Advances in Animal and Veterinary Sciences
Research Article
Ginseng and Moringa Olifera Ameliorated Cognitive Impairments Induced by Aluminium Chloride In Albino Rat
Asmaa K.Abdelghany1*, Fatma Khalil1, Naglaa M Abdel Azeem1, EL-Shymaa EL-Nahass2, Akram M. EL-Kashlan3, Hosny H. Emeash1
1Animal and Poultry Management and Wealth Development Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, 62511, Egypt; 2Pathology Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, 62511, Egypt; 3Hormone Evaluation Department, National Organization for Drug Control and Research, Giza, Egypt.
Abstract | Learning and memory capability are impaired in different neurodegenerative diseases such as Alzheimer’s disease. Since, the efficacy of Ginseng and Moringa Oliefera extracts as a prophylactic for induced Alzheimer’s disease in albino rat was investigated. One hundred rats were equally divided into four groups, (1)Control group (intraperitoneal; IP injected with distilled water), (2)Alzheimer disease model group to be (ADM); (IP injected with Aluminium chloride; AlCl3), (3)AlCl3 (IP) followed by oral gavage of ethanolic extract of ginseng daily and (4)AlCl3 (IP) followed by oral gavage of ethanolic extract of moringa daily. Injection and oral gavage were persisted for seventy consecutive days. Then, learning behavioural tests were performed followed by euthanasia of rats and removal of brains for biochemical measurements (acetylcholinesterase (AChE), monoamines, catalase, malonaldehyde and glutathione activity) and for histopathological examination. In ADM group, cognitive impairment was reported, AChE activity and monoamines levels were decreased significantly, malonaldehyde level and catalase activity were decreased significantly, and neurodegenerative changes were observed. Ginseng and moringa ethanolic extracts ameliorated the reported behavioural, biochemical and histopathological effects of AlCl3 in treated rats. The tested extracts provided efficiently a neuroprotective effect against Alzheimer’s disease.
Keywords | Alzheimer’s disease, Rat, Ginseng, Moringa olifera, Memory, Monoamines , Oxidative stress.
Received | January 18, 2019; Accepted | March 30, 2019; Published | May 11, 2019
*Correspondence | Asmaa K.Abdelghany, Animal and Poultry Management and Wealth Development Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, 62511, Egypt; Email: dr_sma_vet@yahoo.com
Citation | Abdelghany AK, Khalil F, Azeem NMA, El-Nahass ES, El-Kashlan AM, Emeash HH (2019). Ginseng and moringa olifera ameliorated cognitive impairments induced by aluminium chloride in albino rat. Adv. Anim. Vet. Sci. 7(7): 557-565.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.7.557.565
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Abdelghany et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Adequate learning capability is essential for survival and social adaptation of human, animals and birds. Young animals must learn many things rapidly such as escaping from predators (Heckman, 2007). Hence, learning impairment by neurodegenerative disease such as Alzheimer’s disease (AD) may threaten animal life.
Alzheimer’s disease (AD) is an irreversible, progressive neurodegenerative disorder which characterized by learning and memory impairments which caused by diminished level of acetylcholine, reduced biogenic amines, increased
oxidative stress and antioxidant enzyme disturbance. In addition it characterized pathologically by brain size reduction, degeneration and death of hippocampal neurons, aggregations of senile amyloid plaques and tau proteins (Dobhal et al., 2013; Yin et al., 2013; Haider et al., 2014).
Several In vivo studies were performed to assess the potential role of metals in the pathogenesis of AD, where an increase in aluminium (Al3+) and other metals concentrations were detected in several brain areas of rats administered aluminium chloride for six months (Fattoretti et al., 2004).
The multifactorial nature of AD associated with complex pathophysiology, hence concerns directed to develop new therapeutics for treatment of AD. However, recent trends focused on alternative medicine because herbs were found to possess multifaceted action including antioxidant, anti-inflammatory, anti-apoptotic and anti-amyloid properties which can recommend their use for treatment of various neurodegenerative diseases such as AD (Kumar et al., 2017).
