Purification and Characterization of an Acidic Polygalacturonase from Grapes and its Potential to Improve Juice Quality
Purification and Characterization of an Acidic Polygalacturonase from Grapes and its Potential to Improve Juice Quality
Zahra Nazir1, Saba Ijaz1, Roquyya Gul2 and Mahjabeen Saleem1,*
1Institute of Biochemistry and Biotechnology, University of the Punjab, Lahore-54590
2Institute of Biological Sciences, Gulab Devi Educational Complex, Lahore 54590
ABSTRACT
Pectinolytic enzymes, widespread in plants, fungi and microorganism, gain commercial importance for improving the yield and nutraceutical properties of juice processing industry, degumming of fibre plants and maximum oil recovery. These enzymes break down complex polysaccharide polymers of plant tissues into simpler monomer like D-galacturonic acids. In this study, polygalacturonase from grape skin was purified by salting out with ammonium sulfate, gel filtration on Sephadex G-75 column and ion exchange on Q-Sepharose column chromatography. Polygalacturonase was recognised as a protein with 47kDa molecular weight by SDS-PAGE. The optimum pH of purified polygalacturonase activity was found to be 4.5 and stable within pH range 3.5-5.5. Temperature dependent studies revealed temperature optimum of enzyme to be 40°C and stable up to 60°C. Among substrates, polygalacturonic acid was established as the best substrate for polygalacturonase showing its specificity in the hydrolysis of polysaccharide galacturonate chain. The presence of Na+1 and K+1 enhanced polygalacturonase activity up to 110% and 130%, respectively when used at a concentration of 1mM, however, the enzyme was almost completely inhibited by Pb+2 and Hg+2. Purified polygalacturonase showed pectinolytic effect in juice clarification by attaining maximum clarity (95% transmittance) after incubation at 50°C for 60 min. Total amount of phenolic and free radical scavenging activity of juice was improved after clarification. To understand the effect of juice clarification parameters, response plots based on surface response methodology were developed that showed significant impact of independent variables like enzyme concentration, temperature and incubation time on the dependent variables and improved the overall quality of apple juice. Hence, acidic plant polygalacturonase isolated in this study would be useful as a potential candidate for its biotechnological applications in fruit juice clarification.
Article Information
Received 19 December 2018
Revised 20 January 2019
Accepted 02 February 2019
Available online 06 May 2019
Authors’ Contribution
MS designed the research project and wrote the manuscript. ZN and SI did the research work and RG helped in statistical analysis.
Key words
Polygalacturonase, Grapes, Purification, Characterization, Juice clarification.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.4.1387.1402
* Corresponding author: mahjabeensaleem1@hotmail.com
0030-9923/2019/0004-1387 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Propensity for fruit and vegetable juice consumption has been raised in modern age and is predicted to increase in future due to improved mode of life and profound health awareness
(Hyson, 2015). It is now almost practically impossible to achieve quality juice production without the profound use of food processing enzymes in food industries. Pectinolytic enzymes are in practice since olden times but on commercial level, it was first used in wine and fruit juice preparation in 1930 (Tapre and Jain, 2014). Pectinolytic enzymes are routinely used for smooth juice extraction through reduction in viscosity and water binding capacity of pectin (Nakkeeran et al., 2011; Rehman et al., 2013). Enzymatic liquefaction of fruit pulp offer advantages in improvement of soluble components, juice yield, colour, aroma, phenolic components and reduced cloudiness (Buchert et al., 2005).
