiology. I. Rates, case-finding, and interpretation. Rev. Infect. Dis. 3(4): 658–667. http://dx.doi.org/10.1093/clinids/3.4.658 PMid:7339779
Fujita H, Hansen B, Hanel R (2013). Bacterial Contamination of Stethoscope Chest Pieces and the Effect of Daily Cleaning. J. Vet. Intern. Med. 27(2): 354–358. http://dx.doi.org/10.1111/jvim.12032 PMid:23425122
Gaynes RP (1997). Surveillance of nosocomial infections: a fundamental ingredient for quality. Infect. Control Hosp. Epidemiol. 18(7): 475–478. http://dx.doi.org/10.2307/30141186 http://dx.doi.org/10.1086/647651 PMid:9247829
Ghosh A, Dowd SE, Zurek L (2011). Dogs leaving the ICU carry a very large multi-drug resistant enterococcal population with capacity for biofilm formation and horizontal gene transfer. PLoS ONE. 6:e22451. http://dx.doi.org/10.1371/journal.pone.0022451 PMid:21811613 PMCid:PMC3139645
Giannouli M, Antunes LCS, Marchetti V, Triassi M, Visca P, Zarrilli R (2013). Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineage I–II and to the emerging genotypes ST25 and ST78. BMC Infect. Dis. 13: 282. http://dx.doi.org/10.1186/1471-2334-13-282 PMid:23786621 PMCid:PMC3691691
Gibson JS, Morton JM, Cobbold RN, Filippich LJ, Trott DJ (2011). Risk factors for dogs becoming rectal carriers of multidrug-resistant Escherichia coli during hospitalization. Epidemiol. Infect. 139(10): 1511–1521. http://dx.doi.org/10.1017/S0950268810002785 PMid:21156096
Giraffa G (2002). Enterococci from foods. FEMS Microbiol. Rev. 26(2): 163–171. http://dx.doi.org/10.1111/j.1574-6976.2002.tb00608.x PMid:12069881
Goehring LS, Landolt GS, Morley PS (2010). Detection and management of an outbreak of equine herpesvirus type 1 infection and associated neurologic disease in a veterinary teaching hospital. J. Vet. Intern. Med. 24(5): 1176–1183. http://dx.doi.org/10.1111/j.1939-1676.2010.0558.x PMid:20584137
Goni P, Vergara Y, Ruiz J, Albizu I, Vila J, Gomez-Lus R (2004). Antibiotic resistance and epidemiological typing of Staphylococcus aureus strains from ovine and rabbit mastitis. Int. J. Antimicrob. Agents. 23(3): 268–272. http://dx.doi.org/10.1111/j.1939-1676.2010.0558.x PMid:20584137
Grayson ML, Eliopoulos GM, Wennersten CB, Ruoff KL, De Girolami PC, Ferraro MJ (1991). Increasing resistance to beta-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob. Agents Chemother. 35(11): 2180–2184. http://dx.doi.org/10.1128/AAC.35.11.2180 PMid:1803989 PMCid:PMC245356
Gronthal T, Moodley A, Nykasenoja S, Junilla J, Guardabassi L, Thomson K, Rantala M (2014). Large Outbreak Caused by Methicillin Resistant Staphylococcus pseudintermedius ST71 in a Finnish Veterinary Teaching Hospital – From Outbreak Control to Outbreak Prevention. PLoS ONE 9(10): e110084. http://dx.doi.org/10.1371/journal.pone.0110084 PMid:25333798 PMCid:PMC4198203
Guardabassi L, Schwarz S, Lloyd DH (2004). Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrobial. Chemo. 54(2): 321–332. http://dx.doi.org/10.1093/jac/dkh332 PMid:15254022
Gustafsson A, Ba˚ verud V, Gunnarsson A, Pringle J, Franklin A (2004). Study of faecal shedding of Clostridium difficile in horses treated with penicillin. Equine. Vet. J. 36(2): 180–182. http://dx.doi.org/10.2746/0425164044868657 PMid:15038443
Haenni M, Ponsin C, Metayer V, Medaille C, Madec JY (2012). Veterinary hospital-acquired infections in pets with a ciprofloxacin-resistant CTX-M-15-producing Klebsiella pneumoniae ST15 clone. J. Antimicrob. Chemother. 67: 770–771. http://dx.doi.org/10.1093/jac/dkr527 PMid:22178643
Hall IC, O’Toole E (1935). Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Dis. Child. 49(2): 390– 402. http://dx.doi.org/10.1001/archpedi.1935.01970020105010
Hall-Stoodley L, Costerton JW, Stoodley P (2004). “Bacterial biofilms: from the natural environment to infectious diseases”. Nature Rev. Microbiol. 2 (2): 95–108. http://dx.doi.org/10.1038/nrmicro821
Hamilton E, Kruger JM, Schall W, Beal M, Manning SD (2013). Acquisition and persistence of antimicrobial-resistant bacteria isolated from dogs and cats admitted to a veterinary teaching hospital. J. Am. Vet. Med. 243(7): 990–1000. http://dx.doi.org/10.2460/javma.243.7.990 PMid:24050566
Hanselman B, Rousseau J, Kruth S, Weese JS (2006). Methicillinresistant Staphylococcus aureus (MRSA) colonization in veterinary personnel. Emerg. Infect. Dis. 12(12): 1933–1938. http://dx.doi.org/10.3201/eid1212.060231 PMid:17326947 PMCid:PMC3291342
Hardy KJ, Oppenheim BA, Gossain S, Gao F, Hawkey PM (2006). A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients acquisition of MRSA. Infect. Control Hosp. Epidemiol. 27(2): 127-132. http://dx.doi.org/10.1086/500622 PMid:16465628
Harrison WA, Grifith CJ, Ayers T, Michaels B (2003). Bacterial transfer and cross-contamination potential associated with paper-towel dispensing. Am. J. Infect. Control. 31(7): 387–391. http://dx.doi.org/10.1067/mic.2003.81 PMid:14639433
Hartmann FA (1995). West SEH. Antimicrobial susceptibility profiles of multidrug-resistant Salmonella anatum isolated from horses. J. Vet. Diagn. Invest. 7: 159–61. http://dx.doi.org/10.1177/104063879500700128 PMid:7779955
Hartmann FA, Callan RJ, McGuirk SM, West SE (1996). Control of an outbreak of salmonellosis caused by drug-resistant Salmonella anatum in horses at a veterinary hospital and measures to prevent future infections. J. Am. Vet. Med. Assoc. 209(3): 629-631.
