Advances in Animal and Veterinary Sciences
Research Article
Pathological, Clinicopathological and Molecular Investigations on Chickens Experimentally Infected with Avian Leucosis Virus Type J
Ahmed Fotouh1, Hamdy Soufy1*, El-Begawey M.B2, Soad M. Nasr1
1Department of Parasitology and Animal Diseases, National Research Centre, 33 Bohouth Street, Dokki, Post Box 12622, Giza, Egypt; 2Department of Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Post Box 62511, Egypt.
Abstract | Avian leucosis virus type J (ALV-J) causes highly economic losses in the production of meat and egg. This study aimed to investigate the effect of experimental infection of one-day-old chicks with ALV-J strain -local isolate- on hemogram, biochemical constituents, ALV-J antibody titer, and histopathology of the internal organs. Detection of ALV-J DNA in the internal organs was also performed using the PCR technique. One hundred and twenty chicks -one day old- were randomly divided into two equal groups. The first group was infected intra-peritoneally with isolated ALV-J strain at a dose of 0.2 ml of log104 embryo infected dose (EID50). The second group was kept as normal control and was inoculated intra-peritoneally with 0.2 ml of sterile saline. After inoculation, all experimental birds were kept under strict daily observation for recording clinical signs and mortality rates. At the 1st, 2nd, 3rd, 4th and 5th month post-infection (mpi), blood and tissue samples were collected from each group for determination of hemogram, serum biochemical parameters, antibody titer, and histopathological and molecular examinations of the internal organs. The infected group showed dullness, ruffled feathers, droppings, loss of appetite, decrease in feed intake and depression. The mortality rate reached to18.3% in the infected group. Macrocytic normochromic anemia, leukocytosis, heterophilia, and lymphocytosis were recorded. Serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase activities, and creatinine and uric acid levels were markedly (P<0.05) increased. Lymphoid and myeloid leucosis in the heart, liver, spleen, and kidneys and also erythroid leucosis in the heart and osteopetrosis were recorded. In conclusion, the experimental infection with ALV-J caused alteration in the hemogram, dysfunction of liver and kidney as well as histopathological lesions in the internal organs and bone of infected chickens. These changes began at the 1st mpi and can help in the early diagnosis of disease.
Keywords | Myeloid leucosis, Chickens, Hemogram, Serum biochemistry, Pathology
Received | March 04, 2020; Accepted | April 20, 2020; Published | May 15, 2020
*Correspondence | Hamdy Soufy, Department of Parasitology and Animal Diseases, Veterinary Research Division, National Research Centre, 33 Bohouth Street, Dokki, P.O. Box 12622, Giza, Egypt; Email: hamdysoufy@yahoo.com
Citation | Fotouh A, Soufy H, El-Begawey MB, Nasr SM (2020). Pathological, clinicopathological and molecular investigations on chickens experimentally infected with avian leucosis virus type J. Adv. Anim. Vet. Sci. 8(6): 590-600.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.6.590.600
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Fotouh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Avian retroviruses have been a major cause of mortality in poultry (Wang et al., 2017). These viruses, generally referred to as avian leucosis viruses (ALV), mainly induce leucosis affecting the various hemopoietic cells. The virus infection spreads by both vertical and horizontal transmission (Gao et al., 2012). Avian leucosis viruses of chickens are classified into types (A, B, C, D, E, and J) on the basis of interaction between virus specific cell receptors and virus envelop glycoprotein (Payne and Nair, 2012). Although avian leucosis virus (ALV) is capable of inducing a variety of neoplasms, lymphoid leucosis is the most common naturally occurring B-cell lymphoma caused by ALV.
In Egypt, the diagnosis of ALV infection is based on gross pathological lesions and antibody titer in imported 21 weeks old broiler breeder chickens. ELISA titer was 76%. The infection with ALV J causes up to 36% morbidity and 8% mortality (Sultan et al., 2004). ALV J infection causes serological, hematological and pathological changes in 25 weeks old broiler breeder farms suffering from visceral and bone tumor formation, and also causing up to 40% morbidity and 16% mortality (El-Gohary et al., 2000). ALV J infection reported in broilers -28 days old- depending on histopathological lesions and antibody detection. Antibody titer was 26% with 3.5% mortality (Abdel Gayed et al., 2017).
ALV-J infection in breeder flocks is associated with the occurrence of myeloid leucosis (ML) (myelocytomatosis). ML is a tumor condition, which is readily characterized as comprised of transformed white blood cells from the bone marrow. The first recorded case was observed in broiler breeder birds between 25-55 weeks of age (Cai et al., 2013). Gao et al. (2015) recorded that ML tumors appeared in the field as early as 17 weeks. They noted that the timing, however, may vary according to factors such as genetics, environment, management, nutritional status, concomitant infections, immune-competence and the actual form of transmission. Field experience indicates that immunosuppressive infections such as infectious bursal disease, chicken anemia or Marek’s disease viruses are a lethal combination with ALV-J infection (Qian et al., 2018).
Therefore, the objective of this study was to describe the effect of experimental infection of one-day-old chicks with ALV-J strain -local isolate on hematological and biochemical constituents, ALV-J antibody titer and histopathology of the liver, kidney, spleen, heart, and bone. Moreover, detection of ALV-J DNA in the internal organs was performed using the PCR technique.
MATERIAL AND METHODS
Ethics statement
This experiment was carried out according to guidelines for animal experimentation and approved by the Institutional Animal Care and Use Committee, National Research Centre Animal Care Unit, Dokki, Giza, Egypt, the protocol approval No.: 19/123.