Among these herbs, ginseng (GS) and moringa olifera (MO) were used for managing and treatment of AD (Kim and Oh, 2012; Patave and Une, 2016). GS and its active ingredient ginsenosides induce a neuroprotective activity. They have the ability to increase neurons survival improve neurite growth and prevent apoptosis and neuronal cell death, which caused by brain and spinal cord neurotoxicity, ischaemia and oxidative stress (Lim et al., 1997; Liao et al., 2002; Mizumaki et al., 2002). Moreover high ratio of panaxatriol/panaxadiol ginsenoside content in ginseng extract improved memory in rodents (Kennedy and Scholey, 2003).
MO extract possess a nootropic and antioxidant activities because it rich in vitamin C and E, hence it combat oxidative stress and improve memory by using a higher concentrations to treat AD (Mohan et al., 2005; Sutalangka et al., 2013; Roy, 2014).
Based on our knowledge, there are different studies that studies role of GS and MO in treatment of AD (Kim and Oh, 2012; Patave and Une, 2016). However, no report compares their efficacy. Thus, we aimed to compare efficacy of GS and MO extracts in managing of induced Alzheimer’s disease in albino rat using behavioral, biochemical and pathological alterations.
Materials and methods
Chemicals
Aluminium chloride anhydrous (AlCl3) were purchased from Alpha Athero Company imported from central drug house in India.
Ginseng and Moringa Olifera Extract Preparation
Ethanolic Ginseng extract were prepared from dried powder of plant roots, which was extracted with 80% ethanol and stored in darkness at 4°C for one week followed by filtration and storage for final use (Lewis et al., 1999).
Ethanolic moringa olifera extract were prepared from dried leaves of plant, which were dried and ground to obtain fine powder. The powder was extracted with dehydrated alcohol at room temperature for 24hr, then the extract was filtered through Whatman filter paper and vacuum dried to obtain solid mass, which was dissolved in distilled water for final use (Ganguly and Guha, 2008).
Experimental Animals
A total number of 100 male albino rats weighing 70-90g (40-45 days age) were purchased from laboratory house of Al-Nil for Pharmaceuticals Company, Giza, Egypt, and acclimatized for two weeks. The animal house was maintained at a temperature of 19-25.5°c, relative humidity of 40-56%, and lighting system was maintained depending on the natural and artificial lighting using a reversed 12hour light/ dark cycle. The rats were Fed adlibitum twice a day using commercial balanced diet and continuous adequate supply of clean fresh water.
Experimental Design
After acclimatization, rats weighing 160-200g were randomly divided into four groups, twenty five rats for each. (1)Control group (intraperitoneal; IP injected with distilled water), (2)Alzheimer disease model group (ADM) (IP injected with Aluminium chloride; AlCl3), (3)AlCl3 (IP) followed by oral gavage of ethanolic extract of ginseng daily and (4) AlCl3 (IP) followed by oral gavage of ethanolic extract of moringa daily. Injection and oral gavage were persisted for seventy consecutive days.
The study was performed in accordance with Institutional Animal Care and Use Committee of Beni-Suef University (BSU-IACUC) ethical guidelines. The study was approved by the committee (018-50) http://www.bsu.edu.eg/Content.aspx?section_id=3291&cat_id=43&lang=en
In Alzheimer disease model group (ADM), aluminium chloride (AlCl3) freshly prepared weekly by dissolving the powder in distilled water and kept in dark coloured bottle for intraperitoneal injection. Rats were daily intraperitoneally injected with AlCl3 at a dose of 4.2mg\kg b.wt for 70 days (Chakrabarty et al., 2012; Bitra et al., 2014). Control rats were injected with distilled water.
Rats in AlCl3+GS group were subjected daily to oral gavage of GS extract at a dose of 100mg\kg b.wt for consecutive 70 days (Zhao et al., 2009; AKhadrawy et al., 2016).