Heller J, Kelly L, Reid SW, Mellor DJ (2010). Qualitative risk assessment of the acquisition of Meticillin-resistant staphylococcus aureus in pet dogs. Risk Anal. 30(3): 458–472. http://dx.doi.org/10.1111/j.1539-6924.2009.01342.x PMid:20136747
Hird DW, Casebolt DB, Carter JD, Pappaioanou M, Hjerpe CA (1986). Risk factors for salmonellosis in hospitalized horses. J. Am. Vet. Med. Assoc. 188(2): 172–7.
Horan TC, Andrus M, Dudeck MA (2008). CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 36(5): 309–332. http://dx.doi.org/10.1016/j.ajic.2008.03.002 PMid:18538699
Hordijk J, Schoormans A, Kwakernaak M, Duim B, Broens E, Dierikx C, Mevius D, Wagenaar JA (2013). High prevalence of fecal carriage of extended-spectrum ß-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front. Microbiol. 4: 242. http://dx.doi.org/10.3389/fmicb.2013.00242 PMid:23966992 PMCid:PMC3745002
Howard DH, Scott RD, Packard R, Jones D (2003). The Global Impact of Drug Resistance. Clin. Infect. Dis. 36(1): S4–S10. http://dx.doi.org/10.1086/344656 PMid:12516025
Huber H, Zweifel C, Wittenbrink MM, Stephan R (2013). ESBL-producing uropathogenic Escherichia coli isolated from dogs and cats in Switzerland. Vet. Microbiol. 162(2-4): 992–996. http://dx.doi.org/10.1016/j.vetmic.2012.10.029 PMid:23177909
Hugonnard M, Chalvet-Monfray K, Dernis J, Pouzot-Nevoret C, Barthélémy A, Vialard J, Goy-Thollot I (2013). Occurrence of bacteriuria in 18 catheterised cats with obstructive lower urinary tract disease: a pilot study. J. Feline Med. Surg. 15(10): 843-8. http://dx.doi.org/10.1177/1098612X13477414 PMid:23400682
Indra A, Lassnig H, Baliko N, Much P, Fiedler A, Huhulescu S, Allerberger F (2009). Clostridium difficile: a new zoonotic agent? Wien. Klin. Wochenschr. 121(3-4): 91–95. http://dx.doi.org/10.1007/s00508-008-1127-x PMid:19280132
Ikeda JS, Hirsh DC (1985). Common plasmid encoding resistance to ampicillin, chloramphenicol, gentamicin, and trimethoprim-sulfadiazine in two serotypes of Salmonella isolated during an outbreak of equine salmonellosis. Am. J. Vet. Res. 46(4): 769–73.
Jevons MP (1961). ‘‘Celbenin’’-resistant staphylococci. Br. Med. J. 1(5219): 124–125. http://dx.doi.org/10.1136/bmj.1.5219.124-a
Johnson JA (2002). Nosocomial infections. Vet. Clin .Small Anima. Pract. 32(5): 1101-1126. http://dx.doi.org/10.1016/S0195-5616(02)00038-4
Jones SL (2008). Medical disorders of the large colon. In: Smith, B.P. (Ed.), Large Animal Internal Medicine, fourth ed. Mosby Elsevier, St. Louis, USA, pp. 743–744.
Jones ID, Case AM, Stevens KB, Boag A, Rycroft AN (2009). Factors contributing to the contamination of peripheral intravenous catheters in dogs and cats. The Vet. Rec. 164(20): 616–618. http://dx.doi.org/10.1136/vr.164.20.616 PMid:19448253
Jordan D, Simon J, Fury S, Moss S, Giffard P, Maiwald M, Southwell P, Barton MD, Axon JE, Morris SG, Trott DJ (2011). Carriage of methicillin-resistant S. aureus by veterinarians in Australia. Aust. Vet. J. 89(5): 152–159. http://dx.doi.org/10.1111/j.1751-0813.2011.00710.x PMid:21495985
Julian T, Singh A, Rousseau J, Weese JS (2012). Methicillin-resistant staphylococcal contamination of cellular phones of personnel in a veterinary teaching hospital. BMC Res. Notes. 5: 193. http://dx.doi.org/10.1186/1756-0500-5-193 PMid:22533923 PMCid:PMC3393609
Kampf G, Rudolf M, Labadie JC, Barrett SP (2002). Spectrum of antimicrobial activity and user acceptability of the hand disinfectant agent Sterillium gel. J. Hosp. Infect. 52(2): 141–147. http://dx.doi.org/10.1053/jhin.2002.1281 PMid:12392906
Keessen EC, Hensgens MP, Spigaglia P, Barbanti F, Sanders IM, Kuijper EJ, Lipman LJ (2013). Antimicrobial susceptibility profiles of human and piglet Clostridium difficile PCR-ribotype 078. Antimicrob. Resist. Infect. Control. 2:14. http://dx.doi.org/10.1186/2047-2994-2-14 PMid:23566553 PMCid:PMC3651393
Kempf M., Rolain J.M (2012). Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int. J. Antimicrob. Agents. 39(2): 105–114. http://dx.doi.org/10.1016/j.ijantimicag.2011.10.004 PMid:22113193
Kevin JC, Lorraine DR, Katharyn JM, Karin H, Martin W, Patrick ML, Craig A, Lorin DW, Gillian AP (2014). Salmonella enterica Serovar Oranienburg Outbreak in a Veterinary Medical Teaching Hospital with Evidence of Nosocomial and On-Farm Transmission. Vector-Borne Zoonotic Dis. 14(7): 496-502.