Microorganisms and plants are the main sources of pectinolytic enzymes. These enzymes are used to breakdown pectin polymer. The degradation of plant cell wall structures require a large number of enzymes with different specificity and are divided into groups according to their substrates i.e. cellulases, hemicellulases, pectinases and lignases etc. Pectinolytic enzymes belong to huge group of enzymes which help in pectin transformation and degradation and generally classified according to their site of cleavage. Endopolygalacturonase (EC 3.2.1.15) disrupts glycosidic bond within the pectin chain while exopolygalacturonase (EC 3.2.1.67) cleave glycosidic bond at the end of the pectin chain resulting in hydrolysis of pectin chain. Endopolygalacturonase, exopolygalacturonase, pectin or pectate lyase, rhamnogalacturonan hydrolase and lyase, pectin methyl esterase, acetyl esterase and rhamnogalacturonan acetyl esterase act on the main chain of pectin. Enzymes involved in cleavage of hairy region of pectin are arabinofuranosidases, endoarabinase, β-galactosidase, endogalactanase and feruloyl esterase. Rhamnogalacturonase acts on rhamnogalacturonic segment and polygalacturonase acts on galacturonic acid. Polygalacturonase is probably the most studied pectin degrading enzyme (Sakai et al., 1993). Acidic polygalacturonases are mainly used in juice and wine making while alkaline polygalacturonases are used in degumming of plant fibre, paper making, pectic waste water, oil recovery and coffee fermentation (Kashyap et al., 2001).
Polygalacturonase has been isolated from different plants like tomato (Ali and Brady, 1982), strawberry (Nogata et al., 1993), Zimbabwean wild fruits (Muchuweti et al., 2004), Jamaica cherry (Gayathri et al., 2007), mango (Prasanna et al., 2006), ripened banana (Gayathri and Nair, 2015), Solanum macrocarpum L. (Chinedu et al., 2017) and many other fruits.
One of the most popular summer fruits are grapes which belongs to warm and temperate zone. Vitis genus of grapes consists of almost sixty species, though, the cultivated grape are derived from prime species “Vitis vinefera”. Dessert grapes, wine grapes and raisin grapes are three comprehensive divisions of grapes. Grapes are usually consumed as table grapes, juices, wines, raisins, jams, jellies and frozen products. In Pakistan, mostly European grapes are cultivated. Balochistan and KPK provinces are growing 70% of total grapes with annual production of 122 thousand tons. The average of existing yield is 19 tons ha-1 while the possible yield can be 25 tons ha-1 (Khan et al., 2008; Kausar et al., 2017).
Polygalacturonase have been employed for effective clarification in various juice preparations like pear, guava, banana, papaya, carrot, beet (Soares et al., 2001), pine apple juice (Tochi et al., 2009), fresh apple juice (Ajayi et al., 2011) and lemon juice (Maktouf et al., 2014). In the beverage industry, enzymatic juice extraction process followed by clarification and membrane separation is considered as an innovative step for concentration of juice. In enzyme treated juice clarification, pectin breakdown is greatly influenced by enzyme concentration, incubation time and temperature (Rai et al., 2004; Sin et al., 2006).
The aim of current trial is to purify and characterize polygalacturonase from grapes skin and to optimize the PG concentration, incubation time and temperature for apple juice clarification and further characterization of PG treated and untreated juice using response surface methodology (RSM).
Materials and methods
Protein extraction
Polygalacturonase enzyme was extracted from grapes skin by slightly modified method (Singh and Dwivedi, 2008). A total of 100 grams of fresh grapes (Vitis vinifera) were collected from the local market. To achieve powder form, the skin of grapes was peeled off and crushed along with liquid nitrogen in sterilized pestle and mortar. 30% homogenate of grapes powder was prepared in extraction buffer (50mM sodium acetate buffer pH 4.5, 20mM EDTA, 20mM cysteine, 0.1% β-mercaptoethanol and 0.5% triton X-100) and incubated at 4°C for 30 min. Polyvinylpolypyrrolidone was added before homogenization. Mixture was centrifuged at 10,000 rpm (SiGMA® Laborzentrifugen) for 10 minutes at 4°C. Supernatant obtained after passing through the filter paper was utilized as a source of enzyme for further investigation.