Experimental design
One hundred and twenty of one-day-old specific-pathogen-free (SPF) chicks were used in this experiment. These chicks were serologically negative for the ALV-J antibody. These chicks were put under the required hygienic condition, fed on a balanced ration, supplied with clean water in sufficient quantities. The experimental chicks were subjected to traditional vaccinal program for different viral diseases (Avian influenza, Newcastle, infectious bronchitis and infectious bursal disease) until the end of the experimental period.
The chicks were divided into 2 equal groups (each of 60 chicks). The first group (infected) was inoculated intra-peritoneally with 0.2 ml of log104 embryo infected dose (EID50) of locally field isolated ALV-J, (Lin et al., 2017), while the second group (normal control) was inoculated intra-peritoneally with 0.2 ml of PBS. In both groups, clinical signs and mortality rate were recorded for 5 months. At 1st, 2nd, 3rd, 4th, 5th month post-infection (mpi), blood and tissue samples were collected from 10 chickens from each group.
Hemogram, biochemical and serological analyses
At the 1st, 2nd, 3rd, 4th, and 5th mpi, one blood sample was collected from the wing vein of each bird and was divided into two portions. The first portion was collected into vacutainer tubes containing anticoagulant K3EDTA (ethylenediaminetetraacetic acid tri-potassium) and used for evaluation of hemogram. The second portion was placed into a plain vacutainer tube, left to clot then centrifuged at 3000 rpm for 15 min for separation of serum. Sera were stored at -20 °C until used for biochemical analyses and detection of the antibody specific for ALV-J.
Complete blood picture consisted of erythrogram [red blood cell counts (RBCs), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC)], and leukogram [total White blood cell counts (WBCs) and its differential counts (Heterophils, eosinophils, basophils, lymphocytes, and monocytes)]. Red and white blood cells counts were performed according to the method of Weiss and Wardrop (2010) using Natt and Herrick’s solution as a diluent (Natt and Herrick, 1953).
Serum biochemical assessment for alanine aminotransferase (ALT) and aspartate aminotransferase (AST), alkaline phosphatase (ALP), uric acid and creatinine were performed according to the manufacturers’ instructions using commercial Test kits (Biodiagnostic Co, Egypt) using the spectrophotometer (T80 UV/VIS PG instrument Ltd, UK).
Detection of the antibody specific for ALV-J in the serum of all chickens was performed by using IDEXX ALV-J Ab ELISA Test Kit (IDEXX Laboratories, Inc., USA). The interpretation according to the manufacturer’s recommendation: The positive sample O.D. more than or equal 0.60.
Histopathological examination
Tissue specimens were collected from the liver, spleen, heart, kidneys and bones for histopathological examination. These specimens were fixed in neutral buffered formalin 10%. Then the tissue specimens were washed, dehydrated, cleared and embedded in paraffin. Paraffin blocks were sectioned at 5 µm thickness and stained with hematoxylin and eosin (H and E), and Giemsa stain was used when needed (Suvarna et al., 2019).
Molecular diagnosis of ALV-J using polymerase chain reaction (PCR) in tissue
Tissue samples from the liver, spleen, and kidney were kept at -70ºC until used for detection of DNA of ALV-J using PCR. The extraction of DNA from tissue was carried out according to the method of Murray and Thompson (1980). Briefly, 500 µl cell lysis solution was added to 100 mg tissue into 2 ml micro-tube, and the mixture was homogenized then 2 µl of proteinase K solution (20 mg/ml) was added to the lysate and the suspension incubated at 55 °C overnight. DNA of ALV-J was detected in tissues of affected chickens, employing ALV-J specific primers. The sequences of oligonucleotide 2 primers were used in this study which selected from the published sequences (Smith et al., 1998); the first primer H5 was designed against the 3’ region of the pol gene and was conserved across ALV-J subgroup and the second primer H7 was designed from a well-conserved region of the gp85 sequence of the variant viruses and the product size in amplification with primer H5 is expected to give a 545. The primers H5 and H7 were derived from the HPRS-103 sequence (Genbank accession No. Z46390). (Table 1).
Table 1: Sequence of oligonucleotide primers, targets and expected PCR product sizes.
| Primer | Sequence (5’–3’) | Position | Product size with H5 (bp) | Amplification target |
| H5 | 5’-GATGAGGTGACTAAGAAAG-3’ | 5258–5277 | - | - |
| H7 | 5’-CGAACCAAAGGTAACACACG-3’ | 5783–5802 | 545 | Avian leucosis virus type J |
Statistical analysis
All data were subjected to statistical analysis including the calculation of the mean and standard error of the mean. Significance between data of groups was evaluated by student t-test at level P<0.05 (Petrie and Watson, 1999) using SPSS (Statistical Package for Social Sciences) version 20 Computer program (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical signs and mortality rate
Clinical signs demonstrated in the infected group beginning at the 3rd mpi with ruffled feathers and loss of appetite. On the 4th mpi, emaciation and depression were marked. At the end of the experiment, severe emaciation and mortalities (18.3%) among the experimentally infected chicks were recorded (Table 2), while no abnormal signs or mortalities were observed in the group control group during the experimental periods.