Kim LM, Morley PS, Traub-Dargatz JL, Salman MD, Gentry-Weeks C (2001). Factors associated with Salmonella shedding among equine colic patients at a veterinary teaching hospital. J Am Vet Med Assoc. 218(5): 740-748. http://dx.doi.org/10.2460/javma.2001.218.740 PMid:11280409
Konkle DM, Nelson KM, Lunn DP (1997). Nosocomial transmission of Cryptosporidium in a veterinary hospital. J. Vet. Intern. Med. 11(6), 340-343. http://dx.doi.org/10.1111/j.1939-1676.1997.tb00477.x PMid:9470158
KuKanich KS, Ghosh A, Skarbek JV, Lothamer KM, Zurek L (2012). Surveillance of bacterial contamination in small animal veterinary hospitals with special focus on antimicrobial resistance and virulence traits of enterococci. J Am Vet Med Assoc. 240(4): 437–445. http://dx.doi.org/10.2460/javma.240.4.437 PMid:22309016
Kwon NH, Park KT, Moon JS, Jung WK, Kim SH, Kim JM, Hong SK, Koo HC, Joo YS, Park YH (2005). Staphylococcal cassette chromosome mec (SCCmec) characterization and molecular analysis for methicillin-resistant Staphylococcus aureus and novel SCCmec subtype IVg isolated from bovine milk in Korea. J. Antimicrobial. Chemo. 56(4): 624–632. http://dx.doi.org/10.1093/jac/dki306 PMid:16126781
Kwon KH, Moon BY, Hwang SY, Park YH (2012). Detection of CC17 Enterococcus faecium in Dogsanda Comparison with Human Isolates. Zoonoses Public Health. 59(6): 375–378. http://dx.doi.org/10.1111/j.1863-2378.2012.01466.x PMid:22372965
Laarhoven LM, de Heus P, van Luijn J, Duim B, Wagenaar JA, van Duijkeren E (2011). Longitudinal study on methicillin-resistant staphylococcus pseudintermedius in Households. PLoS One. 6:e27788. http://dx.doi.org/10.1371/journal.pone.0027788 PMid:22132141 PMCid:PMC3223215
Landry SL, Donowitz LG, Wenzel RP (1982). Hospital-wide surveillance:perspective for the practitioner. Am. J. Infect. Control. 10(2): 66-67. http://dx.doi.org/10.1016/0196-6553(82)90005-0
Larson EL, Leyden JJ, McGinley KJ, Grove GL, Talbot GH (1986). Physiologic and microbiologic changes in skin related to frequent handwashing. Infect. Control Hosp. Epidemiol. 7(2): 59–63
Lee JH (2003). Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl. Environ. Microbiol. 69(11): 6489–6494. http://dx.doi.org/10.1128/AEM.69.11.6489-6494.2003 PMid:14602604 PMCid:PMC262320
Leonard FC, Abbott Y, Rossney A, Quinn PJ, O’Mahony R, Markey BK (2006). Methicillin-resistant Staphylococcus aureus isolated from a veterinary surgeon and five dogs in one practice. Vet. Rec. 158(5): 155–159. http://dx.doi.org/10.1136/vr.158.5.155 PMid:16461622
Leonard FC, Markey BK (2008). Meticillin-resistant Staphylococcus aureus in animals: A review. Vet. J. 175(1):27–36. http://dx.doi.org/10.1016/j.tvjl.2006.11.008 PMid:17215151
Levin BR, Rozen DE (2006). Non-inherited antibiotic resistance. Nature Rev. Microbiol. 4(7): 556–562. http://dx.doi.org/10.1038/nrmicro1445 PMid:16778840
Lloyd DH (2007). Reservoirs of Antimicrobial Resistance in Pet Animals. Clin Infect Dis. 45: Suppl.2: 148–152. http://dx.doi.org/10.1086/519254 PMid:17683019
Lobetti RG, Joubert KE, Picard J, Carstens J, Pretorius E (2002). Bacterial colonization of intravenous catheters in young dogs suspected to have parvoviral enteritis. J. Am. Vet. Med. Assoc. 220(9): 1321–1324. http://dx.doi.org/10.2460/javma.2002.220.1321 PMid:11991409
Loeffler A, Boag AK, Sung J, Lindsay JA, Guardabassi L, Dalsgaard A, Lloyd DH (2005). Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. J. Antimicrobial. Chemo. 56(4): 692–697. http://dx.doi.org/10.1093/jac/dki312 PMid:16141276
Loeffler A, Pfeiffer DU, Lloyd DH, Smith H, Soares-Magalhaes R, Lindsay JA (2010). Methicillin-resistant Staphylococcus aureus carriage in UK veterinary staff and owners of infected pets: new risk groups. J. Hosp. Infect. 74(3): 282–288. http://dx.doi.org/10.1016/j.jhin.2009.09.020 PMid:20080322
Lombardo J, Burkom H, Elbert E, Magruder S, Lewis SH, Loschen W, Pavlin J (2003). A systems overview of the Electronic Surveillance System for the Early Notification of Community-Based Epidemics (ESSENCE II). J. Urban Health. 80(2 Suppl 1): i32–142.
Low JC, Tennant B, Munro D (1996). Multiple-resistant Salmonella typhimurium DT104 in cats. Lancet. 348: 1391.
Lynch AS, Robertson GT (2008). Bacterial and Fungal Biofilm Infections. Annu. Rev. Med. 59: 415–428. http://dx.doi.org/10.1146/annurev.med.59.110106.132000 PMid:17937586
Ma J, Zeng Z, Chen Z, Xu X, Wang X, Deng Y, Lu D, Huang L, Zhang Y, Liu J, Wang M (2009). High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac(6’)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob. Agents Chemother. 53(2): 519–524. http://dx.doi.org/10.1128/AAC.00886-08 PMid:18936192 PMCid:PMC2630616
Madewell BR, Tang YJ, Jang S, Madigan JE, Hirsh DC, Gumerlockm PH, Silva J (1995). Apparent outbreaks of Clostridium difficile-associated diarrhea in horses in a veterinary medical teaching hospital. J. Vet. Diagn. Invest. 7(3): 343–346. http://dx.doi.org/10.1177/104063879500700308 PMid:7578449
Major RH (1954). A history of medicine. Springfield, IL: Charles C. Thomas. Pp 950-951.
