Tissue Specific Changes in the Antioxidant Enzyme Activity of Manganese Exposed Labeo rohita
Tissue Specific Changes in the Antioxidant Enzyme Activity of Manganese Exposed Labeo rohita
Maliha Ayub1, Muhammad Javed1, Fariha Latif 1*, Faiza Ambreen2, Komal Sabir1
1Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad
2Department of Zoology, Government College Women University, Faisalabad
Abstract | The accumulation of heavy metals in the body organs of aquatic organisms poses serious impacts on the activities of antioxidant enzymes. These enzymes are effective in protecting the bio-molecules against the reactive oxygen species (ROS) and their harmful effects. This study was aimed to evaluate the effects of sub-lethal concentrations of manganese on antioxidant activity in the fish, Labeo rohita. Fish were acclimatized for two weeks in the cemented tanks prior to the start of experiment. Four groups (n=10) of one year old fingerlings of Labeo rohita were exposed to 96-hr LC50 and sub-lethal concentrations (2/3rd,1/4th and 1/5th of LC50) of manganese for a duration of 30 days in the glass aquaria of 70L water capacity. After 30-day exposure, antioxidant (peroxidase) activity was measured in the fish liver, kidney, gills and brain and then compared with control group. The results showed that the antioxidant activity increased significantly in all organs of metal stressed fish as compared to the control fish. Among all test concentrations, the activity of antioxidant enzyme (peroxidase) was significantly higher in the fish liver under 96-hr LC50 manganese exposed fish as evident from its mean value as 0.685±0.005UmL-1, followed by 2/3rd (0.585±0.006 UmL-1), 1/4th (0.480±0.005 UmL-1), 1/5th (0.367±0.007 UmL-1) and control (0.278±0.005 UmL-1) group. Fish liver showed significantly higher (0.479±0.005 UmL-1) activity of peroxidase than that of other organs like kidney (0.396±0.006UmL-1), gills (0.356±0.004UmL-1) and brain (0.278±0.004UmL-1) of Labeo rohita.
Article History
Received: March 04, 2018
Revised: September 05, 2018
Accepted: July 18, 2019
Published: September 16, 2019
Authors’ Contributions
MA did the research work. MJ conceived the research idea. FL worte the manuscript. FA peformed statistical analysis. KS helped in manuscript write-up.
Keywords
Antioxidant enzyme, Peroxidase, Sub-lethal Exposure, Manganese
Corresponding author: Fariha Latif
fariha.latif44@hotmail.com
To cite this article: Ayub, M., Javed, M., Latif, F., Ambreen, F. and Sabir, K., 2019. Tissue specific changes in the antioxidant enzyme activity of manganese exposed Labeo rohita. Punjab Univ. J. Zool., 34(2): 125-132. https://dx.doi.org/10.17582/journal.pujz/2019.34.1.127.132
Introduction
The natural freshwater ecosystems are being polluted due to rapid industrialization and various other activities, which discharge their effluents in the water (Velma and Tchounwou, 2010). Fish occupy the top place in the food chain and sensitive to the water pollution, therefore can be used as a suitable bio-indicators for the assessment of aquatic pollution (Fidan et al., 2007). Fish may take heavy metals from their surroundings through the body surface, gills and digestive tract (Abdullah et al., 2007). Gills play an important role in the ionic and osmotic regulation, can accumulate higher concentration of metals due to their direct contact with the water (Nussey et al., 2000).
In the aquatic habitat, manganese is a naturally occurring micronutrient (Holmes, 2010). Manganese is an important metal which takes part in many metabolic processes such as energy metabolism, specific enzyme activation, function of nervous and immunological system (ATSDR, 2000). Manganese is also a cofactor of different enzymes including antioxidant enzymes such as gluthathione-S-transfarase (GST), catalase (CAT) and lipid peroxidase (LPO) (Aschner et al., 2007). It is a vital micronutrient for the fish but may induce toxicity at higher concentrations (Howe et al., 2004).