Assay of polygalacturonase (PG) activity
PG activity was examined by DNS method described by Miller (1959) using D-galacturonic acid as a standard. 0.5g polygalacturonic acid (PGA) comprising 0.02% sodium azide was soaked in 50mM sodium acetate buffer (pH 4.5) for two hours. Reaction mixture having 0.5ml suitably diluted enzyme and 0.5ml PGA in a test tube was incubated at 40°C for 30 min and then centrifuged at 3000 rpm for 10 min. After addition of DNS reagent in subsequent supernatant, the mixture was heated in boiling water bath for 5 min and cooled till room temperature. Reducing sugars liberated in the reaction mixture were determined at 540nm by using the UV/visible light spectrophotometer (UV-2450; Shimadzu). Polygalacturonase activity (PA) was calculated by using the following formula.
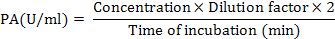
One unit of polygalacturonase activity (U) was defined as the amount of enzyme that catalyzes the release of 1µmol of D-galacturonic acid per minute under standard assay conditions.
PG diffusion assay
For determining the PG activity, staining method was used in which wells were prepared on PGA agar plate (0.5% polygalacturonic acid, 4% agar in 50mM sodium acetate buffer pH 4.5). Samples having different PG activities (0.1-0.3U) were introduced in respective wells and sodium acetate buffer loaded as control. The plate was kept at 37°C for 2 h. Staining of substrate agar plate was done with 0.02% congo red for 15 min. Zones of PG activity were visualized after destaining with 1M NaCl.
Protein estimation
The protein contents in crude extract as well as in purified preparation were determined by Bradford method (Bradford, 1976) with bovine serum albumin (Sigma Chemicals Co., USA) as a standard.
Purification of PG
Ammonium sulphate precipitation
The crude PG extract was obtained after 85% ammonium sulphate saturation. Protein extract was centrifuged at 8,000 rpm for 15 min. The pellet obtained was dissolved in 50mM sodium acetate buffer of pH 4.5 and kept at 4°C. PG activity and protein concentration of the extract was measured according to previously described methods.
Gel Filtration chromatography
Dialysis was carried out to remove the salt from precipitated protein sample. The dialyzed sample was lyophilized until the solution was dry like powder. The powder was dissolved in 50mM sodium acetate buffer (pH 4.5). 1ml of lyophilized sample was loaded on a Sephadex G-75 (Pharmacia Fine Chemicals, Sweden) column (0.25x30cm) which was pre-equilibrated with 50mM sodium acetate buffer of pH 4.5. 1ml of the lyophilized sample was loaded on gel filtration column having the flow rate of 1ml/min. The protein was eluted by using the same buffer and 60 fractions (1ml each) were collected. Polygalacturonase activity and protein concentration in all fractions were determined.
Q-Sepharose chromatography
Fractions having PG activity were combined and filtered in an amicon ultrafiltration cell fixed with 10,000 MW cutoff membrane (Millipore Co., USA) under nitrogen pressure at 4°C. The concentrated enzyme sample was purified on Q-Sepharose (Sigma Chemicals Co.) column with dimensions of 1.6 x 10 cm which was equilibrated with Tris-HCl buffer of pH 8.5 under natural flow rate. The proteins were eluted with the same buffer containing a linear gradient of 0 to 1.5M NaCl. Protein concentration and PG activity of fractions obtained (1ml) was determined.
Polyacrylamide gel electrophoresis
Molecular mass determination of purified polygalacturonase was done by measuring the relative mobility of proteins in 12% SDS-PAGE (Bio-Rad) along with standard protein size marker (Invitrogen) as per method illustrated by Laemmali (1970). After electrophoresis, SDS gel was stained with a solution containing 0.1% Coomassie Brilliant Blue R-250 (Sigma), 10% glacial acetic acid and 30% methanol. The gel was destained in destaining solution comprising 10% glacial acetic acid and 30% methanol in distilled water. The molecular weight of purified polygalacturonase was determined by comparing with protein ladder (Invitrogen).
