Screening of Maize Genotypes against Southern Corn Leaf Blight (Bipolaris maydis) under Artificial Epiphytotic Conditions
Research Article
Screening of Maize Genotypes against Southern Corn Leaf Blight (Bipolaris maydis) under Artificial Epiphytotic Conditions
Azra1* and Shaukat Hussain2
1Amir Muhammad Khan Campus Mardan, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Plant Pathology, Faculty of Crop Protection Sciences, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Thirty-six maize genotypes were evaluated under artificial epiphytotic of southern corn leaf blight during Kharif season 2015 and 2016 to find out new sources of genetic resistance in maize genotypes against southern corn leaf blight. The experiment was laid out as randomized complete block design with three replications. Data on disease severity and grain yield were recorded. There were not significant differences in disease severity among maize genotypes for individual years. However, significant differences were recorded amongst the genotypes when the data were pooled over two years. Based on disease severity scale 14 genotypes were categorized as moderately resistant, 14 moderately susceptible and the remaining eight genotypes were grouped as highly susceptible. Significant differences were recorded for grain yield among all tested genotypes during kharif season 2015 and 2016 as well as data pooled over two years. Moderately resistant genotypes CS-2Y10, EVY-1 and EHY-2 scored highest yield. Conversely, the lowest yield was observed for the highly susceptible genotypes WL-4 and WL-5. Agglomerative hierarchical cluster analysis with grain yield and disease severity categorized the tested genotypes into two major clusters. Cluster 1 is further divided into three sub clusters. In sub cluster I most of the genotypes are moderately resistant with medium to high yielding potential. Sub cluster II includes highly susceptible genotypes with low yield potential. Sub cluster III has moderately susceptible to moderately resistant genotypes. However, genotypes of this sub cluster are high yielding. Genotype CS-2Y10, being moderately resistant, is also the highest yielding genotype.Cluster II includes moderate to highly susceptible genotypes. All genotypes in this cluster are however low yielding.
Received | June 20, 2019; Accepted | September 23, 2019; Published | November 08, 2019
*Correspondence | Azra, Amir Muhammad Khan Campus Mardan, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: azranadeem@aup.edu.pk
Citation | Azra and S. Hussain. 2019. Screening of maize genotypes against southern corn leaf blight (Bipolaris maydis) under artificial epiphytotic conditions. Sarhad Journal of Agriculture, 35(4): 1122-1128.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.4.1122.1128
Keywords | Maize, Genotypes screening, Bipolaris maydis, Southern corn leaf blight, Genetic resistance
Introduction
Maize (Zea mays L.), a member of the tribe Maydae, family Poaceae, is the most cultivated cereal crop after wheat and rice in the world (Singh and Kumar, 2016). In Pakistan, maize is grown as multipurpose food and forage crop. In mountainous region of the country, it is used as staple food and 50% of the total production is directly consumed by the local population. Pakistan ranks at 22nd position in global maize production (FAO, 2013). The fertile land of Punjab and plains of Khyber Pakhtunkhwa (KP) at low and mid to high land ecologies are major maize producing areas of Pakistan. The total area under maize cultivation in Pakistan is 1334 thousand hectares with a production of 6130 thousand tones producing a yield of 4595 kg per hectare (GOP, 2016-2017). The major contribution comes from Punjab with 4019.9 tons with an area of 672.8 thousand hectares under cultivation giving a yield of 5975kg per hectare. Khyber Pakhtunkhwa province contributes 1965 tons from an area of 463 thousand hectares (GOP, 2014-2015). This is however far below the world maize production. The world major maize producing countries produce 563 million metric tons/year (Ranum et al., 2014). The situation indicates several socio economic, biological and physical constrains that limit maize productivity in Pakistan. Approximately 65 pathogens including fungi, bacteria and viruses cause economically important diseases in maize with annual losses amounting to 9.4% (Singh and Gilbreath, 2002). Among fungal diseases, leaf blights, smuts and stalk rots cause significant damage (Rahman et al., 1986).
