Advances in Animal and Veterinary Sciences
Research Article
Short Term Storage of Asian Walking Catfish (Clarias batrachus Linnaeus, 1758) Gametes
Sheikh Mustafizur Rahman1,2*, Ahmed Saud Alsaqufi3, Yousef Ahmed Alkhamis2,3, Md. Moshiur Rahman1, Md. Nazmul Ahsan1, Roshmon Thomas Mathew2, Quazi Zahangir Hossain4
1Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna-9208, Bangladesh; 2Fish Resources Research Center, King Faisal University, P.O Box 420, Al-Ahsa 31982, Saudi Arabia; 3Agriculture and Food Sciences College, King Faisal University, P.O Box 420, Al-Ahsa 31982, Saudi Arabia; 4Environmnetal Science Discipline, Khulna University, Khulna-9208, Bangladesh.
Abstract | The present study was carried out to investigate the viability of gametes from Asian walking catfish (Clarias batrachus) following short-time storage under hatchery condition at room temperature (26–28°C) and at chilled temperature (2–4ºC) using a household refrigerator. Eggs stored at refrigeration for 5 min resulted in a drastic reduction of fertilization success (36%) in comparison with those stored at room temperature (86%). Eggs without water have had significantly higher successes of fertilization than those stored with water in both storage conditions. On the other hand, prolonged potency was observed for sperm stored in refrigerator than those kept outside at room temperature. Sperm kept in refrigerator with both water and dextrose solutions were consistently viable for at least 48 hours. At room temperature, however, sperm diluted with dextrose showed comparatively higher fertilization (up to 50 min, 27–98%) than sperm diluted with water (up to 10 min, 25–87%). Similar results were obtained when the whole testis was stored. Sperm–egg contact time experiment showed a quick fusion and it required only a minute to achieve 100% fertilization. The results of this study provide evidence on the short-time storage of catfish sperm that might be of relevance to hatchery operators including, for example, production of quality seeds by sacrificing less numbers of male individuals.
Keywords | Contact time, Egg, Fertilization, Preservation, Sperm
Received | September 07, 2020; Accepted | September 15, 2020; Published | November 15, 2020
*Correspondence | Sheikh Mustafizur Rahman, Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna-9208, Bangladesh; Email: srahman@kfu.edu.sa; mustafizfrmt@yahoo.com
Citation | Rahman SM, Alsaquft AS, Alkhamis YA, Rahman MM, Ahsan MN, Mathew RT, Hossain QZ (2020). Short term storage of asian walking catfish (clarias batrachus linnaeus, 1758) gametes. Adv. Anim. Vet. Sci. 8(12): 1394-1401.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1394.1401
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Rahman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Walking catfish, Clarias batrachus is one of the most popular food fish among catfish species, which is widely distributed in various fresh water bodies of Asian countries. Because of its unique taste with high nutritive value (Paul et al., 2015; Thorat, 2017) and absence of intra muscular bones, walking catfish stands out as one of the most valued fish species in Asian countries. Besides, unlike other fish food species, Asian walking catfish are easily stored and transported live to the markets thus the consumers do not have to worry about the quality of preservation in sharp contrast to what happens, in recent times, with other fish. Consequently, consumers always willingly pay premium price for this fish.
In many species, the maturation of gonads in the two sexes is not synchronous. Males often show testicular recrudescence earlier during the season. Because of this, male maturity occurs during the beginning of the season while the females are not yet mature and ready for spawning (Pillay and Kutty, 2005; Juntti and Fernald, 2016). The reverse situation occurs during the end of the breeding season. This is particularly true for many catfish species (Pongthana et al., 1995). Catfish commonly do not release semen by hand stripping; male broods have to be sacrificed to obtain macerated testis glands for artificial fertilization of stripped eggs. This practice puts burden on hatchery operators as considerable number of male bloodstocks has to be procured and maintained. Thus, it would be advantageous to have suitable means of preserving and holding spermatozoa for artificial fertilization at the right time without being concerned for precise synchronization of male and female fish.