Rats in AlCl3+MO group were subjected daily to oral gavage of MO extract at a dose of 200mg\kg b.wt for consecutive 70 days (Ganguly and Guha, 2008; Sutalangka et al., 2013).
Memory Tests
All memory tests performed on the sixty day of experiment.
Y-maze test: This test used for assessing working memory in rodents. Test apparatus, procedures and calculation equation performed according to protocol described by Wall et al. (2004); Rasoolijazi et al. (2007) and Baluchnejadmojarad et al. (2012). The apparatus composed of three arms of dark coloured wood; each arm was 40 cm long, 30 cm high and 15 cm wide. A digital camera was used for recording the test days. Arms labeled as A, B and C followed by each rat placed at the end of one arm and allowed to move freely for eight minutes and cleaning maze with 70% alcohol after each rat. The sequence of arm entries was calculated in overlapping triplet sets (i.e ABCCBAABC). Spontaneous alternation behaviour percent (SAP %), Alternate arm returns (AAR) and same arm returns (SAR) were calculated.
Morris water maze (MWM): The Morris water maze is a test which used for assessment of spatial reference memory in rodents depending on using distal cues to locate the platform. MWM is a swimming circular pool painted black measured 150cm in diameter and 50cm height and a platform of 10-12cm in diameter submerged under water by 2cm.Water temperature in the maze is kept at 21-250C (Qi et al., 2009; Chen et al., 2016). Test procedures performed according to protocol described by Vorhees and Williams (2006). A digital camera was used for recording the test days. Test consisted of acquisition phase or training phase where rats placed daily in the maze at a start position facing the maze wall and allowed to locate the platform within 60 second and stand on it for 15-30 second, and then guided if failed to found it. In the first two days of training platform submerged under water by 2cm and in the other two days of training milk powder was used to make the water obaque. Each rat exposed to three training trials from three different starting positions (from three quadrants) where platform was found in the fourth quadrant (target quadrant, Q4). On the final test day 24hr from the last training session (probe trial), platform is removed and each rat allowed searching for the platform for 60second, and time spent in target quadrant was calculated.
Biochemical Measurements
Rats were humanely sacrificed after the end of behavioural tests using diethyl ether anesthesia and brain samples were washed with saline and preserved in deep freezer at -800C.then brain homogenate prepared and following measurements performed at National Organization for Drug Control and Research, Giza, Egypt.
Estimation acetylcholinesterase activity: Acetylcholinesterase activity (AChE) was assayed by the Gorun et al. (1978). This method is a modification of method of Ellman et al. (1961).
Determination of oxidative stress indicators: Total protein was determined according to the method of Lowry et al. (1951) for calculation of oxidative stress parameters as following; reduced g lutathione (GSH) level was determined according to the method of Van Doorn et al., 1978 catalase (CAT) activity was determined according to the method of Aebi, 1984 and malonaldehyde (MDA) level was measured according to the method of Uchiyama and Mihara, 1978.
Estimation of the Monoamine Levels: The estimation of dopamine (DA), noradrenaline (NE) and serotonin (5-HT) levels in the rat brain were carried out according to the fluorometric method described by Ciarlone, 1978.
Pathological Examination
Histological study of hippocampus: Rats were deeply anesthetized using anesthetic ether followed by cardiac perfusion technique where perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer from the left ventricle occurs. The brains were removed and placed in 4% paraformaldehyde 48 h. Routine histological techniques were carried out including dehydration, clearance, embedding and microtomy of sagittal sections of brains. Microtomy of 5 µm sagittal sections using a rotary microtome was performed; these sections were stained by either the Hematoxylin and eosin stain (Gage et al, 2012; Barkur and Bairy, 2016). The rats’ carcasses were hygienically disposed in incineration unit in Faculty of Veterinary Medicine, Beni- Suef University.
Morphometry: Morphometric analysis was carried out using a light microscope (Olympus Pvt. Ltd., Germany). Six sections from each rat were considered for calculations. The numbers of degenerated neurons in the CA1, CA2, CA3, CA4 regions of hippocampus were counted (Barkur and Bairy, 2016).