Zymography for PG activity
To identify the PG activity on the gel directly, modified method described for cellulase enzymes was used (Beguin, 1983). Under native condition, 15% polyacrylamidе gel еlеctrophorеsis was performed. SDS was omitted and 0.3% polygalacturonic acid solution as substrate for polygalacturonase was incorporated in separating gel. After electrophoresis, staining of native gel with 0.02% Congo red dye for 15 min and destaining with 1M NaCl solution was done. The gel was treated with acetic acid to improve the contrast.
Characterization of purified PG
Effect of pH
The influence of pH on purified PG activity was noted by keeping pH of reaction mixtures using 1% PGA as substrate. Sodium acetate buffer with pH range of 2.5-5.0 and sodium phosphate buffer with pH range 5.5-7.0 were used.
pH stability of PG was determined by incubating purified enzyme without substrate in buffer solutions of pH varying from 2.5-7.0 at room temperature for 1 h. The residual enzyme activity was measured under standard conditions.
Effect of temperature
The optimum temperature of purified PG was determined by incubating a known amount of PG activity at various temperatures ranging 10-700C with 1% PGA as substrate prepared in optimized buffer.
The thermal stability of the PG was assessed by incubating it various temperatures (10-70°C) for 1 h prior to the substrate addition. The residual enzyme activity was then measured under standard conditions of pH and temperature.
Substrate specificity
The substrate specificity of PG was determined after incubating it with different concentration (0.5-1.5%) of polygalacturonic acid (PGA), pectin, xylan, cellulose and galactose as substrate. The relative activity of each substrate was measured by taking PGA as a control.
Effect of metal ions
The enzyme was incubated at room temperature with 1mM concentration of different metal ions (Na+1, K+1, Ca+2, Mn+2, Cu+2, Mg+2, Fe+3 Pb+1 and Hg+2). Then, the enzyme activity was determined as mentioned above.
Clarification of apple juice
Unripe apples were picked, cut in cubes and meshed in blender and pressed by twofold of cheese cloth to obtain raw and unclarified juice. Unclarified juice was pasteurized and temperature was immediately brought to 40°C. Apple juice in the absence of purified PG was considered as control and incubated under same condition as that of sample. Purified PG (2.5U/ml and 5.0U/ml) was added to 1ml apple juice (pH 4.0) and reaction was incubated at 40 and 50°C for various time periods (30 and 60 min) in shaking water bath. The reaction was stopped by keeping reaction mixture in boiling water bath for 5 min and centrifuged at 3,000 rpm for 10 min. The supernatant was analyzed for various physic-chemical parameters as such as clarity (% transmittance) at 660nm, color at 440nm and percentage reduction in viscosity. The juice viscosity was measured by Ostwald viscometer. Total soluble solids (°Brix) were measured by refactometer. Turbidity of juice was determined with turbidity meter and results were described in nephelometric turbidity units (NTU). Total phenolic content of apple juice was estimated according to previously reported method (Emmons and Peterson, 2001). The amount of phenolic content was measured as gallic acid equivalent from standard graph of gallic acid and expressed in mg/ml gallic acid equivalent. DPPH method was used to determine the free radical scavenging activity of apple juice (Brand-Williams et al., 1995). 0.1mM DPPH solution in methanol was prepared. 50μl of PG treated juice was mixed with 1.5 ml of DPPH solution in covered test tube for the determination of total scavenging activity. Under optimized conditions of temperature and time, the absorbance was checked at 515 nm. The DPPH scavenging activity of apple juice was determined from the following formula:
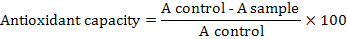
After juice clarification, the effect of three independent variables such as enzyme concentration, temperature and incubation time on dependent variables like yield, viscosity, turbidity, clarity, total phenolic content, total soluble solid and DPPH antioxidant activity was examined by response surface methodology (RSM). Analysis of variance (ANOVA) based on response surface methodology was considered to validate the model and plots were obtained by keeping one variable constant and changing the other two variables within experimental range. With the help of box plots, cumulative effect on the response variables was established.