Southern corn leaf blight (SCLB) caused by Bipolars maydis (Nisikado and Miyake) Shoem also called Drechslera maydis, (Nisikado and Miyake) Subram and Jain, is a serious fungal disease of maize throughout the world. The disease causes significant losses both in quality and quantity of the crop. However, its extent of damage varies from area to area and season to season. The pathogen overwinters in the form of mycelium and spores in plant debris and above soil surface (Ullstrup, 1972). Under favourable environmental conditions, the spores germinate and attack the newly emerging plants. The developing mycelium of the pathogen invades the parenchymatous tissues of leaf and causes browning and collapse of the infected cells, resulting in the loss of chlorophylic area and subsequent reduction in the rate of photosynthesis. The characteristic angular tan lesions will appear on maize leaves. Yield and vigor of the plant is reduced alarmingly if more leaf area is killed. Starch formation in kernels is also affected which results in small chaffy kernels (Payak and Renfro, 1968). Due to lower nutritional value, the leaves are also not suitable for fodder (Harlapur, 2012).
In view of the losses caused by this disease, it is important to develop a proper disease control strategy. Cultural and chemical control practices are admittedly not very effective and the disease remains a persistent problem. Arguably, the best and efficient means to control the disease is the use of resistant germplasm (Ali and Shabeer, 1992). It is the most practical, economical and environment friendly way to reduce the losses caused by this disease. Hence, evaluation of maize germplasm is essential to find out the putative sources of resistance (Williams and Hallauer, 2000). In the present study an attempt was made to find out sources of resistance in available maize germplasm against SCLB.
Materials and Methods
An experiment was conducted at Cereal Crops Research Institute (CCRI), Pirsabak, Nowshera (34oN Latitude, 72oE Longitude and 288m Altitude) KP, Pakistan for two consecutive years during kharif season of 2015 and 2016 to screen 36 maize genotypes (Table 1) against SCLB under artificial epiphytotic conditions. The experiment was laid out using Randomized Complete Block Design (RCBD) with three replications. Maize seeds were planted in each row with a length of 3m, having 25cm plant to plant distance while the row to row distance was maintained as 75cm. A local susceptible check variety was planted around the whole experimental unit in two rows to provide continuous and uniform source of inoculum throughout the course of experiment. Standard cultural practices including irrigation, fertilizer application, hoeing and thinning was carried out throughout the growing season.
Inoculum was prepared by immersing 50mm discs of the pure culture of the pathogen in 150 ml sterilized distilled water, filtering the suspension in double layered muslin cloth and determining the spore concentration with the help of a haemocytometer. Finally, spore concentration was adjusted up to 2x104 spores’ ml-1. Plants at 4-6 leaf stage were inoculated with the help of Ultra Low Volume (ULV) sprayer. Inoculation was performed during evening when optimum temperature and humidity was appropriate for successful infection. Two successive inoculations at three weeks interval were performed for successful infection.
Data were recorded on Disease Severity (DS) and Grain Yield (GY). Disease severity was recorded according to Sharma (1983). According to this scale 0; represents no disease, 1) refers to few lesions on lower leaves; 2) shows moderate lesions on lower leaves only; 3) exhibits increased amount of lesion on lower and moderate on middle leaves; 4) abundant lesion on lower and middle leaves exceeding to the upper leaves; 5) all leaves are severely infected and plant is nearly dead. Maize genotypes with disease severity in the range of 2.1-3.0 were scored as Moderately
Table 1: List of maize genotypes screened against SCLB during Kharif 2015 and 2016.