Metals can induce oxidative stress by generating reactive oxygen species (ROS) in aquatic organisms as they get accumulated in the fish body organs (Sevcikova et al., 2011). Reactive oxygen species may cause cellular damages that alternate the structure, gene expression, cellular proliferation and transcription factor activation (Sabatini et al., 2009). At higher concentration, manganese can increase the production of ROS (Falfushynska et al., 2011) that can cause neurogenic disorders due to inactivation of antioxidant enzyme system and by enhanced production of free radicals (Chen et al., 2006). In the living organisms, imbalance between production of ROS such as hydroxyl radical (OH-1), hydrogen peroxide (H2O2), superoxide radical (O-2) and antioxidant enzyme defense system causes oxidative stress (Hermes, 2004). For the maintenance of cellular homeostasis, there should be a balance between antioxidant enzyme system and ROS (Valavanidis et al., 2007). The fish kidney possesses antioxidant defense systems that protect the fish from oxidative stress (Jorgensen, 2010). The antioxidant defence mechanism of fish includes enzyme system and low molecular weight antioxidants. The antioxidant enzyme system contain superoxide dismutase (SOD), catalase (CAT), Glutathione-S-transferase (GST), lipid peroxidase (LPO) and glutathione peroxidase (GPx) (Di-Giulio and Meyer, 2008). Peroxidase is a well-known antioxidant enzyme present in mitochondrial matrix and cell cytoplasm which converts peroxidase into water and oxygen (Aruljothi and Samipillai, 2014).
Labeo rohita, an Indian major carp, is extensively cultured and consumed in Pakistan due to its better meat quality and it is also frequently used for the monitoring of aquatic toxicity (Ramani, 2002). Therefore, this study was planned to study the effects of sub-lethal doses of manganese on the activity of antioxidant enzyme (peroxidase) in the liver, kidney, gills and brain of Labeo rohita.
Materials and Methods
The proposed research work was performed in the Laboratories of Fisheries Research Farms, Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad. Fingerlings of Labeo rohita were brought to the laboratory and acclimatized prior to the experiments. After acclimatization, the one year old fingerlings of Labeo rohita, having similar weights and lengths, were transferred to the glass aquaria of 70L water capacity. Chemically pure compound of manganese was dissolved in 1000ml deionized water for the preparation of metal stock solution. Four groups of fish (n=10) were exposed to sub-lethal concentrations viz. 96-hr LC50 (73.70±3.64mgL-1) 2/3rd, (49.13±1.52mgL-1), 1/4th (18.42±1.09mgL-1) and 1/5th (14.74±0.89mgL-1) of LC50 values of manganese as determined by Abdullah et al. (2007). The experiment was conducted with three replications for each test concentration. Another group of fish regarded as “control” was kept in the metal free media. After 30 days of iron exposure, the fish were dissected and their liver, kidney, gills and brain tissues were isolated and stored at -4°C for enzyme assay. The physico-chemical parameters of water viz. dissolved oxygen, pH, total ammonia, magnesium, total hardness, carbon dioxide and calcium and monitored on daily basis by following the methods of A.P.H.A. (1998).
Preparation of enzymes extract
The liver, kidney, gills and brain of the fish were rinsed with phosphate buffer of pH 6.5 (0.2M) and homogenized in cold buffer (1:4W/V) by using a blender. This was done for the removal of red blood cells from the tissues (liver, kidney, gills and brain). After homogenization, at 10,000rpm, the organ homogenate was centrifuged for 15 minutes at 4ºC. After centrifugation, the clear supernatant was preserved at -4ºC for the enzyme assay. The samples were subjected to enzyme assay by following the method described by Civello et al. (1995) for the determination of peroxidase activity. The enzyme peroxidase activity was determined spectrophotometrically at a wavelength of 470nm by measuring conversion of guaiacol to tetraguaiacol.
Preparation of 0.2M phosphate buffer (pH 6.5)
For the preparation of 0.2M phosphate buffer, 4g NaH2PO4 and 1g Na2HPO4 were taken in flask and dissolved by adding distilled water. The volume was raised up to 200ml and pH was adjusted to 6.5.