Results and Discussion
Pectinolytic enzymes, abundant in microorganism and plants, have about 25% share of international sale and purchase of food enzymes. Polygalacturonase is extensively studied pectinolytic enzymes and has significant importance in the biotechnological applications (Jayani et al., 2005). Enzyme treatment in industrial applications is ecofriendly, robust, conserves energy and regarded as safe. Enzymes are highly productive as compared to industrial chemicals (Urlaub, 2002).
Instead of microbial enzymes, plant source enzymes are preferably used in food and pharmaceutical industries for multiple reasons (Jach et al., 2010). Unfortunately, plants have not been actively investigated as source of commercial enzymes except very few enzymes like malt amylase, papain and bromelain extracted from barley malt, papaya latex and pineapple, respectively (de Roos et al., 2007).
PG extraction and diffusion Assay
PG enzyme was extracted from grapes skin. Crude extract was suitably diluted and analyzed for PG enzyme activity and total protein content. An agar plate diffusion assay was used to assess the PG activity forming distinctive haloes of PGA hydrolysis (Fig. 1). As a result of diffusion through gel, PG helps to breakdown PGA and producing a light halo around the well, since the Congo red dye reacts only to unhydrolyzed substrate. This PGA plate clearing approach appears to be semi-quantitative as the diameter of hydrolysis area is directly proportional to the amount of enzyme used in the wells.
Purification of PG
Enzyme purification helps to set free the required enzyme from the cluster of proteins present in the crude extract. During purification, the purity of the enzyme is usually checked by increase in its specific activity. Purified enzyme helps to learn about its catalytic activities and its behavior towards regulators that can alter its activity (Kornberg, 2009). Moreover, kinetic and product inhibition studies are conducted with purified enzymes.
Brief description of PG purification is illustrated in Table I. After dialysis and lyophilization, sample was precipitated by ammonium sulphate (85%) and loaded on Sephadex G-75 that was pre-equiliberated with 50mM sodium acetate buffer (pH 4.5). PG activity appeared as single peak as depicted in Figure 2. All the fractions having activity were combined and purified further by Q-Sepharose chromatography. Proteins bound to column were eluted by linear gradient of NaCl (0-1.5M) in Tris-HCl buffer (pH 8.5) as shown in Figure 3. Purified PG enzyme had activity of 128.57 U/mg whereas percentage recovery was 25 and 15.0 times purification fold.
Many researchers have reported multi-step procedure for purification of PG from various sources. PG was purified from ripened banana with specific activity of 79.95U/mg (Gayathri and Niar, 2014) and mango pulp with 34.36U/mg specific activity, 13.6 percent recovery and 19.4 times purification fold (Prasanna et al., 2006) by two chromatographic steps while Gayathri et al. (2007) purified PG from fruit tissues of Jamaica cherry (Muntingia calabura) with 292.9 U/mg specific activity, 7.8 percent recovery and 511.2 times purification fold by using three chromatographic steps. Interestingly, Chinedu et al. (2017) have reported the purification of PG from S. macrocarpum with specific activity 10211.47 U/mg and percentage recovery of 17.85 and 108.3 times purification fold by employing only single chromatographic step.
Properties of purified PG
Molecular weight determination
The molecular weight of purified PG on 12% SDS-PAGE was evaluated as 47 kDa shown in Figure 4A. Under native conditions, PG activity was further confirmed by 15% (w/v) polyacrylamide gel having 0.3% PGA followed by staining with Congo red. Enzyme activity was appeared as prominent band with red background of gel as presented in Figure 4B. Nearly similar molecular mass have been reported in avocado PG isoforms (46 and 48kDa) (Wakabayashi and Huber, 2001) and in mango pulp PG isoforms (45 and 51 kDa) (Prasanna et al., 2006). However, numerous investigators have reported diverse molecular weight of plant polygalacturonase contrasting to our study like tomato (115kDa) by Ali and Brady (1982), papayas (164 kDa and 34 kDa) by Chan and Tam (1982), banana (120, 105 and 65kDa) by Singh and Dwivedi (2008).
