Advances in Animal and Veterinary Sciences
Research Article
Study of the Biochemical Parameters of Pre-Slaughter Stress Response in Bovine Species in Algeria
Sabrina Salhi1, Farida Bouzebda1, Zoubir Bouzebda1, Amel Djaout1,2 , Houria Ouennes1*
1Laboratory of Animal Productions, Biotechnologies and Health (PABIOS), Institute of Agronomic and Veterinary Sciences. Mohammed Cherif Messaadia University, Souk Ahras. 41,000. Algeria; 2National Institute for Agricultural Research of Algeria (INRAA). Setif 19,000, Algeria.
Abstract | There is no doubt that the well-being of cattle, as for other beef species, is a consumer concern. The pre-slaughter period includes a series of potentially stressful interventions to which animals are subject can significantly affect the quality of meat. This is closely related to the disturbances of the physiological state of the animal and its possible reactions to stress. The present study was involved in 38 beef cattle of different ages (<2 years n=19 and >2 years n=19), sexes (male n=20 and female n=18), and breeds (Prim’Holstein n=19, autochthone cattle n=19).The evaluation of stress response of cattle was carried out during their landing in slaughterhouse, before and at the time of slaughter by exploration do some blood biochemical indices (cortisol, testosterone and ACTH) and urinary parameters (cortisol, catecholamines and glucose). The results showed significant breed effect on cortisolemia with a high levels in autochthone cattle compared to Prim’Holstein particularly 20mn before the slaughter (26.8 ng / ml vs 8.6 ng / ml, p <0.05). Similarly, females had high but no significant cortisolemia compared to males before and at bloodletting(21.75 ng / ml vs 18.67 ng / ml at 12h ante mortem, 18.75 ng/ml vs 17 ng/ml at 20 mn before slaughter and 29.25 ng / ml vs. 21.33 ng / ml at bloodletting, P>0.05). Older animals were more reactive to slaughter stress at 20 mn ante mortem and bloodletting (Cortisolemia:21 ng/ml and 29.8 ng/ml) than young, ones who showed more cortisolemia 12h ante mortem (21.2 ng / ml) and also significant difference of testosterone levels (p <0.01) .The urinary parameters did not showed any significant changes during the slaughter preparation stages and bleeding (P>0.05) and they were not significantly influenced by animals characteristics (age, breed and sexe; P>0.05).
Keywords | Blood indices, Bovine species, Slaughter, Stress, Urinary parameters.
Received | June 25, 2019; Accepted | August 19, 2019; Published | December 22, 2019
*Correspondence | Houria Ouennes, Laboratory of Animal Productions, Biotechnologies and Health (PABIOS), Institute of Agronomic and Veterinary Sciences. Mohammed Cherif Messaadia University, Souk Ahras. 41,000. Algeria; Email: webhouria36@hotmail.fr
Citation | Salhi S, Bouzebda F, Bouzebda Z, Djaout A, Ouennes H (2019). Study of the biochemical parameters of pre-slaughter stress response in bovine species in algeria. Adv. Anim. Vet. Sci. 8(1): 46-53.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.1.46.53
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Salhi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The implication of stress before slaughter on major quality defects in meat is well known (Terlouw et al., 2015). Available data show that stress can explain a significant portion of changes in technological and sensory quality of the meat of all the animal species. The current state of data on the subject led to the establishment of a repository (AHW)”Animal Health and Welfare”to select the priority actions to be implemented at three stages of farming, transport and slaughter (Marhin and Marion, 2018). Current studies aim to understand the physiological and molecular mechanisms underlying that seem to vary by species and even according to the animal’s stress disorder at the time of slaughter (Terlouw et al., 2015). Three types of answers are possible: a behavioral response, autonomous nerve response is orchestrated by the central nervous system and structures of the autonomic nervous system and neuroendocrine response.
The main endocrine axes modified by stress are the corticotropic, gonadotropic and thyroid axes as well as the secretions of growth hormone and prolactin (Brisville, 2006).Many studies have examined the stress responses of cattle during the stages of slaughter. However, some procedures are rarely discussed and the assessment of the stress state of cattle is usually based on their physiological responses only (Terlouw et al., 2015).