Malik S, Peng H, Barton M (2006). Partial nucleotide sequencing of the mecA genes of Staphylococcus aureus isolates from dogs and cats. J. Clin. Microbiol. 44(2): 413–416. http://dx.doi.org/10.1128/JCM.44.2.413-416.2006 PMid:16455893 PMCid:PMC1392638
Manian FA (2003). Asymptomatic nasal carriage of mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MRSA) in a pet dog associated with MRSA infection in household contacts. Clin. Infect. Dis. 36(2): e26–e28. http://dx.doi.org/10.1086/344772 PMid:12522764
Mann P (1959). Antibiotic sensitivity testing and bacteriophage typing of staphylococci found in the nostrils of dogs and cats. J. Am. Vet. Med. Assoc. 134(10): 469–470.
Marshal BM, Ochieng DJ, Levy SB (2009). Commensals: unappreciated reservoir of antibiotic resistance. Microbe. 4(5): 231-238.
Marsh-Ng ML, Burney DP, Garcia J (2007). Surveillance of infections associated with intravenous catheters in dogs and cats in an intensive care unit. J. Am. Anim. Hosp. Assoc. 43(1): 13-20. http://dx.doi.org/10.5326/0430013 PMid:17209080
Mathews KA, Brooks MJ, Valliant AE (1996). A prospective study of intravenous catheter contamination. J. Vet. Emerg. Crit. Care. 6(1): 33-43. http://dx.doi.org/10.1111/j.1476-4431.1996.tb00032.x
Metan G, Zarakolu P, Unal S (2005). Rapid detection of antibacterial resistance in emerging Gram-positive cocci. J. Hosp. Infect. 61(2): 93–99. http://dx.doi.org/10.1016/j.jhin.2005.02.020 PMid:16009459
Middleton JR, Fales WH, Luby CD, Oaks JL, Sanchez S, Kinyon JM, Hartmann F (2005). Surveillance of Staphylococcus aureus in veterinary teaching hospitals. J. Clin. Microbiol. 43(6): 2916-2919. http://dx.doi.org/10.1128/JCM.43.6.2916-2919.2005 PMid:15956418 PMCid:PMC1151956
Mielke M (2010). Prevention and control of nosocomial infections and resistance to antibiotics in Europe – Primum non-nocere: Elements of successful prevention and control of healthcare-associated infections. Int. J. Med. Microbiol. 300(6): 346–350. http://dx.doi.org/10.1016/j.ijmm.2010.04.004 PMid:20451448
Moodley A, Stegger M, Bagcigil AF, Baptiste KE, Loeffler A, Lloyd DH, Williams NJ, Leonard N, Abbott Y, Skov R, Guardabassi L (2006). Spa typing of methicillin-resistant Staphylococcus aureus isolated from domestic animals and veterinary staff in theUK and Ireland. J. Antimicrobial. Chemo. 58(6): 1118–1123. http://dx.doi.org/10.1093/jac/dkl394 PMid:17030517
Morley PS (2002). Biosecurity of veterinary practices. Vet. Clin: Food Anim. Pract. 18(1): 133–155.
Morley PS (2004). Surveillance for nosocomial infections in veterinary hospitals. Vet. Clin: Equine Pract. 20(3): 561-576. http://dx.doi.org/10.1016/j.cveq.2004.08.002 PMid:15519818
Morley PS, Apley MD, Besser TE, Burney DP, Fedorka-Cray PJ (2005). Antimicrobial drug use in veterinary medicine. J. Vet. Intern. Med. 19(4): 617–629. http://dx.doi.org/10.1111/j.1939-1676.2005.tb02739.x PMid:16095186
Morley PS Weese JS (2008). Biosecurity and infection control for large animal practice. In B. P. Smith (ed.) Large animal internal medicine. 4th ed. (510-527). New York: Elsevier
Mostashari F, Hartman J (2003). Syndromic surveillance: a local perspective. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 80(2): i1-i7.
Murray A, Dineen A, Kelly P, McGoey K, Madigan G, Nighallchoir E, Gunn-Moore DA (2014). Nosocomial spread of Mycobacterium bovis in domestic cats. J. Feline Med. Surg. Apr 7. [Epub ahead of print].
Murphy C, Reid-Smith RJ, Prescott JF, Bonnett BN, Poppe C, Boerlin P, Weese JS, Janecko N, McEwen SA (2009). Occurrence of antimicrobial resistant bacteria in healthy dogs and cats presented to private veterinary hospitals in southern Ontario: a preliminary study. Can. Vet. J. 50(10): 1047-53.