Table I.- Purification scheme of polygalacturonase from grape skin.
|
Purification steps |
Total activity (U) |
Total protein (mg) |
Specific activity (U/mg) |
Purification fold |
Yield (%) |
|
Crude extract |
360 |
42 |
8.57 |
1.0 |
100 |
|
Ammonium sulphate precipitation |
301 |
30 |
10.03 |
1.17 |
83.16 |
|
Gel filtration chromatography |
200 |
5 |
40 |
4.66 |
55 |
|
Ion exchange chromatography |
90 |
0.7 |
128.57 |
15.0 |
25 |
Effect of pH on activity and stability of purified PG
Tertiary and quaternary structures of enzymes are greatly affected by pH. Enzymes possess both acidic and basic amino acids due to their amphoteric nature. Keeping in view the dissociation constants and pH of surrounding, these charged amino acids have profound influence on overall peripheral charge distribution, thus greatly manipulating the enzyme activity, stability and its solubility (Chaplin and Bucke, 1990).
Purified PG in current study was found to be optimally active at pH 4.5 as appeared in Figure 5. Enzyme exhibited its full activity at pH 4.5 while approximately 55% at pH 6.5. However, further increase in pH reduced the enzyme activity. The optimum pH of purified PG enzyme was similar to the pH optima reported in pears, pH 4.5 (Pressey and Avants, 1976) and papaya, pH 4.6 (Chan and Tam, 1982). According to Chun and Huber (1998), polygalacturonases, usually have optimum pH from 4 to 4.5 However, polygalacturonase reported from various fruits had optimum pH ranging 3.0-6.0; S. macrocarpum had optimum pH 3.0 (Chinedu et al., 2017), ripened banana and mango pulp, pH 3.5 (Gayathri and Niar, 2014; Prasanna et al., 2006), Jamaica cherry and peach, pH 4.0 (Gayathri et al., 2007; Pressey and Avants, 1973), mango PG1 had pH 5.0 (Singh and Dwivedi, 2008), strawberry, pH 5.5 (Nogata et al., 1993) and PGII and PGIII isoforms of mango had pH 6.0 (Singh and Dwivedi, 2008).
Since purified PG in our study showed activity in acidic region, therefore it could possibly be employed in clarification of fruit juice according to previously reported literature (Kashyap et al., 2001).
To study the pH stability of PG, enzyme was added to the buffers of different pH for one hour at room temperature, followed by measuring the residual activity. Purified enzyme was stable in the pH range 3.5 to 5.5, however above and below this pH, the activity decreased as shown in Figure 5. Comparable pH stability profile for Jamaica cherry PG (3.0-6.0) is reported by Gayathri et al. (2007). PG enzymes are also reported exhibiting pH stability even at broader pH range; pH stability of PG from S. macrocarpum was in pH range 3.0 to 9.0 (Chinedu et al., 2017).
Effect of temperature on activity and stability of purified PG
Temperature is an important factor that regulates the enzyme activity. Characteristic properties of proteins like hydrophobic interactions, hydrogen bonding and ionic strength resist the enzymes to thermal denaturation (Voordouw et al., 1976).
In order to determine optimum temperature of purified enzyme, it was incubated for 10 min at temperature ranging 10-70°C. The purified enzyme was optimally active at 40°C as illustrated in Figure 6. Similar optimal temperature are also reported for PG of mango pulp (Prasanna et al., 2006), ripened banana (Gayathri and Niar, 2014) and Jamaica cherry (Gayathri et al., 2007). Low and high temperature optima for PG in different fruits are also reported by Chinedu et al. (2017) for S. macrocarpum PG (30°C), by Chan and Tam (1982) for papaya (45°C) and by Mukherjee (2013) for C. gigantea PG (50°C).
Thermal stability of the PG was evaluated by keeping enzyme at different temperatures before the substrate added and residual enzyme activity was measured under standard conditions. Purified enzyme preserved its activity up to 60°C when incubated for one hour and afterwards declined sharply as shown in Figure 6. Previous reports are comparable to present study like Jamaica cherry (Gayathri et al., 2007) and S. macrocarpum (Chinedu et al., 2017).