Therefore, we try by the present study to answer the following question: How does the Algerian local cattle react to the conditions of local slaughter compared to the improved breed and imported the Prim‘Holstein? How would be the pattern of change of this response to stress according before slaughter and at the time of bleeding and according to cattle age and sex?
MATERIALS AND METHODS
Study Area and Slaughterhouse Location
The study was conducted in the slaughterhouse, located on the coast of Skikda department (36 ° 52 ‘00 “North and 6 ° 54’ 00” East) at 345 km east of the capital (Algiers). The national statistics of animal production indicated that this department is ranked 16th in relation to the production of red meat (8142.17 K) and 4th in relation to the production of beef cattle (MADR, 2012).
Animals
Thirty eight cattle were used for this study. They weredivided in tow sex classes (male n= 20 and female n= 18), tow age groups (<2 years n=19 and >2 years n=19) and tow ethnologic categories (Prim ‘Holstein breed -Batch I- and autochtone cattle -Batch II-) (Table 1). The Prim ‘Holstein animals come from high intensive livestock system and the local cattle were exclusively reared under extensive and pastoral system. All animals were forwarded to slaughter without stunning.
Tables 1: Description of the studied animals
| Race | Male | Female | Total |
| Prim’ Holstein / Batch I | 10 | 9 |
19 |
| Autochtone cattle / Batch II | 10 | 9 | 19 |
| Total | 20 | 18 |
38 |
Samples Design
The blood samples were taken three times per animal over the slaughter day. Firstly, at 12 hours ante mortem (12h AM) (in landing after a transport truck and undeveloped for 30 min), 20 minutes ante-mortem (resting 20 min AM), and at the time of slathering. All blood samples were taken at the jugular vein into plain vacuo.
Serum washarvested and frozen at -4°C. The serum tubes was rigorously labeled with indication sampling times, transported in a cooler to the laboratory for assay of cortisol, ACTH and Testosterone (Brisville, 2006).
In other hand, the urine was collected using a disposal syringe (Brisville, 2006), for cortisol assay, VMA (metabolites degradation of adrenaline and norepinephrine) and urinary glucose (Table 2) which would reflecting these levels in the blood before slaughter (Mormède et al., 2004).
Table 2: Studied variables in ante-mortem (AM) and bloodletting (Sg)
| Variables | Abbreviations | Time |
| Blood Cortisol | Cor. sg (ng/ml) | 12h AM, 20 mn AM and Sg |
| Adreno CorticoTropin Hormone | ACTH ((pg/ml ) | 12h AM, 20 mn AM and Sg |
| Testostérone | Testos (ng/ml) | 12h AM, 20 mn AM and Sg |
| Urinary Cortisol | Cor. ur (ng/ml) | Sg |
| Acidevanylmandelique | VMA (mg/l) | Sg |
| urinary glucose | Glu (g/l) | Sg |
AM : ante mortem, Sg : slathering
Laboratory Analyzes
The frozen were analyzed after thawing in water bath and identification of the tubes containing serum. Plasma cortisol was assayed by a method called AxSYM Cortisol, which is a fluorescence polarization immunoassay (FPIA). ACTH was assayed using a so-called “sandwich” method with an analytical cycle duration of 18 minutes. The assay principle of testosterone is immunoenzymatic by a final fluorescence detection. All the steps are performed automatically by an automaton “Mini Vidas Bio Mérieux”. At the end of the test which lasts 40 minutes, the reading is performed automatically by the device with respect to a stored calibration curve, then printed.The urinary cortisol assay of each thawed sample in water bath should be thoroughly homogenized prior to analysis. Urine specimens that are cloudy or contain suspended particles should be centrifuged before the assay (at a recommended rate of 8000 to 10000 xg for 10 min). The assay principle follows the same method used for the determination of blood cortisol (AxSYM Cortisol).For the determination of vanylmandelic acid (VMA) in the samples is retained by an interchange resin. Once the interfering substances are removed by washing, the VMA is quantified by spectrophotometry. Glycosuria was made by the reflectometer.