National Nosocomial Infections Surveillance (NNIS) System. (1991). Nosocomial infection rates for interhospital comparison: limitations and possible solutions. Infect. Control Hosp. Epidemiol. 12(10): 609-621. http://dx.doi.org/10.2307/30145247 PMid:1664844
Nicholson M, Beal M, Shofer F, Brown DC (2002). Epidemiologic evaluation of postoperative wound infection in clean-contaminated wounds: a retrospective study of 239 dogs and cats. Vet. Surg. 31(6): 577-581. http://dx.doi.org/10.1053/jvet.2002.34661 PMid:12415527
Nienhoff U, Kadlec K, Chaberny IF, Verspohl J, Gerlach GF, Kreienbrock L, Schwarz S (2011). Methicillin-resistant Staphylococcus pseudintermedius among dogs admitted to a small animal hospital. Vet. Microbiol. 150(1-2):191-197. http://dx.doi.org/10.1016/j.vetmic.2010.12.018 PMid:21247709
O’Keefe A, Hutton TA, Schifferli DM, Rankin SC (2010). First detection of CTX-M and SHV extended-spectrum beta-lactamases in Escherichia coli urinary tract isolates from dogs and cats in the United States. Antimicrob. Agents and Chemother. 54(8): 3489–3492. http://dx.doi.org/10.1128/AAC.01701-09 PMid:20479196 PMCid:PMC2916344
O’Mahony R, Abbott Y, Leonard FC, Markey BK, Quinn PJ, Pollock PJ, Rossney AS (2005). Methicillin-resistant Staphylococcus aureus (MRSA) isolated from animals and veterinary personnel in Ireland. Vet. Microbiol. 109(3-4): 285-296. http://dx.doi.org/10.1016/j.vetmic.2005.06.003 PMid:16026939
Oteo J, Cercenado E, Cuevas O, Bautista V, Delgado-Iribarren A, Orden B, Perez-Vazquez M, Garcı´a-Cobos S, Campos J (2010). AmpC beta-lactamases in Escherichia coli: emergence of CMY-2-producing virulent phylogroup D isolates belonging mainly to STs 57, 115, 354, 393, and 420, and phylogroup B2 isolates belonging to the international clone O25b-ST131. Diagn. Microbiol. Infect. Dis. 67(3): 270–276. http://dx.doi.org/10.1016/j.vetmic.2005.06.003 PMid:16026939
Owens CD, Stoessel K (2008). Surgical site infections: epidemiology, microbiology and prevention. J. Hosp. Infect. 70(2): 3-10. http://dx.doi.org/10.1016/S0195-6701(08)60017-1
Pak SI, Han HR, Shimizu A (1999). Characterization of methicillinresistant Staphylococcus aureus isolated from dogs in Korea. J. Vet. Med. Sci. 61(9): 1013–1018. http://dx.doi.org/10.1292/jvms.61.1013 PMid:10535519
Pandya M, Wittum T, Tadesse DA, Gebreyes W, Hoet A (2009). Environmental Salmonella surveillance in The Ohio State University Veterinary Teaching Hospital. Vector Borne and Zoonotic Dis. 9(6): 649-654. http://dx.doi.org/10.1089/vbz.2008.0120 PMid:19272003
Panlilio AL, Culver DH, Gaynes RP, Banerjee S, Henderson TS, Tolson JS, Martone WJ (1992). Methicillin-resistant Staphylococcus aureus in US hospitals, 1975–1991. Infect. Control Hosp. Epidemiol. 13(10): 582–586. http://dx.doi.org/10.2307/30148460 PMid:1469266
Pitout JDD (2010). Infections with extended-spectrum beta-lactamaseproducing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 70(3): 313–333. http://dx.doi.org/10.2165/11533040-000000000-00000 PMid:20166768
Poeta P, Costa D, Rodrigues J, Torres C. (2006). Antimicrobial resistance and the mechanisms implicated in faecal enterococci from healthy humans, poultry and pets in Portugal. Int. J. Antimicrob. Agents. 27(2): 131–137. http://dx.doi.org/10.1016/j.ijantimicag.2005.09.018 PMid:16388931
Pottinger, JM, Herwaldt LA, Perl TM (1997). Basics of surveillance – an overview. Infect. Control Hosp. Epidemiol. 18(7): 513–527. http://dx.doi.org/10.1086/647659 PMid:9247837
Rich M, Roberts L (2004). Methicillin-resistant Staphylococcus aureus isolates from companion animals. Vet. Rec. 154(10): 310–17.
Rich M, Roberts L, Deighton L (2005). Clindamycin resistance in methicillin-resistant Staphylococcus aureus isolated from animals. Vet. Microbiol. 111(3-4): 237-40. http://dx.doi.org/10.1016/j.vetmic.2005.09.011 PMid:16289541
Roberts MC, O’Boyle DA (1981). The prevalence and epizootiology of salmonellosis among groups of horses in south-east Queensland. Aust. Vet. J. 57(1): 27–35. http://dx.doi.org/10.1111/j.1751-0813.1981.tb07081.x PMid:7236142
Rodriguez-Palacios A, Reid-Smith RJ, Staempfli HR, Daignault D, Janecko N, Avery BP, Martin H, Thomspon AD, McDonald LC, Limbago B, Weese JS (2009). Possible seasonality of Clostridium difficile in retail meat, Canada. Emerg. Infect. Dis. 15(5): 802–805. http://dx.doi.org/10.3201/eid1505.081084 PMid:19402975 PMCid:PMC2687045
Rubin JE, Pitout JDD (2014). Extended-spectrum b-lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet. Microbiol. 170(1-2): 10–18. http://dx.doi.org/10.1016/j.vetmic.2014.01.017 PMid:24576841
Ruby R, Magdesian KG, Kass PH (2009). Comparison of clinical microbiologic, and clinicopathologic findings in horses positive and negative for Clostridium difficile infection. J. Am. Vet. Med. Assoc. 234(6): 777–784. http://dx.doi.org/10.2460/javma.234.6.777 PMid:19284345
Ruple-Czerniak A, Aceto HW, Bender JB, Paradis MR, Shaw SP, Van Metre DC, Weese JS, Wilson DA, Wilson JH, Morley PS (2013). Using Syndromic Surveillance to Estimate Baseline Rates for Healthcare-Associated Infections in Critical Care Units of Small Animal Referral Hospitals. J. Vet. Intern. Med. 27(6): 1392–1399. http://dx.doi.org/10.1111/jvim.12190 PMid:24134779
Ruple-Czerniak AA, Aceto HW, Bender JB, Paradis MR, Shaw SP, Van Metre DC, Weese JS, Wilson DA, Wilson J, Morley PS (2014). Syndromic surveillance for evaluating the occurrence of healthcare-associated infections in equine hospitals. Equine. Vet. J. 46(4): 435–440. http://dx.doi.org/10.1111/evj.12190 PMid:24028074
Rupnik M, Wilcox MH, Gerding DN (2009). Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Rev. Microbiol. 7(7): 526-536. http://dx.doi.org/10.1038/nrmicro2164 PMid:19528959
Sabino CV, Weese JS (2006). Contamination of multiple-dose vials in a veterinary hospital. Can. Vet. J. 47(8): 779-782.