Cryogenic preservation could ensure the gametes for prolonged period throughout the years (Tian et al., 2018) however; it requires build-up facilities, skilled manpower and costly cryogenic equipments (liquid nitrogen and its container, straws, power, and so on). One alternative approach is short-term preservation that does not require sophisticated facilities. Short-time storage of fish gametes have been developed for many aquaculture species (Hulata and Rothbard, 1979; Christensen and Tiersch, 1996; Linhart et al., 2001; Gisbert and Williot, 2002; Marques and Godinho, 2004; Vuthiphandchai et al., 2009; Bernáth et al., 2017; Ladoktha et al., 2017; Risopatrón et al., 2018; Santos et al., 2018; Beirão et al., 2019) while dealing with Asian walking catfish, Clarias batrachus is scare. Development of a protocol for short-term storage of gametes of this valuable species could have direct bearings on the hatchery operations in several ways including, for example, addressing asynchrony in maturation, reducing the number of viable male fish sacrificed, transportation of gametes to distant place, reducing inbreeding depression and preserving healthy wild stock (Vuthiphandchai et al., 2009; Rahman et al., 2015; Farago et al., 2017). Therefore, this study was conducted to investigate the viability of Asian walking catfish gametes (eggs and sperm) under different storage conditions. This study also observed the sperm-egg contact time of this species.
MATERIALS AND METHODS
Brood Collection
Mature healthy broods of Asian walking catfish, C. batrachus were collected from the natural sources before the advent of monsoon and maintained in glass fiber tanks (500 L) with constant aeration until experiment. Broods were fed with a commercial pelleted diet (30% protein) at a maintenance level of 8% of their body weight. All experiments were conducted at the laboratory of Fish Physiology and Breeding, Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna. For this experiment, required number of broods corresponding to 130 to 190 g for male and 100 to 160 g for female were used for artificial insemination. All experiments were conducted according to the guidelines for the care and use of animals of Khulna University.
Induced Breeding and Collection of Gonad
For obtaining spawning, the gametes were induced by the procedure described by Indian Council by Agricultural Research (ICAR, 2019). The ovulation took place within 14-18 hours of the first injection. In this study, about 150 individuals (♀: 102 and ♂: 48) were used for induced breeding. Eggs were collected through stripping while male individuals were sacrificed to dissect out testis organs. Immediate after dissection, adhered fat and blood were removed from the testes and then the organs were cut into small pieces followed by passing through a bolting silk. Prior to storage treatment motility of sperm was evaluated (the percentage of spermatozoa showing progressive movement within 10 sec of water addition) by using a microscope at 40x magnification (Ladoktha et al., 2017) and only samples showing higher than 80% motility were used for subsequent experimental treatments.
Experimental Conditions
Viability of preserved gonads was evaluated by measuring their fertilization capacity. Fertilization, judged after 1 hour when the eggs reached to 8-16 cells stage, was considered as an indicator of gamete quality. Unfertilized eggs (with or without water), sperm (one drop of fresh milt diluted with 1 ml of water/dextrose) and dry whole testis were preserved at room temperature (26–28°C) or in a household refrigerator (2–4°C) for several time intervals for the observation of fertilization rates. Our preliminary experiment revealed that sperm dilution used this experiment had 100% fertilization rates. After artificial insemination, fertilized eggs were washed with freshwater several times to remove access sperm, if any and incubated in net bed with continuous water flow and aeration at room temperature. Untreated eggs/sperm were considered as control.