Statistical Analysis
The statistical analysis were performed under SPSS version 19. Descriptive statisticsnamely; Mean, Minimum, Maximum, Error stdand variance of the different serum and urine parameters were planned. The statistical difference in plasma concentrations of the different blood variables between the three study stages (12H before slaughter, 20 min ante-mortem and bloodletting) was studied using the single-factor ANOVA Test. The effect of the breed (Prim’ Holstein and autochthone cattle) gender (male and female) and age (young and adult) were compared using multiple comparison test Student Newman-Keuls (SNK).
Table 3: Overall results of blood biochemical indices in ante-mortem (AM) and slathering (Sg) cattle
| Moyenne | Minimum | Maximum | Erreur std | Ecart type | Variance | p | |
| Cor 12h AM | 19,90 | 6,00 | 34,00 | 3,18 | 10,05 | 100,99 | ns |
| Cor 20 min AM | 17,70 | 1,00 | 42,00 | 4,42 | 13,99 | 195,79 | |
| Cor 5 mn PM | 24,50 | 4,00 | 46,00 | 4,95 | 15,65 | 244,94 | |
| Testos 12h AM | 4,52 | 0,02 | 17,50 | 1,80 | 5,71 | 32,57 |
ns |
| Testos 20 min AM | 1,97 | 0,04 | 6,20 | 0,66 | 2,09 | 4,37 | |
| Testos 5 mn PM | 1,77 | 0,01 | 5,83 | 0,59 | 1,85 | 3,42 | |
| ACTH 12h AM | < 1 | - | - | - | - | - | - |
| ACTH 20 min AM | < 1 | - | - | - | - | - | - |
| ACTH 5 mn PM | < 1 | - | - | - | - | - |
- |
AM : ante mortem, Sg : slathering, ns: no significatif (P >0.05),Cor :cortisol, Testos :Testosterone
Table 4: Comparative blood biochemical indices in ante-mortem (AM) and bloodletting (Sg) cattle according to the breed
| Lot I | Lot II | p | |
| N | 19 | 19 | |
| Cor 12h AM | 15,6±6,84 | 24,2±11,6 | ns |
| Cor 20 min AM | 8,6±7,8 | 26,8±13,1 | * |
| Cor Sg | 23,8±14,3 | 25,2±18,6 | ns |
| Testos 12h AM | 4,16±4,55 | 4,88±7,23 |
ns |
| Testos 20 min AM | 2,69±2,65 | 1,24±1,23 | ns |
| Testos Sg | 2,31±2,42 | 1,22±1,04 | ns |
| ACTH 12h AM | < 1 | < 1 | - |
| ACTH 20 min AM | < 1 | < 1 | - |
| ACTH Sg | < 1 | < 1 | - |
AM : ante mortem, Sg : slathering, h: hours , mn: minute, ns: no significatif (P >0.05), *(P<0.01),Cor : cortisol, Testos :Testosterone
Table 5: Comparative blood biochemical indices in ante-mortem (AM) and slathering (Sg) cattle according to the sex
| Male | Female | Value p | |
| N | 20 | 18 | |
| Cor12h AM | 18.7±8.14 | 21.7±13.6 | ns |