Preparation of buffer substrate solution
Guaiacol (750µL) was added to phosphate buffer (47ml) and mixed well on vortex agitator. After agitation, H2O2 (300µL) was added to buffer solution. Reaction mixture contain buffered substrate solution (300 µL), enzyme extract (60 µL) and blank. A cuvette containing 3ml of blank solution was inserted into the Spectrophotometer and set it to zero at wave length of 470nm. Then a cuvette containing buffered substrate was put into the spectrophotometer, reaction was started by adding 0.06ml enzyme extract. The reaction time was 3 minutes and after that absorbance was recorded and enzyme activity was calculated by using following formula:
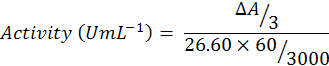
Statistical analyses
The means were compared by employing least square design (LSD) and the correlation and regression analyses were also performed to find out statistical relationships among different parameters under study by using the statistical software Minitab17.
Table 1: Analysis of variance on peroxidase activity (UmL-1) in various tissues of Labeo rohita after sub-lethal exposure of manganese.
|
Organs/Tissues |
Concentrations/Treatments |
||||
|
96-hr LC50 |
2/3rdLC50 |
1/4th LC50 |
1/5th LC50 |
Mean±SD |
|
|
Liver |
0.685±0.005 |
0.585±0.006 |
0.480±0.005 |
0.367±0.007 |
0.479±0.005a |
|
Kidney |
0.584±0.004 |
0.467±0.006 |
0.387±0.004 |
0.322±0.006 |
0.396±0.006b |
|
Gills |
0.465±0.005 |
0.428±0.006 |
0.361±0.002 |
0.311±0.005 |
0.356±0.004c |
|
Brain |
0.363±0.007 |
0.345±0.005 |
0.309±0.004 |
0.206±0.004 |
0.278±0.004d |
|
Mean±SD |
0.524±0.005a |
0.456±0.005b |
0.384±0.003c |
0.301±0.005d |
|
The mean with similar letter in single row and column are statistically non-significant at p<0.05.
Results and Discussion
Manganese is an important metal which takes part in many metabolic processes such as energy metabolism, specific enzyme activation, function of nervous and immunological system. Metals can produce reactive oxygen species which may generate oxidative stress. Under manganese stress significant increase in the antioxidant enzyme (peroxidase) activity in the fish organs were observed than that of control group (Table 1). The peroxidase activity was found higher in the liver at 96-hr LC50 exposure as compared to the other treatments predicting that peroxidase activity can be significantly increased at elevated concentrations of manganese. Comparison of means reveals that the activity of peroxidase was increased at all the exposure concentrations than in the control group (Figure 1). Peroxidase activity was significantly highest (0.524±0.005 UmL-1) at 96-hr LC50 concentration while it was significantly lower (0.222±0.004 UmL-1) in control group (Figure 2). It was also observed that in the liver of fish, the peroxidase activity was more pronounced as compared to the brain showing quick response of liver antioxidant enzyme (peroxidase activity) to prevent the cells from oxidative damage caused by manganese.