Table 2: Mortality rate of chicks experimentally infected with avian leucosis virus type J during the experimental period. (n=60).
| Groups | Periods (months) | Total | % | ||||
|
1st |
2nd |
3rd |
4th |
5th |
|||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infected | 0 | 0 | 1 | 3 | 7 | 11 | 18.3 |
Hematological findings
Macrocytic normochromic anemia was recorded from the 3rd mpi until the end of the experiment. This anemia was manifested by a significant decrease in RBCs, PCV% and Hb concentration associated with a marked increase in MCV (Table 3). Some abnormalities were observed in red cells of the infected group including anisocytosis, poikilocytosis, basophilic stippling, increased number of polymorphic erythroblasts and binucleated erythroblasts.
Significant leukocytosis was recorded from the 2nd mpi until the end of the experiment. The differential leukocytic count revealed significant heterophilia and lymphocytosis associated with a decrease in monocytes and eosinophils count (Table 3).
Serum biochemical findings
Serum ALT and AST activities and creatinine level significantly (P<0.01) increased from the 3rd mpi, and uric acid level markedly (P<0.01) increased from the 4th week until the end of the experiment. While the ALP activity significantly increased at the 5th mpi. (Table 4).
Antibody titer for avian leucosis virus type J (ALV-J)
The result revealed that ALV-J antibody titer was significantly (P<0.01) increased by the time from the 3rd mpi until the end of the experiment (Table 5).
Pathological findings
Macroscopic findings
No gross lesions could be observed in chickens of the normal control group all over the experimental period. However, in the infected group, some livers showed slight congestion at the 1st and the 2nd mpi. At the 3rd mpi, congestion of liver, spleen, and kidney were seen. Also, a small whitish nodule was observed on the border of the
Table 3: Hemogram of chicks experimentally infected with avian leucosis virus type J during the experimental period. (Mean±SE, N=10).
| Parameters | Groups | Periods (months) | ||||
|
1st |
2nd |
3rd |
4th |
5th |
||
|
Red blood cell Counts ( ×106 ∕µl) |
G1 | 2.66± 0.14 | 2.65± 0.09 | 2.74±0.09 | 2.76±0.09 | 2.87±0.12 |
| G2 | 2.40± 0.13 | 2.39± 0.13 | 2.05±0.08** | 1.95±0.09** | 1.69±0.10** | |
|
Packed cell volume ( %) |
G1 | 26.90±0.84 | 26.44±0.44 | 26.40±0.54 | 26.00±0.59 | 26.50±0.45 |
| G2 | 25.60±0.39 | 26.44±0.49 | 23.00±0.24** | 21.33±0.36** | 21.11±0.37** | |
|
Hemoglobin (g/dl) |
G1 | 10.25±0.37 | 9.95±0.12 | 10.47±0.17 | 10.62±0.16 | 10.96±0.28 |
| G2 | 10.22±0.21 | 10.10±0.19 | 8.51±0.28** | 8.14±0.29** | 7.93±0.23** | |
|
Mean corpuscular volume (fl) |
G1 | 103.19±5.67 | 100.73±4.55 | 97.09±2.89 | 95.47±4.97 | 93.87±4.73 |
| G2 | 108.91±5.22 | 113.85±7.45 | 113.73±4.84** | 111.48±5.40* | 129.98±9.87** | |
|
Mean corpuscular Hemoglobin )pg) |
G1 | 39.72±3.00 | 37.80±1.27 | 38.71±1.64 | 38.94±1.76 | 38.54±1.42 |
| G2 | 43.45±2.20 | 43.34±2.57 | 42.29±2.62 | 42.57±2.50 | 48.52±3.47* | |
|
MCHC )g/dl( |
G1 | 38.34±1.55 | 37.74±0.78 | 39.89±1.27 | 41.06±1.24 | 41.50±1.39 |
| G2 | 39.99±1.02 | 38.32±0.96 | 37.01±1.10 | 38.37±1.84 | 37.71±1.39 | |
|
White blood cell Counts (×103 ∕µl) |
G1 | 22.58±1.20 | 22.38±0.55 | 22.24±0.67 | 21.26±0.45 | 21.89±0.44 |
| G2 | 22.86±0.70 | 26.09±0.47** | 30.20±0.69** | 33.08±0.61** | 35.05±0.68** | |
|
Heterophils (×103 ∕µl) |
G1 | 26.90±0.84 | 26.44±0.44 | 26.40±0.54 | 26.00±0.59 | 26.50±0.45 |
| G2 | 25.60±0.39 | 26.44±0.49 | 23.00±0.24** | 21.33±0.36** | 21.11±0.37** | |
|
Lymphocytes (×103∕µl) |
G1 | 14.69±0.89 | 14.78±0.30 | 14.55±0.48 | 13.50±0.28 | 14.01±0.29 |
| G2 | 14.61±0.46 | 17.71±0.36** | 20.04±0.43** | 21.97±0.29** | 23.51±0.47** | |
|
Monocytes (×103∕µl) |
G1 | 1.15±0.04 | 1.30±0.12 | 1.21±0.06 | 1.42±0.06 | 1.14±0.06 |
| G2 | 1.41±0.09 | 0.71±0.16* | 0.64±0.18* | 0.50±0.06** | 0.45±0.06** | |
|
Eosinophils (×103∕µl) |
G1 | 0.41±0.04 | 0.29±0.03 | 0.44±0.05 | 0.45±0.05 | 0.52±0.03 |
| G2 | 0.41±0.05 | 0.26±0.05 | 0.21±0.08* | 0.19±0.07* | 0.18±0.06** | |
|
Basophils (×103 ∕µl) |
G1 | 0.16±0.04 | 0.11±0.03 | 0.22 ± 0.04 | 0.23 ± 0.01 | 0.20 ±0.04 |
| G2 | 0.18 ± 0.03 | 0.07 ±0.03 | 0.12 ±0.03 | 0.09 ±0.05 | 0.10±0.04 | |
G1: Control; G2: Infected; SE: Standard error; *: significant at P<0.05; **: Highly significant at P<0.01. MCHC: Mean corpuscular hemoglobin concentration.