Sanchez S, Stevenson MAM, Hudson CR, Maier M, Buffington T, Dam Q, Maurer JJ (2002). Characterization of Multidrug-Resistant Escherichia coli Isolates Associated with Nosocomial Infections in Dogs. J. Clin. Microbiol. 40(10): 3586–3595. http://dx.doi.org/10.1128/JCM.40.10.3586-3595.2002 PMid:12354850 PMCid:PMC130861
Sax H, Pittet D, and the Swiss-NOSO network (2002). Interhospital differences in nosocomial infection rates. Arch. Intern. Med. 162(21): 2437–2442. http://dx.doi.org/10.1001/archinte.162.21.2437 PMid:12437402
Sax H, Uckay I, Richet H, Allegranzi B, Pittet D (2007). Determinants of good adherence to hand hygiene among healthcare workers who have extensive exposure to hand hygiene campaigns. Infect. Control Hosp. Epidemiol. 28(11): 1267–1274. http://dx.doi.org/10.1086/521663 PMid:17926278
Schelenz S, French G (2000). An outbreak of multidrug-resistant Pseudomonas aeruginosa infection associated with contamination of bronchoscopes and an endoscope washer-disinfector. J. Hosp. Infect. 46(1): 23–30. http://dx.doi.org/10.1053/jhin.2000.0800 PMid:11023719
Schneeberg A, Rupnik M, Neubauer H, Seyboldt C (2012). Prevalence and distribution of Clostridium difficile PCR ribotypes in cats and dogs from animal shelters in Thuringia, Germany. Anaerobe. 18(5):484–488. http://dx.doi.org/10.1016/j.anaerobe.2012.08.002 PMid:22951303
Schott HC, Ewart SL, Walker RD, Dwyer RM, Dietrich S, Eberhart SW, Derksen FJ (2001). An outbreak of salmonellosis among horses at a veterinary teaching hospital. J. Am. Vet. Med. Assoc. 218(7): 1152–1159. http://dx.doi.org/10.2460/javma.2001.218.1152 PMid:11318368
Schwaber MJ, Venezia SN, Masarwa S, Levy ST, Adler A, Chmelnitsky I, Carmeli Y, Klement E, Steinman A (2013). Clonal transmission of a rare methicillin-resistant Staphylococcus aureus genotype between horses and staff at a veterinary teaching hospital. Vet. Microbiol. 162(2-4): 907–911. http://dx.doi.org/10.1016/j.vetmic.2012.11.020 PMid:23265243
Scott GM, Thomson R, Malone-Lee J, Ridgway GL (1988). Cross infection between animals and man: possible feline transmission of Staphylococcus aureus infection in humans? J. Hosp. Infect. 12(1): 29–34. http://dx.doi.org/10.1016/0195-6701(88)90119-3
Seguin JC, Walker RD, Caron JP, Kloos WE, George CG, Hollis RJ, Pfaller MA (1999). Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J. Clin. Microbiol. 37(5): 1459-1463.
Semmelweis I (1848). The etiology, concept, and prophylaxis of childbed fever. Translated by KC Carter. Madison: The University of Wisconsin Press.
Sieber S, Gerber V, Jandova V, Rossano A, Evison JM, Perreten V (2011). Evolution of multidrug-resistant S. aureus infections in horses and colonized personnel in an equine clinic between 2005 and 2010. Microb. Drug Resist. 17(3): 471–478. http://dx.doi.org/10.1089/mdr.2010.0188 PMid:21875361
Simjee S, White DG, McDermott PF, Wagner DD, Zervos MJ, Donabedian SM (2002). Characterization of Tn1546 in vancomycin-resistant Enterococcus faecium isolated from canine urinary tract infections: evidence of gene exchange between human and animal enterococci. J. Clin. Microbiol. 40(12): 4659–4665. http://dx.doi.org/10.1128/JCM.40.12.4659-4665.2002 PMid:12454168 PMCid:PMC154613
Singh A, Walker M, Rousseau J, Monteith GJ, Weese JS (2013). Methicillin-resistant staphylococcal contamination of clothing worn by personnel in a veterinary teaching hospital. Vet. Surg. 42(6): 643-648. http://dx.doi.org/10.1111/j.1532-950X.2013.12024.x PMid:23662728
Shaheen BW, Nayak R, Foley SL, Kweon O, Deck J, Park M, Rafii F, Boothe DM (2011). Molecular characterization of resistance to extended-spectrum cephalosporins in clinical Escherichia coli isolates from companion animals in the United States. Antimicrob. Agents Chemother. 55(12): 5666–5675. http://dx.doi.org/10.1128/AAC.00656-11 PMid:21947397 PMCid:PMC3232758
Smarick SD, Haskins SC., Aldrich J, Foley JE, Kass PH, Fudge M, Ling GV (2004). Incidence of catheter-associated urinary tract infection among dogs in a small animal intensive care unit. J. Am. Vet. Med. Assoc. 224(12): 1936–1940. http://dx.doi.org/10.2460/javma.2004.224.1936 PMid:15230447
Smith MM, Vasseur PB, Saunders HM (1989). Bacterial growth associated with metallic implants in dogs. J. Am. Vet. Med. Assoc. 195(6): 765–767.