|
Entry No |
Genotypes |
Type |
Detail |
|
1 |
WL-1 |
White inbred line |
Derived from a cross between Azam and Pahari OPVs |
|
2 |
WL-2 |
White inbred line |
Derived from Sarhad White OPV |
|
3 |
WL-3 |
White inbred line |
Derived from breeding population (PSEV-3) of Jalal OPV |
|
4 |
WL-4 |
White inbred line |
Derived from breeding population (PSEV-3) of Jalal OPV |
|
5 |
WL-5 |
White inbred line |
Derived from breeding population (PSEV-3) of Jalal OPV |
|
6 |
WL-6 |
White inbred line |
One of the parental inbred lines from Babar hybrid |
|
7 |
YL-1 |
Yellow inbred line |
Derived from Sarhad yellow OPV |
|
8 |
YL-2 |
Yellow inbred line |
Derived from Ghauri hybrid |
|
9 |
YL-3 |
Yellow inbred line |
Derived from Sarhad yellow OPV |
|
10 |
EHW-1 |
White hybrid |
Experimental single cross hybrid |
|
11 |
EHW-2 |
White hybrid |
Experimental single cross hybrid |
|
12 |
EHW-3 |
White hybrid |
Experimental single cross hybrid |
|
13 |
EHW-4 |
White hybrid |
Experimental single cross hybrid |
|
14 |
EHW-5 |
White hybrid |
Experimental single cross hybrid |
|
15 |
CS-220 |
White hybrid |
Commercial single cross hybrid of Petal seed Company, Mardan |
|
16 |
EHY-1 |
Yellow hybrid |
Experimental single cross hybrid |
|
17 |
EHY-2 |
Yellow hybrid |
Experimental single cross hybrid |
|
18 |
Babar |
White hybrid |
Commercial double cross hybrid of CCRI |
|
19 |
CS-240 |
White hybrid |
Commercial single cross hybrid of Petal seed Company, Mardan |
|
20 |
EHW-6 |
White hybrid |
Commercial single cross hybrid of Potential seed Company, Malakand |
|
21 |
P-30K08 |
White hybrid |
Commercial single cross hybrid of Pioneer seed Company |
|
22 |
CMY-107 |
Yellow hybrid |
Commercial single cross hybrid of Carpel seed Company |
|
23 |
CS-2Y10 |
White hybrid |
Commercial single cross hybrid of AB seed Company, Kohat |
|
24 |
EVW-1 |
White population |
Experimental variety derived from cross between inbred and pop.2007 |
|
25 |
EVW-2 |
White population |
Experimental variety derived from cross between pop.1325 and pop.2007 |
|
26 |
EVW-3 |
White population |
Experimental variety derived from cross between inbred and OPV Jalal |
|
27 |
EVW-4 |
White population |
Experimental variety derived from cross between inbred and pop.2011 |
|
28 |
EVW-5 |
White population |
Experimental variety derived from cross between pop.1325 and OPV Jalal |
|
29 |
EVW-6 |
White population |
Experimental variety derived from cross between Shaheen and Pahari OPVs |
|
30 |
EVW-7 |
White population |
Experimental variety derived from cross between inbred and Sarhad(W) opv |
|
31 |
Jalal |
White variety |
Approved commercial OPV of CCRI presently in cultivation |
|
32 |
Iqbal |
White variety |
Approved commercial OPV of CCRI presently in cultivation |
|
33 |
Azam |
White variety |
Approved commercial OPV of CCRI presently in cultivation |
|
34 |
Pahari |
White variety |
Approved commercial OPV of CCRI presently in cultivation |
|
35 |
EVW-8 |
White variety |
Approved commercial OPV of CCRI not cultivated on large scale |
|
36 |
EVY-1 |
Yellow variety |
Approved commercial OPV of CCRI not cultivated on large scale |
Resistant (MR), 3.1-3.5 as Moderately Susceptible (MS) and above 3.5 as Susceptible (S) according to Chandrashekara et al. (2014).
Grain yield (tons per hectare) was calculated by using the following formula.
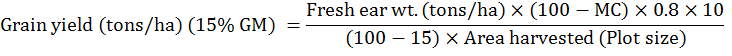
Where;
GM: Grain Moisture; MC: Moisture content (%) in grains at harvest; 0.8: Shelling Coefficient; Plot size: 3.75m2.
Analysis of individual and two years pooled data was performed using generalized analysis of variance RCBD with Genstat (12th Ed) using 5% level of significance. For comparing means, Fisher’s protected least significant difference test was applied wherever significant differences were observed among means. Agglomerative hierarchical cluster analysis using social statistical package SPSS was performed based on pooled DS and GY data to construct clusters of related genotypes.