Table 4: Serum biochemical changes of chicks experimentally infected with avian leucosis virus type J during the experimental period. (Mean±SE, N=10).
| Parameters | Groups | Periods (months) | ||||
|
1st |
2nd |
3rd |
4th |
5th |
||
| Alanine amino transferase (IU/L) | G1 | 24.00±0.18 | 23.00±0.64 | 26.00±0.88 | 26.00±0.45 | 30.00±0.94 |
| G2 | 25.00±0.73 | 25.00±0.73 | 36.00±0.89** | 50.00±0.95** | 50.00±2.55** | |
| Aspartate amino transferase (IU/L) | G1 | 134.70±1.32 | 149.70±2.86 | 151.20±2.13 | 156.9±1.26 | 171.00±2.10 |
| G2 | 146.80±1.96 | 146.60±1.63 | 160.40±2.02** | 171.90±1.58** | 173.70±1.61* | |
| Alkaline phosphatase (IU/L) | G1 | 166.30±1.80 | 169.60±2.77 | 172.00±1.09 | 177.50±1.70 | 174.30±1.65 |
| G2 | 167.9±2.77 | 172.5±1.88 | 169.7±1.95 | 176±1.22 | 203.2±3.30** | |
| Uric acid (mg/dl) | G1 | 4.26±0.13 | 4.02±0.18 | 4.01±0.20 | 4.21±0.15 | 4.21±0.19 |
| G2 | 4.06±0.19 | 4.12±0.19 | 4.35±0.22 | 5.76±0.10** | 5.97±0.09** | |
| Creatinine (mg/dl) | G1 | 0.95±0.01 | 0.97±0.01 | 1.01±0.02 | 0.97±0.01 | 1.07±0.05 |
| G2 | 0.96±0.02 | 0.98±0.01 | 1.45±0.10** | 2.01±0.05** | 2.62±0.10** | |
G1: Control; G2: Infected; SE: Standard error; *: significant at P<0.05; **: Highly significant at P<0.01.
liver (Figure 1A).
At 4th mpi, some livers and spleens showed diffuse enlargement (Figure 1B) and friable inconsistency. Also, thickening of proventricular wall was detected. At the 5th mpi, grayish-yellow nodules of variable size were observed on the surface of the spleen (Figure 1C), in addition, diffuse enlargement of the liver, spleen (Figure 1D), and kidney (Figure 1E) were seen. Heart showed cardiomegaly with slight hydro pericardium were the constant lesion seen in the heart at 3rd mpi, while whitish nodules appeared at the heart apex by the 5th mpi (Figure 1F).
Table 5: Results of antibody titer detection by ELISA of chicks experimentally infected with avian leucosis virus type J during the experimental period. (Mean±SE, N=10).
| Periods (month) | Groups | |
| Control | Infected | |
|
1st |
0.28 ± 0.03 | 0.52 ± 0.02 |
|
2nd |
0.35 ± 0.04 | 0.58 ± 0.05 |
|
3rd |
0.37 ± 0.04 | 0.69 ± 0.05** |
|
4th |
0.38 ± 0.04 | 1.01 ± 0.04** |
|
5th |
0.44 ± 0.02 | 1.52 ± 0.09** |
SE: Standard error; *: significant at P<0.05; **: Highly significant at P<0.01.
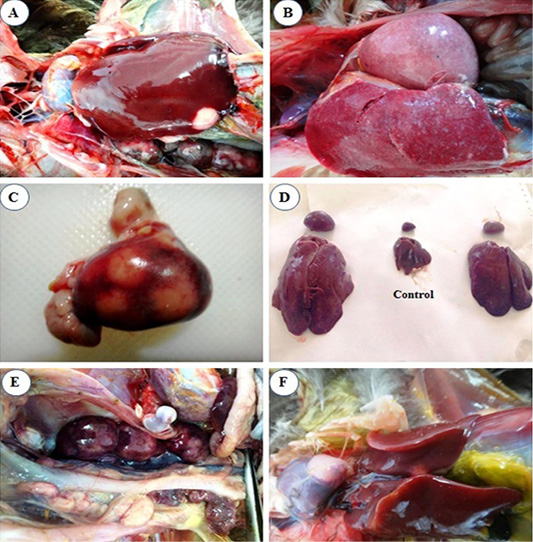
Figure 1: Macroscopic lesions from infected chicks with avian leucosis virus-J; (A) Liver at the 3rd month post infection (mpi), showing small white nodular growth on the border; (B) Liver and spleen at the 4th mpi, showing diffuse enlargement; (C) Spleen at the 5th mpi, showing severe enlargement with 3 nodular growths on surface; (D) Liver and spleen at the 5th mpi, showing severe diffuse enlargement (at left and right) and the control in the center; (E) Kidney at the 5th mpi, showing severe diffuse enlargement and (F) Heart at the 5th mpi, showing white nodular growth at the heart apex.
Microscopic findings
No microscopic alterations could be observed in the liver, spleen, heart, bone, and kidneys of the normal control group.