Smith BP (2004). Evolution of equine infection control programs. Vet. Clin: Equine Pract. 20(3): 521–530. http://dx.doi.org/10.1016/j.ajic.2004.04.002
Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G (2013). Cohabiting family members share microbiota with one another and with their dogs. Elife. 2: e00458. http://dx.doi.org/10.7554/eLife.00458
Steneroden KK, Van Metre DC, Jackson C, Morle PS (2010). Detection and control of a nosocomial outbreak caused by Salmonella Newport at a large animal hospital. J. Vet. Intern. Med. 24(3): 606–616. http://dx.doi.org/10.1111/j.1939-1676.2010.0484.x PMid:20337913
Stolle I, Prenger-Berninghoff E, Stamm I, Scheufen S, Hassdenteufel E, Guenther S, Bethe A, Pfeifer Y, Ewers C (2013). Emergence of OXA-48 carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in dogs. J. Antimicrobial. Chemo. 68(12): 2802–2808. http://dx.doi.org/10.1093/jac/dkt259 PMid:23833179
Stone AB, Hautala JA (2008). Meeting report: panel on the potential utility and strategies for design and implementation of a national companion animal infectious disease surveillance system. Zoonoses and Public Health. 55(8-10): 378–384. http://dx.doi.org/10.1111/j.1863-2378.2008.01129.x PMid:18811903
Suthar N, Roy S, Call DR, Besser TE, Davis MA (2014). An Individual-Based Model of Transmission of Resistant Bacteria in a Veterinary Teaching Hospital. PLoS ONE. 9(6): e98589. http://dx.doi.org/10.1371/journal.pone.0098589 PMid:24893006 PMCid:PMC4043964
Tillotson K, Savage CJ, Salman MD, Gentry-Weeks C, Rice D, Fedorka-Cray PJ, Traub-Dargatz JL (1997). Outbreak of Salmonella infantis infection in a large animal veterinary teaching hospital. J Am Vet Med Assoc. 211(12): 1554–1557.
Traub-Dargatz JL, Dargatz DA, Morley PS, Dunowska M (2004). An overview of infection control strategies for equine facilities, with an emphasis on veterinary hospitals. Vet. Clin: Equine Pract. 20(3): 507–520. http://dx.doi.org/10.1016/j.cveq.2004.07.004 PMid:15519814
Tremblay CL, Charlebois A, Masson L, Archambault M (2013). Characterization of hospital-associated lineages of ampicillin resistant Enterococcus faecium from clinical cases in dogs and humans. Front Microbiol. 4(245): 1–11.
Van Dijke B, Koppen H, Wannet W, Huijsdens X, de Neeling H, Voss A (2006). Methicillin-resistant Staphylococcus aureus and pig farming (abstract). In: Proceedings of the 16th European Congress of Clinical Microbiology and Infectious Diseases Conference,Nice,France,April14,2006,p.474. http://www.blackwellpublishing.com/eccmid16/PDFs/clm_1247.pdf.
Van den Berg RWA, Claahsen HL, Niessen M, Muytjens HL, Liem K, Voss A (2000). Enterobacter cloacae outbreak in the NICU related to disinfected thermometers. J. Hosp. Infect. 45(1): 29–34. http://dx.doi.org/10.1053/jhin.1999.0657 PMid:10917779
van Duijkeren E, Moleman M, Sloet van Oldruitenborgh-Oosterbaan MM, Multem J, Troelstra A, Fluit AC, van Wamel WJB, Houwers DJ, de Neeling AJ, Wagenaar JA (2010). Methicillin-resistant S. aureus in horses and horse personnel: an investigation of several outbreaks. Vet. Microbiol. 141(1-2): 96–102. http://dx.doi.org/10.1016/j.vetmic.2009.08.009 PMid:19740613
Van Metre DC, Barkey DQ, Salman MD, Morley PS (2009). Development of a syndromic surveillance system for detection of disease among livestock entering an auction market. J. Am. Vet. Med. Assoc. 234(5): 658–664. http://dx.doi.org/10.2460/javma.234.5.658 PMid:19250046
Vaneechoutte M, Devriese LA, Dijkshoorn L (2000). Acinetobacter baumannii-infected vascular catheters collected from horses in an equine clinic. J. Clin. Microbiol. 38(11): 4280–4281.
Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M (2005). Methicillin-resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 11(12): 1965–1966. http://dx.doi.org/10.3201/eid1112.050428 PMid:16485492 PMCid:PMC3367632
Wagner S, Gally DL, Argyle SA (2014). Multidrug-resistant Escherichia coli from canine urinary tract infections tend to have commensal phylotypes, lower prevalence of virulence determinants and ampC-replicons. Vet Microbiol 169(3-4) :171–178. http://dx.doi.org/10.1016/j.vetmic.2014.01.003 PMid:24485933 PMCid:PMC3969583
Wall PG, Threllfall EJ, Ward LR, Rowe B (1996). Multiresistant Salmonella typhimurium DT104 in cats: a public health risk. Lancet. 348(9025): 471. http://dx.doi.org/10.1016/S0140-6736(96)24033-4
Weber DJ, Raasch R, Rutala WA (1999). Nosocomial infections in the ICU: the growing importance of antibiotic-resistant pathogens. Chest. 115(Suppl 3): 34–41. http://dx.doi.org/10.1378/chest.115.suppl_1.34S
Weese JS, Archambault M, Willey BM, Dick H, Hearn P, Kreiswirth BN, Said-Salim B, McGeer A, Likhoshvay Y, Prescott JF, Low DE (2005a). Methicillin-resistant Staphylococcus aureus in horses and horse personnel, 2000–2002. Emerg. Infect. Dis. 11(3): 430–435. http://dx.doi.org/10.3201/eid1103.040481 PMid:15757559 PMCid:PMC3298236
Weese JS, Rousseau J (2005b). Attempted eradication of methicillinresistant S. aureus colonization in horses on two farms. Equine. Vet. J. 37(6): 510–514. http://dx.doi.org/10.2746/042516405775314835 PMid:16295927
Weese JS Armstrong J (2003). Outbreak of Clostridium difficile-associated disease in a small animal veterinary teaching hospital. J. Vet. Intern. Med. 17(6): 813–816. http://dx.doi.org/10.1111/j.1939-1676.2003.tb02519.x PMid:14658717