| Cor 20 min AM | 17±14.3 | 18.7±15.6 | ns |
| Cor Sg | 21.3±12.9 | 29.3±20.2 | ns |
| Testos 12h AM | 7.5±5.72 | 0.12±0.12 | * |
| Testos 20 min AM | 3.18±1.85 | 0.14±0.14 | * |
| Testos Sg | 2.86±1.61 | 0.13±0.18 | * |
| ACTH 12h AM | < 1 | < 1 | - |
| ACTH 20 min AM | < 1 | < 1 | - |
| ACTH Sg | < 1 | < 1 |
- |
AM: ante mortem, Sg: slathering, h: hours , mn: minute, ns: no significatif (P > 0.05), *(P<0.05), Cor: cortisol, Testos :Testosterone
RÉSULTS
The results of blood cortisol, ACTH and blood testoster
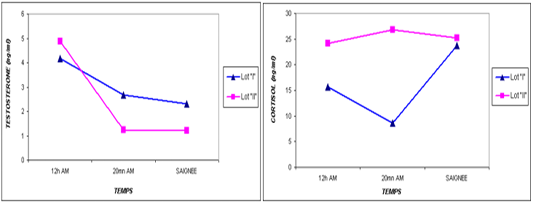
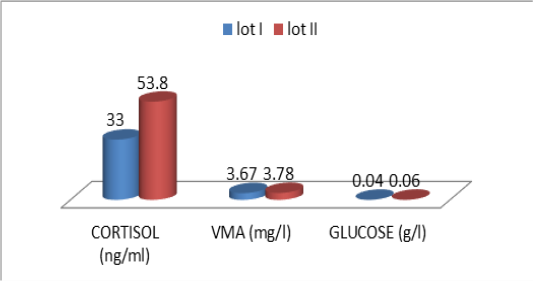
Figure 1: Comparative blood biochemical indices in ante-mortem (AM) and slathering (Sg) cattle according to the breed
one are shown in Table 3. For the overall results, it appears that the plasma levels of these parameters were not significantly changed during the three stages of the stress analysis of the slaughtered animals (P >0.05). However, significant difference (P<0.05) of stress response between the two studied breeds had resulted in a pronounced cortisol secretion in autochthone cattle at 20 minutes AM (Figure 1, Table 4). Similarly, male and young cattle had significantly (P<0.01) higher scores of testosterone during all stages of sampling (Figures 2, 3 and Tables 5, 6).
Table 6: Comparative blood biochemical indices in ante-mortem (AM) and bloodletting (Sg) cattle according to the age
| Young (<2 years) | Adult (>2 years) | p | |
| N | 19 | 19 | |
| Cor12h AM | 21.2±5.89 | 18.6±13.7 | ns |
| Cor 20 min AM | 14±13.7 | 21.4±14.8 | ns |
| Cor Sg | 19.2±13.22 | 29.8±17.5 | ns |
| Testos 12h AM | 8.4±5.86 | 0.64±1.18 | * |
| Testos 20 min AM | 3.63±1.67 | 0.31±0.39 | ** |
| Testos Sg | 3.18±1.56 | 0.35±0.52 | ** |
| ACTH 12h AM | < 1 | < 1 | - |
| ACTH 20 min AM | < 1 | < 1 | - |
| ACTH Sg | < 1 | < 1 | - |
AM : ante mortem, Sg : slathering, h: hours , mn: minute, ns : no significatif (P > 0.05), *(P<0.05), **(P<0.01), Cor :cortisol, Testos :Testosterone
Table 7: Overall results of urinary parameters of the studied cattle
| Average | Minimum | Maximum | Error std | Standard deviation | Variance | |
| Cor | 36.80 | 0.00 | 118.00 | 12.35 | 39.04 | 1524.18 |
| VMA | 3.73 | 0.91 | 9.56 | 0.86 | 2.73 | 7.47 |
| Glu | 0.05 | 0.02 | 0.23 | 0.02 | 0.06 | 0.00 |