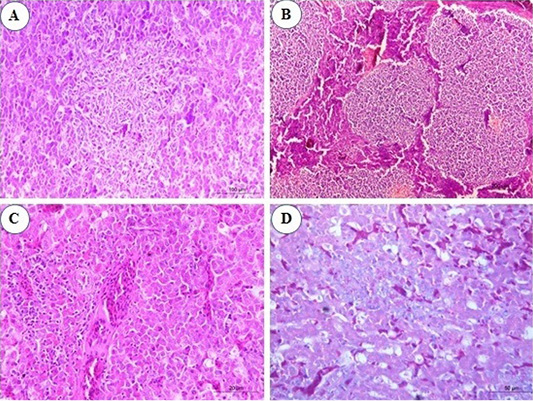
Figure 2: Photomicrograph from liver of chicken infected with avian leucosis virus type J during the experimental period; (A) At the 3rd month post infection (mpi), marked activation of Kupffer cells with aggregation of some reticular cell between hepatocytes (H and E, bar 100); (B) At the 4th mpi, expanding of neoplastic nodules, displacing and compressing surrounding parenchyma. The tumors are composed of pleomorphic lymphoid cells (H and E, bar 200); (C) At the 5th mpi, the hepatic parenchyma was compressed and replaced by immature myeloid cells (H and E, bar 50); (D) At the 4th mpi, focal aggregation of few myeloid cells with characteristic eosinophilic granules were noticed (Giemsa stain, bar 50).
In the infected group, microscopic examination of liver sections revealed that at the 1st and the 2nd mpi, there was congestion of hepatic sinusoids, central veins and portal BVs with multiple areas of diffuse hemorrhages replaced the hepatic parenchyma. Degenerative changes of hepatic cells and focal areas of hepatic necrosis associated with thickening of the hepatic capsule were also seen. At the 3rd mpi, marked activation of Kupffer cells with aggregation of some reticular cells between hepatocytes were detected (Figure 2A). Aggregations of granulocytic myeloid cells with characteristic red cytoplasmic granules (myeloblasts) around a terminal hepatic venule were noticed. At the 4th mpi, neoplastic cells proliferate, neoplastic nodules expand, compressing and displacing surrounding parenchyma rather than infiltrating between hepatocytes. Uniform population of large lymphoid cells (lymphoblast) (Figure 2B) show a slight variation in size detected in a focal manner. Few myeloid cells were observed between hepatic tissues when stained with Giemsa stain (Figure 2D). At the 5th mpi, neoplastic myeloid cells accumulate around blood vessels and extend into the hepatic parenchyma. The hepatic parenchyma was almost totally effaced and replaced by immature myeloid cells with characteristic cytoplasmic eosinophilic granules and round, non-lobulated nuclei (Figure 2C).
Congestion of splenic red pulp was the constant finding in the spleen by the end of the 2nd mpi. At the 3rd mpi, hyperplasia of ellipsoidal reticular cells in the subcapsular sinuses and around capillaries was seen (Figure 3A). At the 4th mpi, pleomorphic lymphoid population around arteriole which show mitotic activity are observed (Figure 3B). Periarteriolar lymphoid sheaths expansion by pleomorphic lymphoid cells was seen. Nodular collection of neoplastic lymphoid cells is separated from the adjacent parenchyma by a thin band of connective tissues were marked in the spleen. At the 5th mpi, the lesion advances, expanded periarteriolar lymphoid sheaths coalesce and form more diffuse sheet of lymphoid cells that replaces splenic tissue (Figure 3C). When the splenic tissues stained by Giemsa stain, myeloid cells was seen with their eosinophilic granules especially by the 3rd mpi (Figure 3D).
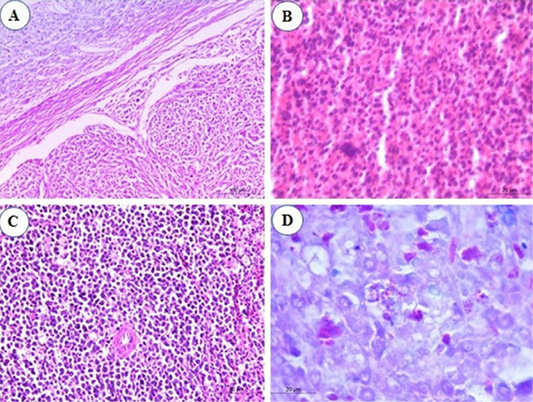
Figure 3: Photomicrograph from spleen of chicken infected with avian leucosis virus type J during the experimental period; (A) At the 3rd month post infection (mpi), marked hyperplasia of ellipsoidal reticular cells at subcapsular sinuses (H and E, bar 100); (B) At the 4th mpi, collection of lymphocytes showing pleomorphism and mitotic activity (H and E, bar 20); (C) At the 5th mpi, pleomorphic lymphoid cells in splenic tissue with mitotic figures (H and E, bar 50) and (D) At the 4th mpi, few myeloid cells with eosinophilic granules (Giemsa stain, bar 20).
Histopathological examination of kidney sections revealed congestion of inter-tubular blood vessels at the 1st and the 2nd mpi. Also, degenerative changes in the renal epithelium at the renal tubules were seen. At the 3rd mpi, hyaline degeneration of some renal tubules was noticed. Kidney tissue was infiltrated by a focal aggregation of neoplastic lymphocytes at periglomerular and inter-tubular areas (Figure 4A). At the 4th mpi, neoplastic myelocytes with characteristic eosinophilic cytoplasmic granules were infiltrating and replacing the renal parenchyma (Figure 4B). By the end of 5th mpi, diffuse granulated myeloid cells were seen infiltrate the renal parenchyma causing pressure atrophy and loss of renal tubules when stained with Giemsa stain (Figures 4C and 4D).