Statistical Analysis
Data was analyzed using one way analysis of variance (One way ANOVA, LSD) using SPSS version 20 statistical software (IBM Corp. Released 2011. IBM spss statistics for window, version 20.0 Armonk, NY: IBM corp). Data was significant at P<0.05.
Results
Figure 1 (A and B) declared that ADM rats showed increased escape latency to reach the platform significantly (P˂0.05) in comparison with control, GS and MO groups during the four training days. In the test day (probe trial) the ADM rats showed increased escape latency to reach the site of removed platform significantly (P=0.000) compared to other groups. In addition, AlCl3 treated rats showed decreased retention latency significantly (P=0.01) in comparison with control and GS groups.
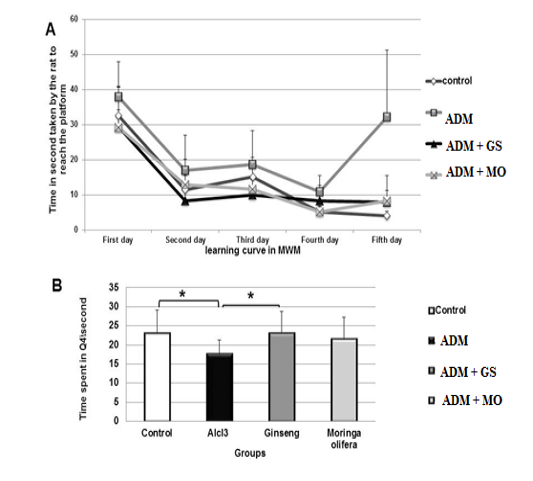
Figure 1: (A) Effect of ginseng and moringa olifera on time/second taken by rat to reach the platform in MWM during training days (learning curve). (B) Effect of ginseng and moringa olifera on time/second spent by rats in target quadrant (Q4) in MWM in probe trial.
* Superscript are significant at P<0.05.
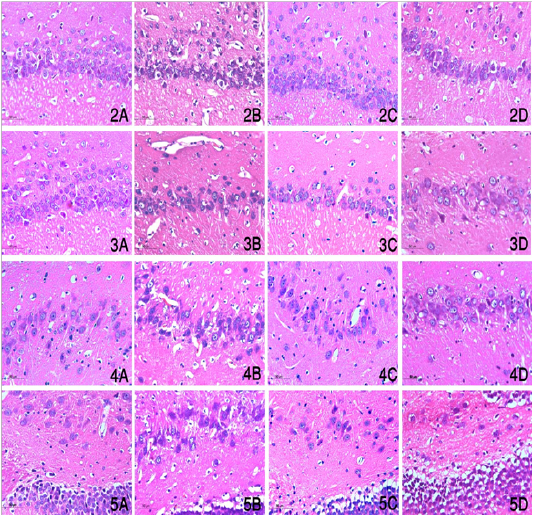
Figures (2-5): Sagittal section of brains in different groups showed hippocampus proper including Cornu Ammonis portions including CA1, CA2, CA3 and CA4 (Figures 2,3,4,5 respectively) in control, ADM, Ginseng and Moringa groups (Figures A, B, C, D respectively) (magnification power X400).
Figure (2-5) showed that the number of degenerated neurons in CA1, CA2, CA3 and CA4 showed a significant difference between different groups. Significant increase of this number in ADM group in comparison with other groups, and also significantly increased in MO group in relation to control group. In contrast no significant difference could be found between control and Ginseng groups.
Based on statistical analysis, SAP decreased and AAR increased significantly (P=0.000, 0.01) in ADM group compared to control group. In addition treatment with GS and MO increased SAP significantly at (P=0.000, 0.001) in comparison with ADM group (Table 1).