Egg and Sperm Storage
Unfertilized eggs (with or without water) stored at room temperature or in refrigerator for 0 (control), 5, 10, 20, 30, 40, 50, 60, 120 min were inseminated with newly obtained sperm. None of the stored eggs was survived with fresh sperm after 30 min of exposure and therefore, data were not shown in the respective figures. On the other hand, sperm diluted either in water or dextrose and whole testis without dilution stored at room temperature [for 0 (control), 5, 10, 20, 30, 40, 50, 60, 120 min] and in refrigerator [for 0 (control), 1, 4, 8, 12, 24, 48, 72, and 120 hours] were inseminated with newly obtained eggs. Each experiment consisted of five replicates with approximately 500 eggs each. Incubation and estimation of fertilization was done according to the methods described previously.
Sperm-Egg Contact Time
The effects of the duration of sperm contact on fertilization success were assessed by manipulating the time when the eggs were in contact with a diluted sperm. A 1 ml of diluted fresh sperm (one drop of fresh milt plus 1 ml of water) was placed in a beaker containing five hundred eggs. At each time intervals (10, 20, 40, 60, 80, 120, 240, 300 sec), inseminated eggs were gently pipetted into plastic cylinders, the bottom of which had been replaced with fine cloth. The cylinder was then rinsed 4–5 times with freshwater to remove excess sperm and the eggs were re-suspended in freshwater for one hour to observe the fertilization rates. Each experiment was conducted with the replication of five.
Statistical Analyses
All analyses were performed using ‘R’ version 3.5.2 (R Core Team 2018). The descriptive statistics (means, SD, SEs, etc.) were calculated using the ‘psych’ package. The Shapiro-Wilk test of normality and the Levene’s tests for homogeneity of variance were done with the ‘onewaytests’ package. Since the response variable was about ‘percentage data’ (fertilization rate in percent) which did not comply with the assumptions of any parametric model, the non-linear poisson regression model was used that is usually suggested for count and percent data. The ‘quasi-poisson’ regression model was applied here using ‘pscl’ package which is useful since it has a variable dispersion parameter to minimize over-dispersion of data (Mangiafico, 2016). In the model, ‘percentage of fertilization rate’ was included as ‘response variable’, while ‘egg or sperm’ storage time or condition was incorporated as ‘fixed factor’. The subsequent post-hoc tests for pair comparison were done using ‘multcompView’ and ‘emmeans’ packages. All graphs were made using ‘ggplot2’ package.
RESULTS
Egg Storage
Analysis of the results revealed that the viability of eggs in terms of fertilization rates was reduced significantly (p<0.001) with the increase of storage time. At room temperature, fertilization rates were decreased to 41% and 14% when eggs were stored only for 5 min with and without water, respectively comparing to their respective controls (100% at 0 min). This reduction trend in hatching was more drastic after 10 min and none of the eggs were fertilized after 20 and 30 min of storage with and without water, respectively at room temperature (Figure 1).
It was also observed that eggs were more sensitive to cold storage than stored at room temperature. Most of the eggs (with or without water) stored in household refrigerator immediately lost their viability and none of them remained viable after 20 min (Figure 1). Eggs without water had shown significantly (p<0.001) better fertilization success than eggs in water throughout the exposure time. For instance, eggs in water had fertilization rates of about 36%, while it was increased up to 86% when kept without water at room temperature for 5 min (Figure 1). Similar patterns were also evident for refrigerated eggs (Figure 1). In all experimental conditions, eggs became opaque with ruptured cell structure within 30 min of storage.
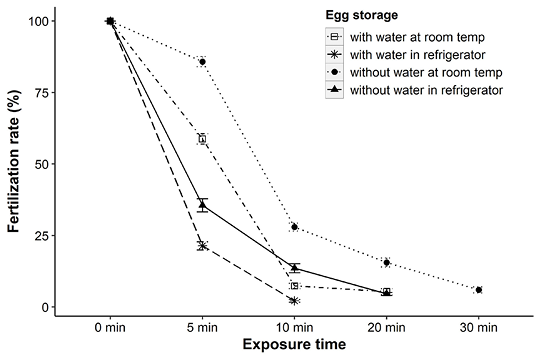
Figure 1: Fertilization rates (mean ± SE) of Clarias batrachus eggs preserved at room temperature (26-28°C) and in refrigerator (2-4°C).