Cor :cortisol, VMA : vanylmandelic acid,Glu :Glucose
Table 8: Comparative urinary parameters according to the breed
| Lot I | Lot II | P | |
| N | 19 | 19 | |
| Cor | 19.8±19.8 | 53.8±48.1 | ns |
| VMA | 3.67±3.54 | 3.78±2.06 | ns |
| Glu | 0.04±0.02 | 0.06±0.09 |
ns |
ns: no significatif (P > 0.05) Cor :cortisol, VMA : vanylmandelic acid,Glu :Glucose
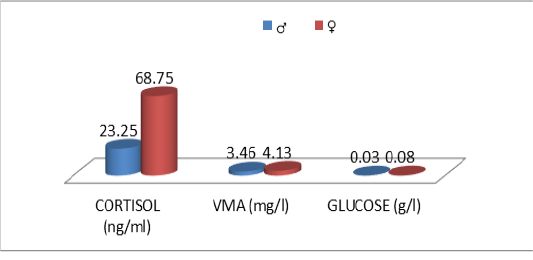
Table 9: Comparative urinary parameters according to the sex
| Male | Female | p | |
| N | 20 | 19 | |
| Cor | 15.5±14.5 | 68.8±44.2 | ns |
| VMA | 3.46±3.17 | 4.13±2.29 | ns |
| Glu | 0.03±0.02 | 0.08±0.10 | ns |
ns: no significatif (P > 0.05), Cor :cortisol, VMA : vanylmandelic acid,Glu :Glucose
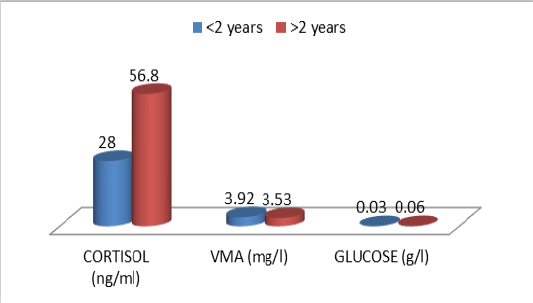
Table 10: Comparative urinary parameters according to the age
| Young(<2ans) | Adulte (>2 ans) | p | |
| N | 19 | 19 | |
| Cor | 16,8±15,8 | 56,8±46,7 |
ns ns ns |
| VMA | 3,92±3,31 | 3,53±2,40 | |
| Glu | 0,03±0,02 | 0,06±0,09 |
ns: no significatif (P > 0.05), Cor :cortisol, VMA : vanylmandelic acid,Glu :Glucose
The descriptive statistics of the urinary parameters, namely, cortisol, VMA and urinary glucose are shown in Table 7. The statistical analysis did not show any significant difference (P >0.05) of these parameters according to the breed (Table 8), sex (Table 9), and age (Table 10), (Figures 4, 5 and 6).
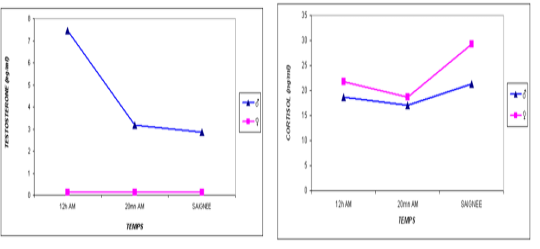
Figure 2: Comparative blood biochemical indices in ante-mortem (AM) and slathering (Sg) cattle according to the sex
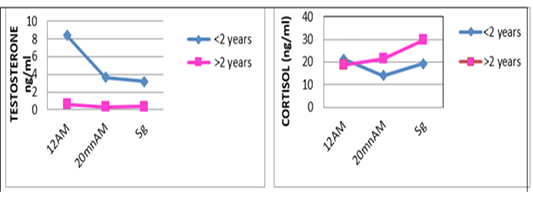
Figure 3: Comparative blood biochemical indices in ante-mortem (AM) and slathering (Sg) cattle according to the age
DISCUSSION
The results are discussed according to the order presented in the results section. According to the analysis of the results of blood parameters, the most stress studies using plasma cortisol and testosterone levels as markers (Cooper and al., 1995), their level expresses an increase during stress (Terlouw and al., 2012; Terlouw and al., 2007; Brisville, 2006; Terlouw, 2002).
Our results (Table 3) showed plasma cortisol levels of 19.90 and 17.70 ng / ml at the two doses of ante mortem, 12h (after unloading) and 20mn before slaughter, whereas this concentration did not increase significantly at time of bleeding (24.5 ng / ml, P >0.05).