Table 1: Effect of ginseng and moringa olifera extracts on impaired spontaneous alternation behaviour in Y-maze test
| Behavioural scoring in Y-maze test | |||
| Group | SAP% | SAR | AAR% |
| Control |
67.94±3.34a |
1.08±0.31 |
22.89±3.05b |
| ADM |
46.96±3.48b |
1.00±0.33 |
35.44±4.73a |
| AlCl3+GS |
66.43±3.05a |
0.58±0.23 | 26.22±3.00 |
| AlCl3+MO |
60.42±1.82a |
1.00±0.21 |
25.63±2.80 |
Results are expressed as means ± standard error.
a, b,and c superscripts in the same column, values with different letters are significant at P<0.05.
ADM: Alzheimer disease model group GS: Ginseng extract treated group MO: Moringa Olifera extract treated group
SAP%: spontaneous alternation percent. SAR: same arm return. AAR%: Alternative arm return.
Table (2) showed that acetylcholinesterase activity (AChE) was decreased significantly (P=0.000) in ADM group compared to control, GS and MO groups, however the AChE enzyme activity decreased significantly (P=0.000, 0.002) in MO group in comparison with control and GS groups. In addition, CAT activity decreased and (MDA) level increased significantly (P=0.000, 0.001) in ADM group inrelation to control group. Furthermore, treatment with GSE and MOE increased catalase activity significantly (P=0.007, 0.000) and decreased significantly (P=0.000, 0.001) elevated MDA level in comparison with AlCl3 group, however MO increased catalase activity significantly (P=0.03) in comparison with control group. In addition no significant changes noticed in glutathione level (GSH) between the all groups.
Table (3) demonstrated that noradrenaline level decreased significantly (P=0.007) in ADM group in comparison with control group and treatment with GS and MO extracts (P=0.04, 0.000). Also dopamine level was decreased significantly (P=0.000) in ADM group related to control group and that treated with GSE significantly (P=0.000). However, rats given MOE showed reduced dopamine lev el significantly (P=0.000) in comparison with control and GSE groups. The serotonin level decreased significantly (P
Table 2: Effect of ginseng and moringa olifera extracts on acetylcholivesterase activity and oxidative stress indicators in rat brain
| Parameter/Group |
Acetylcholinesterase (ACHE) (µMSH/min/g tissue) |
Glutathione (µM/mg protein) |
Catalase (CAT) (IU/min/mg protein) |
Malonaldehyde (MDA) (nM/mg protein) |
| Control |
43.40±1.61a |
42.44±2.59 |
0.017±0.001b |
1.52±0.13b |
| ADM |
26.36±0.96ab |
40.06±3.05 |
0.007±0.001ab |
2.24±0.23a |
| AlCl3+GS |
40.53±0.72a |
40.64±0.86 |
0.017±0.001b |
1.42±0.12b |
| AlCl3+MO |
34.39±1.46ab |
36.04±1.58 |
0.021±0.003ab |
1.51±0.05b |
Results are expressed as means ± standard error.
a, b,and c superscripts in the same column, values with different letters are significant at P<0.05.
ADM: Alzheimer disease model group GS: Ginseng extract treated group MO: Moringa Olifera extract treated group
=0.002) in ADM group in relation to control group. GS increased the reduced serotonin level significantly caused by AlCl3(P=0.000) in comparison with ADM, control and MO groups.
Table 3: Effect of ginseng and moringa olifera extracts on brain monoamines levels
|
Parameter /Group |
Noradrenalin (µg/gm tissue) |
Dopamine (µg/gm tissue) |
Serotonin (µg/gm tissue) |
| Control |
2.07±0.04a |
1.22±0.02a |
0.89±0.06b |
| ADM |
1.63±0.03ab |
0.55±0.02b |
0.57±0.02c |
| AlCl3+GS |
2.22±0.09a |
1.32±0.02a |
1.38±0.10a |
| AlCl3+MO |
1.94±0.12b |
0.53±0.04b |
0.73±0.06b |
Results are expressed as means ± standard error.
a, b,and c superscripts in the same column, values with different letters are significant at P<0.05.