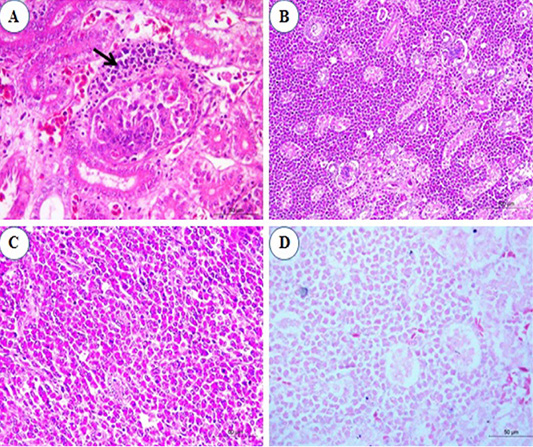
Figure 4: Photomicrograph from kidney of chicken infected with avian leucosis virus type J during the experimental period; (A) At the 3rd month post infection (mpi), periglomerular focal aggregation of pleomorphic lymphocytes (arrow) which showing mitotic activity (H and E, bar 50); (B) At the 4th mpi, heavy infiltration with myeloid cells that replace and compress the renal parenchyma (H and E, bar 100); (C) At the 5th mpi, diffuse infiltration of renal parenchyma by granulated myeloid cells. The renal tubules appear atrophied and completely loss of tubules (H and E, bar 50) and (D) At 5th mpi, diffuse infiltration of renal parenchyma by granulated myeloid cells (Geimsa stain, bar 50).
Examination of heart sections revealed at the 1st and the 2nd mpi, mild to severe congestion of the vasculature of myocardium and epicardium together with mild interstitial edema. By the 3rd mpi, focal lymphocytic aggregation between muscle bundles of myocardium was observed which showing pleomorphism (Figure 5A). Erythroid leucosis represented by severely dilated and engorged coronary artery packed with numerous erythroblasts were markedly seen and hemosiderin pigments could be observed (Figure 5B). At the 4th mpi, massive infiltration of myocardium with leucocytes which compress the myocardial fibers causing its atrophy, degeneration, and dissociation were seen. The lymphocytes show pleomorphism (Figure 5C). At the 5th mpi, massive infiltration of myocardium by myeloid cells with eosinophilic granules were detected when stained by Giemsa stain (Figure 5D).
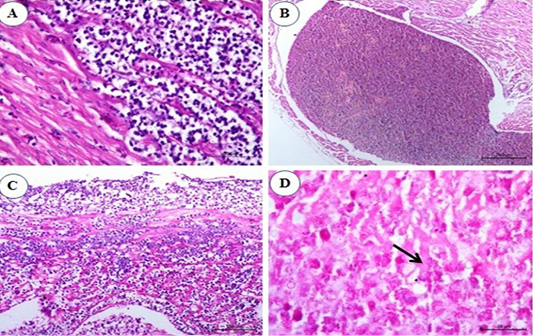
Figure 5: Photomicrograph from heart of chicken infected with avian leucosis virus type J during the experimental period; (A) At the 1st and 2nd month post infection (mpi), focal lymphocytic aggregation between muscle bundles of myocardium. The lymphocytes showing pleomorphism and mitosis (H and E bar 50); (B) At the 3rd mpi, severely dilated engorged coronary packed with numerous erythroblasts. Hemosiderin deposits could be observed (H and E bar 100); (C) At the 4th mpi, massive infiltration of myocardium with leucocytes which compress the myocardial fibers causing its atrophy, degeneration and dissociation. The lymphocytes show pleomorphism (H and E bar 100) and (D) At the 5th mpi, massive infiltration of myocardium by myeloid cells (arrow) with eosinophilic granules (Giemsa stain bar 20).
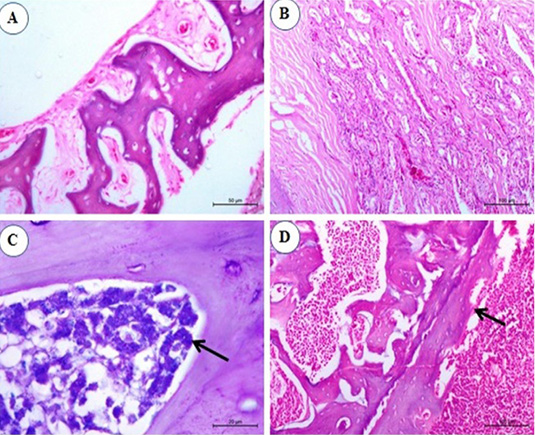
Figure 6: Photomicrograph from sternal bone of chicken infected with avian leucosis virus type J during the experimental period; (A) At the 1st and 2nd month post infection (mpi), normal sternal bone with normal periostium (H and E bar 50); (B) At the 3rd mpi, acinar structure with hyperplasia of cell wall with newly formed acini (H and E bar 100); (C) At the 4th mpi, increasing numbers of granulated myeloid cells (arrow) (Giemsa stain bar 20) and (D) At the 5th mpi, osteopetrosis with dentation in the periostium (arrow) (H and E bar 100).
No histopathological changes could be observed in the sternal bones of infected chicks at 1st and 2nd mpi (Figure 6A). By the 3rd mpi, the acinar structure with cellular hyperplasia and newly formed acini were observed (Figure 6B). At the 4th mpi, increasing numbers of granulated myeloid cells were markedly observed (Figure 6C). By the end of the experiment at the 5th mpi, osteopetrosis in which thickening of the wall and narrowing of the lumen of sternal bone together with dentation in the periosteum was also detected (Figure 6D).