Aquatic ecosystems are polluted due to different heavy metals which released from different sources such as mining, use of pesticides, fertilizers, erosion and other anthropogenic activities (Ambedkar and Muniyan, 2011). Metals can produce reactive oxygen species which may cause oxidative stress. Contamination of freshwater habitats due to metals can be checked by the assessment of oxidative damage and antioxidant defence system of the fish (Luoma and Rainbow, 2008). Alteration in antioxidant enzyme activities can cause oxidation of protein and lipid which leads towards the oxidative tissue damage (Lushchak and Bagnyukova, 2006). Defence mechanism of the fish against ROS is the presence of antioxidant enzymes (Otitoloju and Olagokee, 2011). During the present study, the antioxidant enzyme (peroxidase) activity was found significantly (p<0.05) higher in the various tissues of fish under all metal exposure treatments/concentrations as compared to the control group. Manganese exposed fish showed higher antioxidant enzyme activity due to enhanced production of ROS as compared to the control fish. Zikic et al. (2001) recorded increased antioxidant enzymes activities in the erythrocytes of Carassius auratusafter exposure to cadmium for 15 days. Similarly, Nayak et al. (1999) found an increase in peroxidase enzyme activity in Channa punctatus under exposure to manganese chloride and their results are in line with our findings. Viera et al. (2012) concluded that the level of lipid peroxidase (LPO) increased by 2.25% in the gills of gold fish (Carassius auratus) after exposure to manganese for 96-hrs. Arabi and Alaeddini (2005) observed the effect of copper (400 to 1000µM) in the gills of rainbow trout and found that the level of lipid peroxidase was increased in the gills as compared to control. Increased activity of antioxidant enzyme was observed by Enghild et al. (1999) in the gills of Classman macropomumunder manganese exposure Romeo et al. (2000) observed the elevated level of lipid peroxidase in the kidney of sea bass, Dicentrarchus labrax, when treated with cadmium. Significant increase in the lipid peroxidase level in the gills of Calarias gariepinus were observed by Farombi et al. (2007) due to exposure of various heavy metals viz. Zn, Cd, Cu, and Pb. Similar results were also observed by Aruljothi and Samipillai (2014) who reported that the level of lipid peroxidase (LPO) was increased in the gills of Labeo rohita at 96-hr exposure of arsenic. The results showed that the high level of lipid peroxidase was due to the excess production of ROS by arsenic toxicity. Jena et al. (1998) found significant decreased in antioxidant activity in the brain homogenate of Channa striata after in vitro treatment with MnCl2. Increased peroxidase activity was observed by Helliwell and Gutteridge, (2006) in the kidney (220%) and gills (130%) of Carassius auratusunder exposure of manganese when compared with the control. Gabriel et al. (2013) studied the antioxidant activity in the kidney and gills of exposed Colossoma macropomum and concluded that manganese exposure caused significant increase in the peroxidase enzyme activity in gills while this activity was decreased significantly in kidney. Falfushynska et al. (2011) found decrease in peroxidase activity in the gills of Carassius auratus under exposure to 1.7mgL-1 of manganese for 14 days that are in contrast with present results. Velma and Tchounwou (2010) also observed an increased level of antioxidant activity in the liver, kidney and gills of Anguilla Anguilla after manganese exposure. Aslam et al. (2017) reported a dose dependent increase in peroxidase activity in zinc exposed Catla catla.
Conclusion
The antioxidant activity (peroxidase) was found significantly increased in selected fish organs after exposure to manganese due to all concentrations/treatments than that of control group which shows that increasing exposure concentration of manganese cause increase in reactive oxygen species (ROS) production. To neutralize the harmful effects of these ROS, the synthesis of peroxidase enzyme increases.
References
Abdullah, S., Javed, M. and Javid, A., 2007. Studies on acute toxicity of metals to the fish (Labeo rohita). Int. J. Agric. Biol.,9: 333-337.
A.P.H.A. (American Public Health Association). 1998. Standard method for examination of water and waste water (20th Ed.) New York, pp. 1193.
Ambedkar, G. and Muniyan, M., 2011. Accumulation of metals in the five commercially important fishes available in velar river, Tami Nadu. Ind. Sch. Res. Lib. Arch. App. Sci. Res., 3: 261-264.
Arabi, M. and Alaeddinei, M.A., 2005. Metal-ion mediacted oxidative stress in the gills homogenates of rainbow trout (Oncorhynchus mykiss): antioxidant potential of manganese, selenium and albumin. Biol. Trace Elem. Res.,108: 155-168. https://doi.org/10.1385/BTER:108:1-3:155
Aslam, M., Javed, M., Ambreen, F. and Latif, F., 2017. Effect of various concentrations of zinc on peroxidase activity of Catla catla. Biologia (Pakistan), 63: 125-130.
Aschner, M., Guilarte, T.R.J., Schneider, S. and Zheng, W., 2007. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol., 221: 131-147. https://doi.org/10.1016/j.taap.2007.03.001
Aruljothi. B. and Samipillai, S., 2014. Effect of arsenic on lipid peroxidation and antioxidants system in freshwater fish, Labeo rohita. J. Mod. Res. Rev., 2: 15-17.