ADM: Alzheimer disease model group GS: Ginseng extract treated group MO: Moringa Olifera extract treated group
Discussion
The results of the present study suggest that aluminium chloride induced behavioural, biochemical and pathological abnormalities similar to that occurs in Alzheimer disease which manifested by impaired short term, spatial working and spatial reference memory (Cao et al., 2017).
Our results showed that AlCl3 (in ADM group) impaired short term working memory, where spontaneous alternation behaviour (SAP) was reduced. This result supported with Xing et al. (2018) and Zghari et al. (2018) found that exposure of rat and mice to Al or AlCl3 and D-galactose reduced spontaneous alternation percentage in Y-maze test. While treatment with GS and MO enhanced the reduced SAP and this were in agreement with Kim et al. (2017) and Omotoso et al. (2018) who reported that red ginseng and ginsenoside moringa olifera increased alternation percent in Y-maze.
In addition, the present work revealed impaired long term memory in AlCl3 group in MWM, Justin Thenmozhi et al. (2015) and Iqbal et al. (2016) demonstrated that treated rats took more time to reach platform and express improvement in performance over training days, while in probe trial treated rats spent less time in target quadrant containing platform in comparison with control rats. However treatment with GSE and MOE improved long term memory in MWM. Our results with MOE partially in agreement with Sutalangka et al. (2013) who found that administration of MOE (100, 200, 400mg) decreased escape latency and increased retention time in MWM test. The findings by of GSE treatment were supported by Zhu et al. (2018) who reported that Panax ginseng extract administration (50,100mg) in dementia animal model significantly decreased escape latency and increased time spent in target quadrant in a dose dependent manner.
Cholinergic function decline with the advanced age is suggested to be associated with short term and long term memory impairments (Jha and Rizvi, 2009; Papandreou et al., 2011). In this study it was obvious that AChE activity decreased signficantly in AlCl3 group which run in parallel with Singh et al. (2014) who reported that intraperitoneal administration of AlCl3 to rats at a dose of 4.2mg\kg for 28 days resulted in significant decrease in AChE activity.
Different studies on the effects of aluminium on AChE activity revealed that the effect of aluminium is controversial since it has biphasic effects and thus, induce potentiating and inhibitory effects on enzyme activity due to multiple routes and doses of aluminium administration, length of exposure, variation in biological sample assayed and the metal speciation (Exley and Birchall, 1992; Kumar, 1999; Kaizer et al., 2005). Treatment with GS and MO increased the AChE activity significantly compared to ADM group which supported with Ganguly (2005) and Roy (2014) who found that treatment with MO ameliorated the adverse effect of Colchicine and increased cholineacetyltransferase and acetylcholinesterase activity in AD animal model. In addition Mohamed et al. (2007) found that GS administration increased cholinesterase activity in hypothalamus, thalamus, mid brain and cerebral cortex, however significant decrease in cerebellar cholinesterase recorded and non-significant increase was recorded in medulla in diabetic rats. In addition AKhadrawy et al. (2016) reported that administration of panax ginseng in a rat model of Parkinson’s disease increased acetylcholinesterase activity in mid brain and striatum to non-significant difference from control and model groups.
The brain is rich in peroxidizable fatty acids which may be mostly vulnerable to oxidative stress which contributes to incidence of neuronal damage and death in AD as a result of excessive production of free radicals. Aluminium is considered a pro-oxidant agent and interfering with pathways involved in normal iron metabolism and homeostasis resulting in increased reactive oxygen species and incidence of lipid peroxidation (Kaizer et al., 2005).