Detection of ALV-J DNA in tissues
All examined samples from different periods of the infected group showed positive results (Lanes 1-5). No band product was detected for normal control group samples (Lane 6) and control negative DNA (Lane 7). The control positive gave positive result (Strong band, Lane 8). The bands were seen at 545 bp (Figure 7).
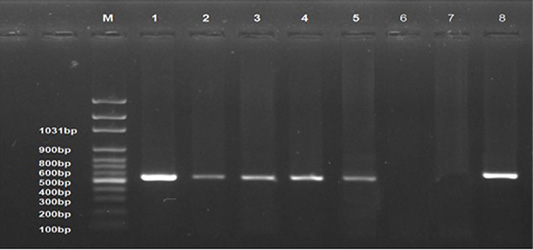
Figure 7: Ethidium bromide stained 1% agarose gel of PCR products showed avian leucosis virus type J (+ve) samples at different experimental periods, (lanes 1-5) of 545 bp PCR products, (-ve) normal control sample (lane 6), and (-ve) control (Lane 7) and (+ve) control (Lane 8). M represents a 100-bp ladder as a size standard.
DISCUSSION
This study was conducted to investigate the hematological, biochemical, and pathological alterations as well as DNA detection in tissues of one-day-old chicks experimentally infected with locally isolated ALV-J strain.
Since ALV-J was first isolated in 1988 in United Kingdom it has spread rapidly all over the world (Thapa et al., 2004) and causes economic losses in white meat type breeder farms. However, ALV-J spread to other many countries including Egypt. In Egypt, ALV-J was detected in native and foreign breeds layer chickens (El-Gohary et al., 2000).
ALV-J is a tumor disease. The tumor progress is a complicated process that is related to many factors as genetic background, immune competence, and viral infection factor. The process of promoting carcinogenesis is still unknown, but the integration of the ALV-J provirus may interfere with the function of host endogenous gene (Desfarges and Ciuffi, 2010). Tumor development is a multi-step process including abnormal expression of an apoptotic gene, inactivation of tumor suppressor genes or activation of proto-oncogenes.
Clinical signs demonstrated in the infected group began at the 3rd mpi with ruffled feathers and loss of appetite. These early signs indicated infection accompanied by a highly significant antibody titer. By 4th mpi, emaciation and depression were marked with gross lesions. At the end of the experiment, t severe emaciation and the total mortalities (18.3%) among the experimentally infected chicks. These findings agree with (Lin et al., 2013) who reported total mortality (26.4%) in chicks experimentally infected with ALV-J.
Data of erythrogram showed significant decreases in RBCs, PCV% and Hb concentration beginning at 3rd mpi in which macrocytic normochromic anemia was recorded. It may be attributed to the infection of these chickens with ALV-J. This virus has (v-myc) gene which plays a role similar to those of (v-erbB) gene of erythroblastosis viruses in blocking erythroid precursor cell differentiation resulting in anemia (Pattison et al., 2008). Also, suppression of the hematopoiesis as a result of marked proliferation of tumor cells in the bone marrow was recorded by Liu et al. (2019). The stained blood film of experimentally infected chicks revealed some erythrocytes abnormalities including anisocytosis, poikilocytosis, binucleated erythroblasts and increased number of polymorphic erythroblasts. These abnormalities may appear as a result of hypochromic anemia. These results were similar to those mentioned previously by El-Baky (2002) and Qian et al. (2018).
The mean values of leukogram revealed significant leukocytosis which was attributed to lymphocytosis and heterophilia. Fadly and Nair (2008) mentioned that hematopoietic neoplasms may appear to involve two cell lines and sometimes more, that can explain why myeloid leucosis caused a significant increase in lymphocytes. Immunosuppression caused by ALV-J makes infected chickens vulnerable to secondary bacterial infection leading to heterophilia. Wu et al. (2017) mentioned that the HPRS-103J virus shows tissue tropism that may relate to their ability to cause myeloid leucosis. In myeloid leucosis, the tumor cells were proliferated adjacent to the bone. The cell invaded the adjacent tissues and may be detected in the blood stream, which is the most characteristic feature in myeloid leucosis (Zhou et al., 2019). Lymphocytosis was found to be the cause of the higher count of leukocytes. Lymphocytosis can occur with antigenic stimulation and with lymphocytic leukemia (Thrall et al., 2004). In some cases of lymphocytic leukemia, immature lymphocytes were detected in the blood film. It has been found that a marked lymphocytosis in which most lymphocytes appear as small, mature lymphocytes with scalloped cytoplasmic margins is associated with lymphoid neoplasia (Murakami and Sassa, 2018).
Significant increases in serum enzymes (AST and ALT) activities from the 3rd mpi till the end of the experimental period in the infected group were recorded. These increases could be suggestive of hepatic affection as AST and ALT are good indicators of hepatocellular damage (Thrall et al., 2004). This was proved by the histopathological changes of liver which indicate the presence of necrosis, and degeneration of hepatocytes. However, in infected chickens, serum ALP activity markedly elevated at 5th mpi due to osteopetrosis formation where thickening of the wall and narrowing of the lumen of sternal bone were observed. Similar result was recorded by Banes and Smith (1977).
The present work showed a significant increase in serum uric acid and creatinine levels that may be attributed to kidney damage caused by infection. This result agrees partially with the results of El-Baky (2002) who reported a significant increase in serum uric acid and creatinine in experimentally infected chicks with ALV-J. The level of creatinine and uric acid is known to reflect the state of glomerular filtration rate and kidney functions (Wang et al., 2018).