ATSDR. 2000. Toxicological profile for manganese. United states department of health and human services, Public health service, Agency for toxic substances and disease registry, Atlanta, GA.
Aureliano, M., Joaquim, N., Sousa, A., Martins, H. And Coucelo, J.M., 2002. Oxidative stress in toadfish (Halobactrachus didactylus) cardiac muscle: Acute exposure to vanadate oligomers. J. Inorg. Biochem, 90: 159-165. https://doi.org/10.1016/S0162-0134(02)00414-2
Chen, M.T., Cheng, G.W., Lin, C.C., Chen, B.H. and Huang, Y.L., 2006. Effects of acute manganese chloride exposure on lipid peroxidation and alteration of trace metals in rat brain. Biol. Trace Elem. Res., 110: 163-177. https://doi.org/10.1385/BTER:110:2:163
Civello, P.M., Arting, G.A., Chaves, A.R. and Anan, M.C., 1995. Peroxidase from strawberry fruit by partial purification and determination of some properties. J. Agric. Food Sci., 43: 2596-2601. https://doi.org/10.1021/jf00058a008
Di-Giulio, R.T. and Meyer, J.N., 2008. Reactive oxygen species and oxidative stress. In: Di- Giulio R. T., Hinton DE (Eds.): The Toxicology of Fishes. Boca-Raton: CRC Press, Taylor and Francis Group, pp. 273-324. https://doi.org/10.1201/9780203647295.ch6
Enghild, J.J., Thogersen, I.B., Oury, T.D., Valnickova, Z., Hojrup, P. and Crapo, J.D., 1999. The herparin-binding domain of extracellular superoxide dismutase is proteolytically processed intracellularly during biosynthesis. J. Biol. Chem., 274: 14818-14822. https://doi.org/10.1074/jbc.274.21.14818
Falfushynska, H.I., Gnatyshyna, L.L., Stoliar, O.B. and Nam, Y.K., 2011. Various responses to copper and manganese exposure of (Carassius auratus) from two populations. Comp. Biochem. Physiocol. Toxicol. Pharmacol., 154: 242-253. https://doi.org/10.1016/j.cbpc.2011.06.001
Farombi, E.O., Adelowo, O.A. and Ajimoko, Y.R., 2007. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Public health, 4: 158-165. https://doi.org/10.3390/ijerph2007040011
Fidan, A.F., Cigeric, I.H., Konuk, M., Kucukkurt, I., Aslan, R. and Dunder, Y., 2007. Determination of some heavy metal levels and oxidative status in Carassius carassius L., 1758 from Eber Lake. Environ. Monit. Assess., 147: 35-41. https://doi.org/10.1007/s10661-007-0095-3
Gabriel, D., Riffel, A.P.K., Finamor, I.A., Saccol, E.M.H., Ourique, G.M., Goulart, L.O., Kochaan, D., Cunha, M.A., Garcia, L.O., Parvanto, M.A., Val, V.L., Badisserotto, B. and Llesuy, S.F., 2013. Effects of subchronic manganese chloride exposure on tambaqui (Colossoma macropomum) tissues, oxidative stress and antioxidant defenses. Arch. Environ. Contam. Toxicol., 64: 659-667. https://doi.org/10.1007/s00244-012-9854-4
Halliwell, B. and Gutteridge, J.M.C., 2006. Free radicals in the biology and medicine fourth ed. Oxford Univ. Press, UK.
Hermes, L.M., 2004. Oxygen in biology and biochemistry: role of free radicals. In: Storey, K.B., (Ed.), Functional metabolism: Regulation and adaptation. Wiley-Liss, Hoboken, pp. 319-368. https://doi.org/10.1002/047167558X.ch12
Holmes, R., 2010. The potential for metal contamination in the Maitai river. Cawthron report. pp. 1850-1852.