It was suggested that aging associated cognitive impairments is attributed to increased oxidative stress in aging brain (Bagheri et al., 2011) which run in harmony with our result where AlCl3 group showed reduced catalase activity and enhanced MDA level which was in agreement with Bitra et al. (2014) recorded that exposure to intraperitoneal injection of AlCl3 resulted in significantly increased MDA level in various brain areas, and partially agree with Lakshmi et al. (2015) stated that exposure to aluminium resulted in significantly decreased activity of CAT and glutathione. On the other hand treatment with GS increased the reduced CAT and decreased the elevated MDA which run in parallel with Sutalangka et al. (2013) who found that oral administration of moringa olifera extract attenuate the decreased catalase activity and decreased the elevated MDA level which induced by a cholinotoxin AF64A. As well as Kim et al. (2017) found thatoral administration of ginsenoside Re increased activity of glutathione peroxidase and decreased MDA level when combating oxidative stress.
No significant difference could be detected between groups in the glutathione (GSH) level, because it may play a minor role in combating oxidative stress that keeps its level unaffected in ADM group. The unaffected GSH to some extent run with El-Gendy, 2011 and Yuan et al. (2012) who reported an increased glutathione peroxidase activity (GPx) after aluminium chloride administration which used the GSH as a substrate during its action in combating oxidative stress. Another explanation may be due to binding of aluminium with transferrin and brain iron overload through Fenton reaction (Muckenthaler et al., 2008; Ighodaro and Akinloye, 2018).
The decreased brain monoamines levels are also linked with aging decline in memory (Lee et al., 2010), in our study AlCl3 significantly decreased the noradrenaline, dopamine and serotonin levels in brain which reviewed with Foster (2000) who mentioned that aluminium neurotoxicity elevates neopterin levels in the brain of AD patients and at the same time induces reduction of brain neurotransmitters such as dopamine, norepinephrin and serotonin via depressing cerebrospinal fluid tetrahydrobiopterin levels which is required for the synthesis of those neurotransmitters. On the other hand our treatment with GSE significantly increased all monoamines levels in brain as achieved by Zhang et al. (2016) who reported that oral administration of ginsenosides in a rat model of AD resulted in restoration of function of various neurotransmitters involving glutamate, aspartate, gamma-aminobutyric acid, acetylcholine, glycine, dopamine and serotonin. Also Xiang et al. (2011) found that Panax notoginseng extract increasing levels of serotonin, dopamine and norepinephrine in animal models of depression.
In addition, treatment with MO increased noradrenaline and serotonin levels significantly, whi decreased dopamine which to some extent agree with Ray et al. (2003) who demonstrated that aqueous extract of moringa olifera also affect level of brain monoamines by raising serotonin level and reduced dopamine level in cortex, cerebellum and caudate nucleus and reduced cortical norepinephrine level.
Concerning histopathological examination, in the present exposure of aluminium via intraperitoneal route, led to severe and marked histopathological alterations in hippocampus, that represented by neuronal degeneration in different regions of hippocampus and cytoplasmic vacuolization, haemorrhage, and gliosis that was similar to the prevuious record by the finding of (Matyia, 2000).
In AD, the hippocampus is the first and mostly affected area in brain as it is contain glutamatergic, monoaminergic and cholinergic axon terminals which are positively correlated with AD (Bingman, 1992; Cao et al., 2017). Our results showed that the number of degenerated neurons in CA1, CA2, CA3 and CA4 showed a significant difference between different groups. Significant increase of this number in ADM group in comparison with other groups, and also significant increase in MO group in relation to control group which may attribute to decreased dopamine level and decreased AChE activity in moringa in comparison with control group. On contrast, no significant difference could be found between control and GS groups which could be supported by (Bingman, 1992; Cao et al., 2017).
Conclusion
Treatments with ginseng and moringa olifera ethanolic extracts ameliorated cognitive impairments enhancedacetyl cholinesterase enzyme activity and monoamines levels and reduced oxidative stress and possess a neuroprotective effect. Moreover, ginseng extract had more potent effect than that of moringa extract in managing Alzheimer’s disease.
Acknowledgement
We acknowledge Projects Funding and Granting unit belonging to the Scientific Research Developing Unit in Beni-Suef University, Egypt for funding this work.
Conflict of interest
No conflict of interest.
Authors Contribution
All authors contributed equally, however the first two authors did much efforts in working and writing the manuscript.
References