Sperm Storage
Walking catfish sperm stored in various experimental conditions showed various degree of fertilization success (Figure 2). At room temperature, sperm in dextrose performed well (around 50% at 30 min) with newly ovulated eggs than sperm in water (only 25% at 10 min). Fertilization rates gradually decreased significantly (p<0.001) over exposure times in all cases. Sperm in dextrose showed fertilization success of about 98, 68, 51, 47, 42, 27, 12, and 0% when stored for 5, 10, 20, 30, 40, 50, 60, and 120 min, respectively (Figure 2a), whereas fertilization rate of about 87, 25, 0.6, and 0% were obtained with sperms diluted in water and stored for 5, 10, 20, and 30 min, respectively (Figure 2a). Whole testis without external media performed significantly (p<0.001) better than sperm in water but not in dextrose (Figure 2a).
On the other hand, prolonged potency was obtained for sperm stored in household refrigerator for all experimental conditions. Fertilization rates were unchanged (about 100%) until 24 hours for sperm diluted with both dextrose and water, but declined gradually up to 120 hours (Figure 2b). Between external solutions, refrigerated sperm in water were found to be slightly better than sperm in dextrose. The subsequent post-hoc tests revealed that no significant variations (p=0.1) were noticed up to 24 hours storage of sperm in both water and dextrose, but it showed significant variations (p<0.001) for further exposure (48, 72, 96, 120 hours) (Figure 2b). Almost 100% fertilization was observed for whole testis up to 12 hours of storage but it reduced to 86, 41, and 2% after 24, 48, and 72 hours of storage, respectively (Figure 2b). No fertilization was observed for the whole testis stored for 96 hours and beyond.
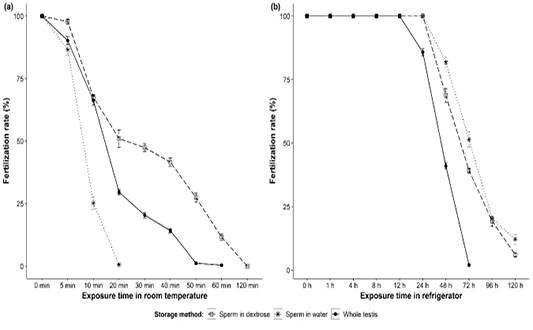
Figure 2: Fertilization rates (mean ± SE) of Clarias batrachus sperm (in dextrose or water and as whole testis) stored (a) at room temperature (26-28°C) and (b) in household refrigerator (2-4°C).
Sperm-Egg Contact Time
Sperm-egg contact time experiment revealed that fertilization followed by cleavage occurred immediate after insemination (Figure 3). After 10 sec of insemination, this species showed 93% fertilization rates and complete fertilization occurred after 60 sec of insemination. No significant differences (p=0.1) were observed between the time intervals (99 to 100%) except 10 (93%) and 20 (96%) sec.

Figure 3: The fertilization success (mean ± SE) based on eggs and sperm contract time in Clarias batrachus.
DISCUSSION
The viability of fish eggs after ovulation remains fertilizable from minutes to few days depending on species and storage condition (Samarin et al., 2015). In the present study, the viability of walking catfish eggs in term of fertilization success was assessed under different ex situ storage conditions. Unfertilized eggs preserved in water had lost their viability within minutes, which is generally in agreement with findings from other fish species (Espinach et al., 1984; Legendre et al., 2000; Linhart et al., 2001) but the results of the present study revealed that the loss of egg viability in Asian walking catfish following storage was considerably faster than in other catfish reported. For example, after 20 min of storage of eggs with water in room temperature Asian walking catfish had only 5% fertilization whereas in case of Asian catfish, Pangasius hypophthalmus it started to decline after 2 hours of storage (Legendre et al., 2000) and in South American catfish, Rhamdia sapo the fertilization rate started to decline after 5 and 9 hours of storage at 24°C and 20°C, respectively of storage (Espinach et al., 1984). Immediate after ovulation, eggs undergo several morphological, physiological, histological cellular and molecular changes that ultimately deteriorate the ovulated egg quality and decrease the ability of the eggs to be fertilized and subsequent embryo development (Rime et al., 2004; Aegerter et al., 2005). Even in successful hatchings, such changes followed by egg ageing increase the malformation rates of hatched larvae (Gisbert and Williot, 2002).