The transportation and slaughter of animals is associated with a multitude of potentially stressful factors (Terlouw and al., 2012). These factors are physical, such as lack of food, restriction of water intake, truck movements, shocks caused by loss of balance, blows received from other animals, or manipulator, and of psychological origin, because any change in the usual situation is likely to cause fear in the animal (Terlouw and al., 2012; Mounier et al., 2007). The departure from the usual environment, the change of the social environment, the introduction into unfamiliar environments and the presence of unfamiliar people are examples (Terlouw and al., 2012). More; in a slaughterhouse that does not meet standards and lacks a waiting room, the situation is further aggravated. Table 3 reports decreasing average plasma testosterone levels, from 4.52 ng / ml to 1.79 ng / ml then to 1.77 ng / ml (at 12h AM, at 20mn AM and at the time bleeding respectively). The results are in accordance with those obtained by Agoud, (2016); Terlouw et al. (2015); Grigor et al. (2004); Van de Water et al. (2003); Lensik et al. (2001a) and Sartorelli et al. (1992). Indeed, these studies have demonstrated the effect of transport and duration of ante mortem on the elevation of cortisolemia in cattle. Similarly, in poultry, the corticosterone level increases with the duration of transport Debut and al. (2005); Mitchell et al. (1992). More; Individuals in the autochtone cattle (Table 4, Figure 1) show higher mean cortisol levels than those measured in the imported breed during the three sampling times; in particular at 20mn (26.8 vs 8.6 ng / ml respectively), which difference is significant (p <0.05) .However, the testosterone level is lower in the last two samples in the autochtone cattle (batch II), including 1.24 ng / ml against 2.69 ng / ml (batch I) at 20 nm and 1.22 ng / ml. ml against 2.31 ng / ml (lot I) , which difference is not significant (P >0.05) (Table 4). The effect of race on stress reactions also exists in the bovine species (Terlouw et al., 2015; Terlouw and al., 2012; Terlouw and al., 2007; Terlouw, 2002): Salers heifers, for example, have more pronounced behavioral responses to isolation than Frisians (Le Neindre, 2003).
In the Limousin breed it has been found that the ease of handling of heifers depends partly on that of the father with a heritability of 20% (Le Neindre et al.,1995). At the time of slaughter, pronounced reactions to separation from congeners and manipulation by humans could, of course, have negative consequences on meat qualities (Bourguet, 2010; Terlouw, 2002). Also, mean cortisol levels in females (Table 5) and older individuals (Table 6) are more pronounced than those observed in males and juveniles in all stages studied. In cattle, several studies have shown that the mode of rearing and the type and frequency of contact with the breeder have a much greater effect than genetics on the ease with which animals can be manipulated (Le Neindre et al., 1995; Boivin and Braastad, 1996; Boissy and al., 2007).Our results; according to the various characteristics of the animals; are in agreement with those obtained by Agoud (2016). The conditions of evolution of the muscular metabolism at the time of slaughter are largely influenced by the physiological state of the animal and in particular of its possible reactions to stress (Terlouw et al., 2015; Strappini et al., 2009). The latter are a function of the genetic type (Terlouw, 2002), the situation previously experienced (Deiss et al 2007), and the individual (Bourguet, 2010). It is important to note that the stress state of the animal depends not on the situation, but on its assessment of the situation (Bourguet, 2010; Terlouw, 2002), each individual is uniquely shaped by their genetic heritage and past experience, so an animal’s state of stress is an individual and subjective experience of how it responds to different life situations. Stress is called stress reactivity, so stress reactivity is a characteristic of the animal (Terlouw, 2002). The ACTH assay recorded nil values (Table 3); this may be related to the early onset of the ACTH response that occurs earlier than that of the cortisol response (Agoud, 2016; Veissier et al., 1999). In addition, variations in ACTH require blood tests at very short intervals to be interpretable, since the half-life of this parameter is short. It may be preferable to use a catheter to avoid bias during blood collection (Hay and al., 2000). Our assay was performed on a single sample which makes the obtained results uninterpretable, in fact, in the face of moderate stimulations, plasma variations of ACTH are very discrete or even undetectable (Mormede et al., 2004).