Weese JS, Caldwell F, Willey BM, Kreiswirth BN, McGeer A, Rousseau J, Low DE (2006a). An outbreak of methicillin-resistant Staphylococcus aureus skin infections resulting from horse to human transmission in a veterinary hospital. Vet. Microbiol. 114(1-2): 160–164. http://dx.doi.org/10.1016/j.vetmic.2005.11.054 PMid:16384660
Weese JS, DaCosta T, Button L, Goth K, Ethier M, Boehnke K (2004). Isolation of methicillin-resistant Staphylococcus aureus from the environment in a veterinary teaching hospital. J. Vet. Intern. Med. 18(4): 468–470. http://dx.doi.org/10.1111/j.1939-1676.2004.tb02568.x PMid:15320581
Weese JS, Rousseau J, Willey BM, Archambault M, McGeer A, Low DE (2006b). Methicillin-resistant Staphylococcus aureus in horses at a veterinary teaching hospital: frequency, characterization, and association with clinical disease. J. Vet. Intern. Med. 20(1): 182–186. http://dx.doi.org/10.1111/j.1939-1676.2006.tb02839.x
Weese JS, Staempfli HR, Prescott JF (2000). Isolation of environmental Clostridium difficile from a veterinary teaching hospital. J. Vet. Diagn. Invest. 12(5): 449–452. http://dx.doi.org/10.1177/104063870001200510 PMid:11021433
Weese JS (2008). Investigation of Enterobacter cloacae infections at a small animal veterinary teaching hospital. Vet. Microbiol. 130(3-4): 426–428. http://dx.doi.org/10.1016/j.vetmic.2008.02.009 PMid:18374521
Weese JS, Duijkeren EV (2010). Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 140(3-4): 418–429. http://dx.doi.org/10.1016/j.vetmic.2009.01.039 PMid:19246166
Weese JS, Finley R, Reid-Smith RR, Janecko N, Rousseau J (2010). Evaluation of Clostridium difficile in dogs and the household environment. Epidemiol. Infect. 138(8): 1100–1104. http://dx.doi.org/10.1017/S0950268809991312 PMid:19951453
Weese JS (2008a). Review of multidrug resistant surgical site infections. Vet. Comp. Orthop. Traumatol. 21(1): 1–7.
Weese JS (2008b). A review of post-operative infections in veterinary orthopedic surgery. Vet Comp Orthop Traumatol. 21(2): 99–105.
Weese JS, Stull J (2013). Respiratory disease outbreak in a veterinary hospital associated with canine parainfluenza virus infection. Can. Vet. J. 54(1): 79–82.
Wenzel RP, Osterman CA, Donowitz LG, Hoyt JW, Sande MA, Martone WJ, Miller GB (1981). Identification of procedure-related nosocomial infections in high-risk patients. Rev. Infect. Dis. 3(4): 701–707. http://dx.doi.org/10.1093/clinids/3.4.701 PMid:6951236
Wenzel RP, Osterman CA, Hunting KJ, Gwaltney JM (1976). Hospital-acquired infections: I. Surveillance in a university hospital. Am. J. Epidemiol. 103(3): 251–260.
Wilberger MS, Anthony KE, Rose S, McClain M, Bermudez LE (2012) Beta-Lactam Antibiotic Resistance among Enterobacter spp. Isolated from Infection in Animals. Adv. Microbiol. 2(2): 129–137. http://dx.doi.org/10.4236/aim.2012.22018
Willems RJ, Top J, VanSchaik W, Leavis H, Bonten M, Siren J (2012).Restricted gene flow among hospital subpopulations of Enterococcus faecium. MBio. 3(4): e00151–00112. http://dx.doi.org/10.1128/mBio.00151-12 PMid:22807567 PMCid:PMC3413404
Willems RJ, VanSchaik W (2009). Transition of Enterococcus faecium from commensal organism to nosocomial pathogen. Future Microbiol. 4(9): 1125–1135. http://dx.doi.org/10.2217/fmb.09.82 PMid:19895216
Winnefeld M, Richard MA, Drancourt M, Grubb JJ (2000). Skin intolerance and effectiveness of two hand decontamination procedures in everyday hospital use. Br. J. Dermatol. 143(3): 546–550. http://dx.doi.org/10.1111/j.1365-2133.2000.03708.x PMid:10971327
Wise LA, Jones RL, Reif JS (1990). Nosocomial canine urinary tract infections in a veterinary teaching hospital (1983 to 1988). J. Am. Anim. Hosp. Assoc. 26(2): 148–152.
Wright JG, Tengelsen LA, Smith KE, Bender JB, Frank RK, Grendon JH, Angulo FJ (2005). Multidrug-resistant Salmonella typhimurium in four animal facilities. Emerg. Infect. Dis. 11(8): 1235–1241. http://dx.doi.org/10.3201/eid1108.050111 PMid:16102313 PMCid:PMC3320505
Zarrilli R, Pournaras S, Giannouli M, Tsakris A (2013). Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents. 41(1): 11–19. http://dx.doi.org/10.1016/j.ijantimicag.2012.09.008 PMid:23127486
Zhao S, White DG, McDermott PF, Friedman S, English L, Ayers S, Meng J, Maurer JJ, Holland R, Walker RD (2001). Identification and expression of cephamycinase blaCMY genes in Escherichia coli and Salmonella isolated from animals and food. Antimicrob. Agents Chemother. 45(12): 3647–3650. http://dx.doi.org/10.1128/AAC.45.12.3647-3650.2001 PMid:11709361 PMCid:PMC90890
Zhang C, Qiu S, Wang Y, Qi L, Hao R, Liu X, Shi Y, Hu X, An D, Li Z, Li P, Wang L, Cui J, Wang P, Huang L, Klena JD, Song H (2013). Higher isolation of NDM-1 producing Acinetobacter baumannii from the sewage of the hospitals in Beijing. PLoS One. 8: e64857. http://dx.doi.org/10.1371/journal.pone.0064857 PMid:23755152 PMCid:PMC3670931
Zordan S, Prenger-Berninghoff E, Weiss R, van der Reijden T, van den Broek P, Baljer G, Dijkshoorn L (2011). Multidrug-resistant Acinetobacter baumannii in veterinary clinics, Germany. Emerg. Infect. Dis. 17(9): 1751–1754. http://dx.doi.org/10.3201/eid1709.101931 PMid:21888812 PMCid:PMC3322069