Results and Discussion
Thirty-six maize genotypes were evaluated against SCLB under artificial epiphytotic conditions during kharif season 2015 and 2016. Disease severity (DS) data for individual years was non-significant. However, significant differences (p=0.03) were recorded amongst the genotypes when data were pooled over two years (Table 2). Two years pooled averages of DS were in the range of 2.7-4.0. Minimum DS (2.7) was observed for genotype P-30K08 followed by WL-1, EHW-3 and EVW-6 (2.9). Conversely, maximum (4.0) DS was noticed for genotype WL-5 followed WL-4 and YL-3 (3.9). Of the 36 genotypes, 14 including WL-1, WL-6, EHW-3, EHW-4, EHW-5, EHY-2, CS-240, P-30K08, CS2Y10, EVW-2, EVW-4, EVW-6, EVY-1 and Jalal exhibited disease severity score in the range of 2.1- 3.0 and were accordingly classified as Moderately Resistant (MR). Other fourteen genotypes namely WL-2, YL-1, EHW-2, CS-220, EHY-1, Babar, EHW-6, CMY107, EVW-1, EVW-3, EVW-5, Iqbal, Pahari and EVW-8 were categorized as Moderately Susceptible (MS) since their disease severity means of the two years fell in the range of 3.1-3.5. The remaining eight genotypes including WL-3, WL-4, WL-5, YL-2, YL-3, EHW-1, EVW-7 and Azam were grouped as highly susceptible (HS) with DS value greater than 3.5 (Table 3).
Significant differences (p= 0.01) were recorded for grain yield (GY) among all tested genotypes during kharif season 2015 and 2016, respectively (Table 2). During kharif 2015, the highest yield was recorded for moderately resistant genotypes such as CS-2Y10 (6.254 tons/ha) and P-30K08 (6.015 tons/ha). Genotypes YL-3 and WL-5, being highly susceptible, produced the lowest yield of 2.569 tons/ha and 2.764 tons/ha, respectively. During kharif 2016, the susceptible genotypes WL-2 and WL-3 produced, the lowest grain yield of 1.320 tons/ha and 1.615 tons/ha, respectively. However, the highest grain yield was recorded for moderately resistant genotypes EVY-1 (5.141 tons/ha), EHW-4 (4.574 tons/ha), EVW-8 (4.554) and EHY-2 (4.511 tons/ha).
Table 2: Disease Severity (DS) and Grain Yield (GY) of 36 maize genotypes under artificial epiphytotic of southern corn leaf blight during kharif 2015 and 2016.
|
S. No |
Genotypes |
DS |
GY (tons/ha) |
|||||
|
2015 |
2016 |
Poo-led |
Reaction Type |
2015 |
2016 |
Pooled |
||
|
1 |
WL-1 |
3.0 |
2.8 |
2.9 |
MR |
4.867 |
2.830 |
3.849 |
|
2 |
WL-2 |
3.2 |
3.3 |
3.3 |
MS |
4.192 |
1.320 |
2.756 |
|
3 |
WL-3 |
3.5 |
3.7 |
3.6 |
HS |
5.402 |
1.615 |
3.509 |
|
4 |
WL-4 |
4.0 |
3.8 |
3.9 |
HS |
2.921 |
1.650 |
2.286 |
|
5 |
WL-5 |
4.0 |
4.0 |
4.0 |
HS |
2.764 |
1.982 |
2.373 |
|
6 |
WL-6 |
3.3 |
2.7 |
3.0 |
MR |
3.532 |
1.800 |
2.666 |
|
7 |
YL-1 |
3.5 |
3.5 |
3.5 |
MS |
3.512 |
2.154 |
2.833 |
|
8 |
YL-2 |
3.7 |
3.8 |
3.8 |
HS |
5.055 |
2.288 |
3.672 |
|
9 |
YL-3 |
4.0 |
3.8 |
3.9 |
HS |
2.569 |
3.493 |
3.031 |
|
10 |
EHW-1 |
4.0 |
3.7 |
3.8 |
HS |
3.683 |
4.266 |
3.975 |
|
11 |
EHW-2 |
3.3 |
3.2 |
3.3 |
MS |
4.294 |
4.215 |