The viability of eggs also varies with the incubation temperature (Samarin et al., 2015) as well as external media. In current results showed that the eggs of walking catfish preserved at room temperature responded better than those of preserved in refrigerator. Eggs stored in refrigerated condition lost their potency immediately with or without presence of water. Mass rupture of cell structure of the eggs was evident; the storage solution became very viscous and laden with cell fragments. The exact reason for such rapid and extensive disruption of cells is currently unknown. Perhaps it is related to the fact of dehydration inside the cell (Loeffler and Lovtrup, 2000; Diwan et al., 2020) or oxygen deficiencies deserve additional study. The present study further proved that unfertilized eggs (both at room temperature and in refrigerator) without external water had the better fertilization capabilities to that of eggs in water. Micropyle closure due to chorion expansion or egg viability loss due to protein breakdown limits the fertilization success when eggs are kept in water (Hsu and Goetz, 1993).
In general, eggs from cold water species are more tolerant to chilling than those from tropical or sub-tropical fish species (Robles et al., 2019). Working with Eurasian perch, Samarin et al. (2017) reported that unfertilized eggs stored at low temperature remained viable for 48 hours while Niksirat et al. (2007) reported even more (9 days) for salmonids eggs. Such variation in cold tolerance could be the presence of antifreeze protein that prevent freezing of these species inhabits in cold region (Robles et al., 2019) or the protective mechanism as for example of Eurasian perch that contain gelatinous layer for the protection of eggs from the direct contact with the air and surrounding media (Samarin et al., 2017).
In nature, the duration of sperm viability is very short for most of the teleost species with external fertilization (Kholodnyy et al., 2020). The current results revealed that sperm of walking catfish stored in water at room temperature remained active in terms of fertilization only for 5 min but showed more active when sperm stored in dextrose (50 min) or as whole testis (30 min). Previous studies on sperm viability also reported short life spans for other catfish species: 30 to 120 sec for African catfish Clarias gariepinus (Mansour et al., 2002), 60 to 70 sec for stinging catfish Heterobranchus longifilis (Otémé et al., 1996) and 100 to 180 sec for S. glanis sperms (Billard et al., 1997). Similar results were reported for most of the marine species (Suquet et al., 1995; Rurangwa et al., 1998). The loss of sperm viability diluted in water might be due to the combined effects of decreased oxygen, increased carbon dioxide and lowered pH (Rahman et al., 2001). Prolonged sperm potency (for about 60 min) was observed for Atlantic cod that inhabits in temperate region (Trippel and Morgan, 1994).
Numerous physiological solutions were tested as the extender for prolonging sperm viability for weeks. Sperms diluted with a number of extenders including dextrose as the simple one were found to be viable after storage for different times (Lahnsteiner et al., 1997; Vuthiphandchai et al., 2009; Santos et al., 2018). The present study also showed that when stored at room temperature sperm diluted in dextrose remained substantially more viable than those in water. On the other hand, the difference in fertilization success of sperms diluted in these two media was not significant when stored at refrigerated temperature. Interestingly, however, sperm stored in refrigerator performed better than those stored at room temperature when water was used instead of dextrose. Such variation might be due to slow metabolic rates of sperm at low temperature although the efficacy of other extenders should be investigated to test this hypothesis.