Howe, P.D., Malcolm, H.M. and Dobson, S., 2004. Manganese and its compound: Environmental aspects. Concise international chemical assessment document, 63rd ed. World health organization, New York.
Jorgensen, S.W., 2010. A derivative of encyclopedia of ecology. Ecotoxicol. Academic press, London, pp. 390-392.
Jena, B.S., Nayak, S.B. and Patnaik, B.K., 1998. Age-related changes in catalase activity and its inhibition by manganese (II) chloride in the brain of two species of poikilothermic vertebrates. Arch. Gerontol. Geriatr., 26: 119-129. https://doi.org/10.1016/S0167-4943(97)00038-1
Luoma, S.N. and Rainbow, P.S., 2008. Sources and cycles of trace metals. In: metal contamination in aquatic environment: Science and management. Camb. Univ. Press. Camb., pp. 47-66.
Lushchak, V. I. and Bagnyukova, T.V., 2006. Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes: Comparative biochemistry and physiology Part C: Toxicol. Pharmacol., 143: 36–41.
Nayak, S.B., Jena, B.S. and Patnaik, B.K., 1999. Effects of age and manganese (II) chloride on peroxidase activity of brain and liver of the teleost, Channa punctatus. Exp. Gerontol., 34: 365-374. https://doi.org/10.1016/S0531-5565(99)00021-2
Nuseey, G., Van-Vern, J.H.J. and Dur-preez, H.H., 2000. Effect of copper on the hematology and osmoregulation of Mosambique tilapia (Oreochromis niloticus). Comp. Biochem. Physiol., 111: 369-380. https://doi.org/10.1016/0742-8413(95)00063-1
Otitoloju, A. and Olagoke, O., 2011. Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons. Environ. Monit. Assesss., 182: 205-213. https://doi.org/10.1007/s10661-010-1870-0
Ramani, M.B., Anna-Mercy, T.V., Nair, R.J. and Sherief, P.M., 2002. Changes in the proximate composition of (Labeo rohita) (Ham.) exposed to sub-lethal concentrations of monocrotophos. Ind. J. Fish., 49: 427-432.
Romeo, M., Bennani, N., Gnassia-Barelli, M., Lafaurie, M. and Girard, J.P., 2000. Cadmium and copper display different responses towards oxidative stress in the gills of the sea bass Dicentrarchus labrax. Aquat.Toxicol., 48: 185-194.
Sevcikova, M., Modra, H., Blahova, J., Dobsikova, R., Plhalova, L., Zitka, O., Hynek, D., Kizek, R., Skoric, M. and Svobodova, Z., 2016. Biochemical, haematological and oxidative stress responses of common carp (Cyprinus carpio L.) after sub-chronic exposure to copper. Vet. Med., 61: 35-50. https://doi.org/10.17221/8681-VETMED
Sabatini, S.E., Juarez, A.B., Eppis, M.R., Bianchi, L., Luquet, and Molina, M.C., 2009. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol. Environ. Safe., 72: 1200-1206. https://doi.org/10.1016/j.ecoenv.2009.01.003
Valavanidis, A., Vlahogianni, T., Dassenakis, M. and Scoullos, M., 2007. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Safe., 46: 178-189. https://doi.org/10.1016/j.ecoenv.2005.03.013
Velma, V. and Tchounwou, P.B., 2010. Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish (Carassium auratus). Mutat. Res., 698: 43-51. https://doi.org/10.1016/j.mrgentox.2010.03.014
Viera, C.M., Torronteras, R., Cordoba, F. and Canalejo, A., 2012. Acute toxicity of manganese in goldfish (Carassius auratus) is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol. Environ. Safe., 78: 212-217. https://doi.org/10.1016/j.ecoenv.2011.11.015
Zikic, R.V., Stajn, A.S., Pavlovic, S.Z., Ognjanovic, B.I. and Saicic, Z.S., 2001. Activities of superoxide dismutase and catalase in erythrocytes and plasma transaminases of goldfish (Carassius auratus gibelio) exposed to cadmium. Physiol. Res.,50: 105-111.
To share on other social networks, click on any share button. What are these?