4.254 |
|
12 |
EHW-3 |
3.0 |
2.8 |
2.9 |
MR |
3.321 |
3.923 |
3.622 |
|
13 |
EHW-4 |
3.2 |
2.8 |
3.0 |
MR |
3.887 |
4.574 |
4.230 |
|
14 |
EHW-5 |
2.8 |
3.2 |
3.0 |
MR |
5.901 |
3.063 |
4.482 |
|
15 |
CS-220 |
3.0 |
3.2 |
3.1 |
MS |
5.206 |
3.931 |
4.568 |
|
16 |
EHY-1 |
3.0 |
3.3 |
3.2 |
MS |
4.242 |
3.085 |
3.663 |
|
17 |
EHY-2 |
3.2 |
2.8 |
3.0 |
MR |
4.705 |
4.511 |
4.608 |
|
18 |
Babar |
3.0 |
3.3 |
3.2 |
MS |
4.749 |
3.124 |
3.936 |
|
19 |
CS-240 |
3.2 |
2.8 |
3.0 |
MR |
3.136 |
3.739 |
3.437 |
|
20 |
EHW-6 |
2.7 |
3.7 |
3.2 |
MS |
4.250 |
3.959 |
4.104 |
|
21 |
P-30K08 |
2.7 |
2.7 |
2.7 |
MR |
6.015 |
3.168 |
4.592 |
|
22 |
CMY-107 |
2.8 |
3.5 |
3.2 |
MS |
4.909 |
3.473 |
4.191 |
|
23 |
CS-2Y10 |
2.5 |
3.5 |
3.0 |
MR |
6.254 |
3.759 |
5.007 |
|
24 |
EVW-1 |
3.3 |
3.0 |
3.15 |
MS |
3.639 |
4.343 |
3.991 |
|
25 |
EVW-2 |
3.0 |
2.7 |
3.0 |
MR |
3.856 |
4.112 |
3.984 |
|
26 |
EVW-3 |
3.3 |
3.0 |
3.15 |
MS |
2.830 |
3.496 |
3.163 |
|
27 |
EVW-4 |
3.0 |
2.7 |
3.0 |
MR |
3.786 |
4.192 |
3.989 |
|
28 |
EVW-5 |
3.5 |
3 |
3.25 |
MS |
4.096 |
4.818 |
4.457 |
|
29 |
EVW-6 |
2.8 |
3.0 |
2.9 |
MR |
4.054 |
4.35 |
4.202 |
|
30 |
EVW-7 |
3.7 |
3.5 |
3.6 |
HS |
3.425 |
3.999 |
3.712 |
|
31 |
Jalal |
2.7 |
3.3 |
3.0 |
MR |
3.895 |
4.191 |
4.043 |
|
32 |
Iqbal |
3.2 |
3.0 |
3.1 |
MS |
2.984 |
3.408 |
3.196 |
|
33 |
Azam |
3.7 |
3.5 |
3.6 |
HS |
3.307 |
3.713 |
3.510 |
|
34 |
Pahari |
3.3 |
3 |
3.15 |
MS |
2.819 |
3.001 |
2.910 |
|
35 |
EVW-8 |
3.5 |
3.3 |
3.4 |
MS |
4.316 |
4.554 |
4.435 |
|
36 |
EVY-1 |
3.3 |
2.7 |
3.0 |
MR |
4.549 |
5.141 |
4.845 |
|
CV % |
10.4 |
5.9 |
8 |
11.8 |
2.5 |
7.1 |
||
|
Standard error |
0.3386 |
0.19 |
0.26 |
0.4713 |
0.0894 |
0.2675 |
||
|
LSD (0.05) |
Ns |
Ns |
0.75 |
1.9846 |
0.4509 |
1.0366 |
||
Table 3: Evaluation of maize genotypes based on their response to southern corn leaf blight.
|
Reaction |
Disease Severity |
Genotypes |
|
|
Resistant |
R |
≤ 2 |
|
|
Moderately Resistant |
MR |
2.1-3.0 |
WL-1, WL-6, EHW-3, EHW-4, EHW-5, EHY-2, CS-240, P-30K08, CS2Y10, EVW-2, EVW-4, EVW-6, EVY-1, Jalal |
|
Moderately Susceptible |
MS |
3.1-3.5 |
WL-2, YL-1, EHW-2, CSA-220, EHY-1, Babar, EHW-6, CMY107, EVW-1, EVW-3, EVW-5, Iqbal, Pahari, EVW-8 |
|
Highly Susceptible |
HS |
>3.5 |
WL-3, WL-4, WL-5, YL-2, YL-3, EHW-1, EVW-7, Azam |
The pooled grain yield data over two years of all genotypes were also significantly different (p<0.001) under disease epiphytotic (Table 2). The highest yield of 5.007 tons/ha was given by genotype CS-2Y10 followed by EVY-1 and EHY-2 with GY of 4.845 tons/ha and 4.608 tons/ha, respectively. Conversely, the lowest yield was observed for the genotypes WL-4 and WL-5 giving 2.286 tons /ha and 2.373 tons /ha, respectively. These low yielding genotypes were highly susceptible to the disease. In general, grain yield and disease severity ratings of the genotypes were inversely related. Grain yield decreased with increase in disease severity among all tested genotypes (Figure 1).