The current study showed that the sperm stored in household refrigerator were more successful than stored at room temperature. Sperm of small-scaled pacu Piaractus mesopotamicus and common carp Cyprinus carpio L can be stored in domestic refrigerator for 30 and 45 hours, respectively (Hulata and Rothbard, 1979; Ferraz de Lima et al., 1989) which is in accordance with the findings of the present study. In this study, sperm of walking catfish tolerated well in refrigerated conditions for 48 hours. Chilled storage of sperm has also been successfully reported in striped bass Morone saxatilis, channel catfish Ictalurus punctatus, Neotropical Characiformes fish species and several species of salmonids (Scott and Baynes, 1980; Christensen and Tiersch, 1996; Marques and Godinho, 2004). Sperm from fish inhabiting cold region such as Atlantic cod and haddock were found to be viable for as long as 40 days in refrigerated condition (DeGraaf and Berlinsky, 2004). Such prolonged storage of sperm, which is attributable to the presence of antifreeze proteins in cold adapted fish species, was also reported for a number of fish including rainbow trout and whitefish (Ciereszko and Dabrowski, 1994), striped bass (Jenkins-Keeran et al., 2001), sea trout and yellowtail tetra (Yasui et al., 2015), rainbow trout (Trigo et al., 2015), Atlantic salmon (Parodi et al., 2017).
The length of time eggs remain in contact with a sperm suspension influences the fertilization success and it is species specific. For example, fertilization occurred (93%) within 10 sec of gamete mixing and complete fertilization (100%) occurred after 60 sec of mixing. Our results are similar to the findings of Rahman et al. (2001), Rosenthal et al. (1988), Suquet et al. (1995) for sea urchin E. mathaei, Herring C. harengus and turbot Scophthalmus maximus, respectively. Much longer time was required for Atlantic cod G. morhua even at high sperm concentration (Butts et al., 2009) while Babcock and Keesing (1999) did not find any significant effect of contact time on fertilization success and concluded that a rapid attachment of sperm to eggs occurred in abalone H. laevigata. In hatchery conditions, fertilization success also varies with sperm numbers, the higher the better (Rahman et al., 2001; Butts et al., 2009) whereas in the nature sperm is diluted once fish ovulate in water. Additional sperm not only block the entry of micropyle (Rosenthal et al., 1988) but also increase the possibility of polyspermy (Debus et al., 2002; Iegorova et al., 2018) that ultimately decreases the fertilization success as well as increases the deformed embryos.
CONCLUSION
The present study showed that walking catfish sperm could be preserved in household refrigerator for two days without losing the viability while, on the other hand, eggs were more sensitive to refrigerated conditions than room temperature. Eggs stored without any external media performed substantially better than those with water or dextrose under both normal and refrigerated conditions. In contrast, sperm diluted in dextrose and kept at room temperature remained more viable whereas in refrigerated condition the effects of external media were insignificant. It was also found that ensuring egg-sperm contact time for at least 60 second after artificial insemination could achieve the maximum fertilization. The results of the present study suggest that short-time storage of catfish gamete is feasible, which might offer some practical benefits to hatchery operators.
ACKNOWLEDGEMENTS
This study was supported by a grant from Research Cell, Khulna University, Bangladesh to the prime author of this manuscript. Authors are indebted to all staffs of Fish Resources Research Center for their kind support during statistical analyses and manuscript preparation.
Conflict of interest
The authors have declared no conflict of interest.
authors contribution
Sheikh Mustafizur Rahman: Conceptualization, methodology, investigation, data curation, writing-original draft, visualization. Ahmed Saud Alsaqufi: Writing and editing data, visualization. Yousef Ahmed Alkhamis: Supervision, project administration, visualization. Md. Moshiur Rahman: Formal analysis, data curation, writing-review and editing. Md. Nazmul Ahsan: Conceptualization, supervision, project administration and fund acquisition, writing-review. Roshmon Thomas Mathew: Conceptualization, software, investigation, and editing data, visualization. Quazi Zahangir Hossain: Investigation, resources.
References