From the standpoint to urinary parameters, the urinary cortisol is estimated at 36.80 ng / ml and the standard deviation (± 39.04) reflects high variability among cattle. Our results are different from those of Brisville, (2006), who observed very low urinary glucocorticoid levels in cattle. The comparison with the average urinary cortisol level is twice the plasma level (Table 3), measured 20 minutes before slaughter (17.70 ng / ml). In swine, an equivalence between these two rates is reported (Mormede et al., 2004). The average level of AMV is in the range of 3.73 ± 2.73 mg / l (Table 7). Variations in plasma catecholamine levels are very sensitive markers correlated with stressor intensity, but are tricky to use and of limited interest in cattle (Brisville, 2006). The autonomic nervous system influences the functioning of all organs, including muscles, through the action of adrenaline, norepinephrine and acetylcholine (Terlouw et al., 2015). In fact, their release into the bloodstream is rapid and fleeting, and for this reason, specific conditions such as in dwelling catheters make it possible to measure plasma catecholamine levels (Hay et al., 2000); all these reasons led us to test the common derivative of adrenaline and norepinephrine degradation, which is vanylmandelic acid (VMA).
The average level of glucose in the urine is estimated at 0.05 g / l (Table 7); however, no difference is significant, depending on race (Table 8), sex (Table 9), and age (Table 10). Glycosuria is normally negative because there is no glucose in the urine. A positive glycosuria is in favor of a diagnosis of diabetes. Indeed, glucose appears in the urine when its blood level exceeds a certain threshold (Brisville, 2006). Glucocorticoids mainly cortisol in cattle; the first important action is the action gluconeogenesis (Brisville, 2006). It increases the production of glucose from non-carbohydrate substrates, and in particular from proteins. This action is beneficial in the short term, but it is expensive in the long term, since this energy intake is at the expense of structural proteins (Mormede et al., 2004) and from glycerol and free fatty acids circulating in the blood. This action of gluconeogenesis takes place in the liver (Tritschler, 2006). It is associated with the reduction of membrane transport of glucose (anti-insulin action), which causes hyperglycemia, potentiated by catecholamines and glucagon (Agoud, 2016; Mormede et al., 2004).
According to Tables 8, 9 and 10, it was deduced that the urinary parameters studied; are not significantly influenced by cattle characteristics; race (Figure 4), sex (Figure 5) and age (Figure 6). However, an individual variability of average rates is recorded. In interaction with the genetic component, individual experiences also play a role in how animals react to a given situation. For example, several studies have shown that calves exposed to positive contact with humans are subsequently less reactive to handling (Boissy et al., 2007; Lensink et al., 2001b; Boivin and Braastad, 1996). Thus, the perception and evaluation of a given situation, as well as the resulting reactions, are the result of genetic heritage and individual experiences (Terlouw et al., 2015). These two components, added to the emotional and physiological state of the animal at a given moment, result in a unique combination explaining at least partly the interindividual variability in the reactions (Terlouw et al., 2015; Terlouw et al., 2012; Bourguet et al., 2010; Bourguet et al., 2008).
CONCLUSION
This study highlights the complexity of the conditions of slaughter bovine species by the multitude and variety of intervening stressors. Therefore, the features in conjunction with the animal, such as race, sex and age and their effect on the stress response were explored. The autochtone cattle respond significantly more than Prim’Holstein to slaughter stress especially some minutes before the slaughter. However, the means of urinary parameters had not showed significant differences according to the sex, breed and age. It was suggested that heterogeneous breeds, ages and sex of beef cattle existing in slaughterhouses is a figure of the lacked organization of the beef industry. Therefore, in order to reduce the stress level of cattle during the slaughter period, it is possible to act by grouping homogeneous beef cattle according to their characteristics (breed, age, weight, sex) and adapt the preparation and slaughter procedures to limit environmental sources of direct and indirect stress. It is also possible to act on the animal level considering its previous experience and genetics.
CONFLICT Of INTEREST
None of the authors have any conflit of interest to declare.
AUTHORS’ CONTRIBUTION
All authors have been involved in constructing the experimental design, analysing data and preparing.
REFERENCES