Agglomerative hierarchical cluster analysis categorized the 36 maize genotypes into two major clusters based on Grain Yield (GY) and Disease severity (DS) data (Figure 2). Cluster 1 is further divided into three sub clusters. Sub cluster I includes 14 genotypes namely EVW-2, EVW-4, Jalal, WL-1, EHW-4, EVW-6, EHW-6, CMY-107, EHW-2, Babar, EVW-1, EHW-3, CS-240 and EHY-1. Most of the genotypes included in this sub cluster are moderately resistant while the remaining genotypes are moderately susceptible with medium to high yielding potential. Sub cluster II includes five genotypes such as WL-3, Azam, YL-2, EVW-7 and EHW-1. All genotypes in this sub cluster are highly susceptible with low yield potential. Sub cluster III has eight genotypes including CS-2Y10, EVY-1, EVW-5, EVW-8, EHW-5, CS-220, EHY-2 and P30K08. Two candidates in this sub cluster are moderately resistant while the others are moderately susceptible. However, genotypes of this sub cluster are high yielding. Genotype CS-2Y10, being moderately resistant, is also the highest yielding genotype.
Cluster II includes nine genotypes namely, EVW-3, Iqbal, WL-2, Pahari, YL-1, WL-6, WL-4, WL-5 and YL-3. Genotypes included in this cluster are moderate to highly susceptible. All genotypes in this cluster are however low yielding.
Differences in the genetic make-up of the genotypes were evident in the present study. Hallauer and Williams (2000) and Kraja et al. (2000) attributed the differences in disease severity to diversity in genetic make-up of the maize genotypes. In our study, complete resistance was not found in any of the genotypes; however, a moderately resistant response was exhibited by a group of fourteen genotypes. Moderate levels of resistance have previously been reported in maize by Wang et al. (2014) and Rijal et al. (2017). The highest grain yield was recorded for the genotypes with moderately resistant response to the disease. Similarly, highly susceptible genotypes produced minimum grain yield. Sharma and Rai (2000) and Shivankar and Shivankar (2000) also found direct association between losses in grain yield and levels of susceptibility in a genotype. A decrease in grain yield in susceptible genotypes is apparently due to the destruction of photosynthetic area (Kim et al., 1974), since soon after penetration in the chlorenchyma tissue of the plant, the hyphae cause destruction of the chloroplast. Reduction in grain yield due to the disease is also reported previously (Shah et al., 2006; Chandrashekara et al., 2014; Mubeen et al. (2017)). The results emphasize the role of resistance gene in disease control and improved yield.
Agglomerative hierarchical cluster analysis proved useful for grouping high yielding and disease resistant genotypes. This allows selection of ideal genotypes with desirable resistant genes and grain yield potential (Chandrashekra et al., 2014).
Conclusions and Recommendations
This study has successfully identified various partially resistant genotypes including hybrids, open pollinated varieties and parental lines that can be used as a source of resistance by plant breeders to evolve new varieties. Based on this study the genotypes CS-2Y10, EVY-1 and EHY-2 are recommended for breeding programs as these are moderately resistant and high yielding.
Acknowledgements
The authors acknowledge Higher Education Commission for providing financial support during the completion of the study.
Novelty Statement
Maize genotypes well adapted to the region were investigated and some new sources of disease resistance and high yield potential are documented.
Author’s Contributions
Azra designed and conducted the experiment. Shaukat Hussain provided conceptual frame work and helped in development of the manuscript.
References
Ali, K. and A. Shabeer. 1992. Genotype assay of maize for resistance to Maydis leaf blight under artificial field epiphytotics of Peshawar region. Sarhad J. Agric. 8: 547-549.