ELISA is a good accurate method for detecting antibodies in infected birds. Analysis of our results revealed a significant increase in antibody titer from the 3rd mpi till the end of the experiment. The gradual increases of the antibody titer indicated the active infection of the ALV-J. These results were similar to those recorded by Sani et al. (2012) and Hou et al. (2016).
Regarding post-mortem examinations among the infected group, no gross lesions were observed at the 1st and 2nd mpi. However, small whitish nodules in the liver were seen at the 3rd mpi. These results agree with Zhou et al. (2019) who observed diffuse enlargement of visceral organs especially liver and spleen due to heavy infiltration of myeloid cells giving the liver mottled appearance at the 4th mpi. Also, a great thickening of proventriculus and gizzard was observed. The aforementioned lesions were coincided to those described by Meng et al. (2016). At the 5th mpi, diffuse enlargement of the most visceral organs with nodules of different sizes on the surface was seen. This result was partially similar to that described by Meng et al. (2018) who detected similar lesions at 20 weeks post-infection.
ALV-J is best known for its induction of myeloid neoplasia, also it has a high cardiac tropism. The heart begins one of the most important organs involved in the infection. However, transformation of host cells in avian lymphoid leucosis by ALV-A because multiple rounds of infection must occur in infected birds before a provirus inserts itself in the vicinity of a cellular proto-oncogene with appropriate activation (Nakamura et al., 2014). In our study, cardiac lesions begin early at 3rd mpi by cardiomegaly with clear, light yellow fluid. Histopathologically, the myocardium and epicardium were mild to severe congested with interstitial edema. These results were similar to that by Stedman and Brown (2002). ALV infection recorded to cause dilated cardiomyopathy (DCM). The ALV-J associated DCM is more likely due to a direct viral effect on the myocardium than to an immune-mediated response. ALV-J predominantly induces myeloid leucosis because of its tropism to the cells of the myeloid rather than lymphoid. A case of erythroblastosis was recorded in the present study in which massive erythroblasts in the coronary artery of the heart. These results agree with Wang et al. (2013) that reported the incidence of erythroblastosis in birds inoculated with the virus after hatching. At the 4th and 5th mpi, infiltration of the myocardial fiber by eosinophilic myeloid cells which confirmed by Giemsa stain were seen.
ALV-J has a tropism for chicken bone marrow cells and induces their neoplastic transformation. Myeloid neoplasia arises from primordial hematopoietic cells that originate myeloid lineage cells (erythrocytes, granulated leukocytes, monocytes, and thrombocytes). The common aspect in the heterogeneous group of myeloid tumors is the origin of a progenitor cell that normally produces terminal differentiation cells of the myeloid series (Aster and Kumar, 1999). In our study, massive increase of bone marrow of sternal bone with myeloid cells at the 4th mpi and at the end of experiment, osteopetrosis with dentation of periosteum were seen. The periosteum is greatly thickened from an increase in number and size of basophilic osteoblasts. An increase occurs in size and irregularity of the Haversian canals, and there is an increase in the number, size and an alteration in the position of lacunae. These results partially agree with El-Gohary et al. (2000) and Liu et al. (2019) who described similar bone lesions in naturally infected chickens with ALV-J at the 27th week old.
Microscopically, in infected group with ALV-J, slight degenerative changes, necrosis and edema were detected at the 1st and the 2nd mpi. These changes could be attributed to viremia. At the 3rd mpi, marked activation of Kupffer cells with aggregation of some reticular cell between hepatocytes were seen. Also, aggregations of granulocytic myeloid cells with characteristic red cytoplasmic granules (myelocytes) were noticed. This result was coincided by Li et al. (2018). The morphology of the cellular type involved in myeloid leucosis was compatible with that of the myelocytes; the cytoplasm filled with eosinophilic granules and voluminous nuclei of different shapes with evident nucleoli. Such features were also observed by Witter and Fadly (2001). At the 4th and 5th mpi, neoplastic myelocytes with characteristic eosinophilic cytoplasmic granules were infiltrating and effacing the renal, hepatic and splenic parenchyma. Complete loss of tubules and pressure atrophy were observed. Similar result was typically founded by Ma et al. (2017).
Analysis of PCR assay with ALV-J specific primers revealed positive reaction with DNA obtained from liver, spleen and kidney indicating the infection with ALV-J. Early positive results were recorded at 1st mpi. It may be attributed to the viremia that persists along the experimental period (Smith et al., 1998). PCR assay is easily performed and rapid accurate results obtained within one day. So, this assay can be used for the tentative diagnosis of ALV-J. The histopathological picture besides the molecular identification gave accurate diagnosis for the disease (Wang et al., 2018).
CONCLUSION
Experimental infection with ALV-J in chickens induced macrocytic normochromic anemia, leukocytosis, heterophilia, lymphocytosis, and disturbance in the liver and kidney functions. ALV-J antibody titer was increased from the 3rd mpi till the end of the experiment. The histopathological changes began from the 1st mpi which aids in early diagnosis. All examined internal organs showed different lesions specially heart (lymphoid, myeloid and erythroid leucosis), and lymphoid and myeloid infiltration of liver, spleen, and kidneys, in addition to characteristic bone lesion as osteopetrosis. ALV-J DNA in tissues was detected from 1st mpi.
AUTHORS CONTRIBUTION
All authors shared equally in designing, conducting the study and writing the manuscript.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
REFERENCES