Chandrashekara, C., S. Jha, R. Arunkumar and P. Agrawal. 2014. Identification of new sources of resistance to Turcicum Leaf Blight and Maydis Leaf Blight in maize (Zea mays L.), Sabrao J. Bread Gen. 46: 44-55.
FAO. 2013. Food and agriculture organization of the United Nations, http://faostat.fao.org/default.aspx.
GOP. 2014-2015. Ministry of agriculture and livestock, www.mnfsr.gov.pk.
GOP. 2016-2017. Ministry of food, agriculture and livestock Pakistan bureau of statitistics. www.pbs.gov.pk.
Harlapur, S. 2012. Studies on maydis leaf blight of maize caused by Drechslera maydis (Nisikado) Subram and Jain. UAS, Dharwad.
Kim, S., A. Hooker and S. Lim. 1974. Corn seedling root and top growth as affected by three Helminthosporium leaf blights, Plant Dis. Rep. 58(3): 219-225.
Kraja, A., J.W. Dudley and D.G. White. 2000. Identification of tropical and temperate maize populations having favorable alleles for disease resistance, Crop Sci. 40: 948-954. https://doi.org/10.2135/cropsci2000.404948x
Mubeen, S., M. Rafique, M.F.H. Munis and H.J. Chaudhary. 2017. Study of southern corn leaf blight (SCLB) on maize genotypes and its effect on yield. J. S. Soc. Agric. Sci. 16: 210-217. https://doi.org/10.1016/j.jssas.2015.06.006
Payak, M. and B. Renfro. 1968. Combating maize disease. Ind. Far. Dis. 1: 53-58.
Rahman, H., H. Javed and H. Rehman. 1986. Maize diseases and their control, maize production manual. Pak. Agric. Res. Counc. Islamabad.
Ranum, P., J.P. Peña-Rosas and M.N. Garcia-Casal. 2014. Global maize production, utilization, and consumption, Annals of the N. Y. Acad. Sci. 1312: 105-112. https://doi.org/10.1111/nyas.12396
Rijal, T.R., K.B. Koirala and M. Karki. 2017. Evaluation of maize genotypes against southern leaf blight (Bipolaris maydis) during summer seasons at Rampur, Chitwan. Int. J. App. Sci. Biotech. 5: 532-536. https://doi.org/10.3126/ijasbt.v5i4.18778
Shah, S.S., I. Khalil and A. Rafi. 2006. Reaction of two maize synthetics to maydis leaf blight following recurrent selection for grain yield. Sarhad J. Agric. 22: 263-265.
Sharma, R. 1983. Techniques of scoring for resistance to important diseases of maize, All India coordinated maize improvement project. Indian Agric. Res. Inst. New Delhi.
Sharma, R.C. and S.N. Rai. 2000. Assessment and quantitative analysis of losses due to Drechslera maydis in maize. Proc.International Conference on Integrated Plant Dis. Management for sustainable Agriculture. Indian Phytopathol. Soc. Div. Plant Pathol. pp. 246-248.
Shivankar, S.K. and R.S. Shivankar. 2000. Losses in the grain yield of maize due to Helminthosporium turcicum leaf blight disease. Agric. Sci. Dig. 20(3): 201-202.
Singh, M. and S. Kumar, 2016. Broadening the genetic base of grain cereals. Springer. https://doi.org/10.1007/978-81-322-3613-9
Singh, R. and G. Gilbreath. 2002. A real-time information system for multivariate statistical process control, Int. J. Prod. Eco. 75: 161-172. https://doi.org/10.1016/S0925-5273(01)00189-X
Ullstrup, A. 1972. The impacts of the southern corn leaf blight epidemics of 1970-1971. Ann. Rev. Phytopath. 10: 37-50. https://doi.org/10.1146/annurev.py.10.090172.000345
Wang, X., Y. Zhang, X. Xu, H. Li, X. Wu, S. Zhang and X. Li. 2014. Evaluation of maize inbred lines currently used in Chinese breeding programs for resistance to six foliar diseases. Crop J. 2: 213-222. https://doi.org/10.1016/j.cj.2014.04.004
Williams, T. and A. Hallauer. 2000. Genetic diversity among maize hybrids. Maydica. 45. 163-171.
To share on other social networks, click on any share button. What are these?