Enzymes having wide pH range and temperature stability are favorite candidate for many biotechnological applications as a lot of biotechnological processes require high temperature and acidic or basic pH values (Antranikian, 2009).
Substrate specificity of purified PG
Substrate specificity of purified enzyme was found by employing various substrates. Polygalacturonic acid at its 1% concentration was found excellent substrate while appreciable activity (58%) was also achieved when pectin used as shown in Figure 7. Yang et al. (2011) have reported an endo-PGA1 showing maximum activity on PGA substrate (100%), followed by pectin (47.7%). Nevertheless, cellulose, xylan and galactose substrates exhibited poor substrate specificity towards purified PG enzyme even enzyme concentration increased from 0.5 to 1.5%.
Inhibition and activation studies
Various metal ions influence the enzyme by activating or inhibiting its activity, therefore, the effect of some potential metal ions on PG activity was determined in a reaction mixture containing 1mM of each chemical. Na+1 and K+1 enhanced PG activity upto 110% and 130%, respectively when employed at 1mM concentration (Fig. 8). The activation of PG by monovalent metal ions in banana and Jamaica cherry were reported by Pathak and Sanwal (1998) and Gayathari et al. (2007) while contrasting results in mango were observed as PG activity was inhibited or slightly affected by monovalent metal ions (Singh and Dwivedi, 2008).
Table II.- Effect of PG concentration, time and temperature on juice yield, total soluble solids, transmittance, viscosity, turbidity, total phenolic content and % DPPH antioxidant activity of apple juice.
|
PG enzyme concen-tration U/ml |
Temp (°C) |
Time (h) |
Juice yield (%) |
Total soluble solids (°Brix) |
% transm-ittance (660nm) |
Visco-sity (m Pa s) |
Visco-sity reduc-tion (%) |
Turbi-dity (NTU) |
Total phenolic content (mg/ml) |
% DPPH antiox-idant activity |
|
Control |
40 |
1 |
20 |
11.1 |
22 |
2.0 |
- |
33.9 |
1.26 |
74 |
|
40 |
2 |
25 |
11.3 |
30 |
1.90 |
5 |
32.8 |
1.56 |
74.5 |
|
|
50 |
1 |
30 |
11.4 |
38 |
1.90 |
5 |
31.7 |
2.05 |
74 |
|
|
50 |
2 |
55 |
11.6 |
45 |
1.85 |
7.5 |
31.5 |
2.12 |
76 |
|
|
PG enzyme concen-tration 2.5U/ml |
40 |
1 |
56 |
12.1 |
52 |
1.60 |
20 |
28.5 |
3.5 |
75 |
|
40 |
2 |
62 |
12.3 |
68 |
1.51 |
24.5 |
26.4 |
7.5 |
75.5 |
|
|
50 |
1 |
68 |
12.6 |
70 |
1.48 |
26 |
22.4 |
7.9 |
75.7 |
|
|
50 |
2 |
72 |
13.4 |
74 |
1.27 |
36.5 |
20.3 |
10.2 |
75.9 |
|
|
PG enzyme concen-tration 5U/ml |
40 |
1 |
71 |
13.4 |
70 |
1.24 |
38 |
20.2 |
11.2 |
75 |
|
40 |
2 |
73 |
14.4 |
85 |
1.17 |
41.5 |
10.9 |
11.9 |
75.4 |
|
|
50 |
1 |
75 |
14.4 |
90 |
1.15 |
42.5 |
5.5 |
12.5 |
75.7 |
|
|
50 |
2 |
77 |
14.8 |
95 |
1.09 |
45.5 |
3.9 |
13.82 |
77 |
In our case, PG activity was partially inhibited by Ca+2, Mn+2, Cu+2, Mg+2, Fe+3 and almost completely inhibited by Pb+1 and Hg+2. Nevertheless, contrasting results obtained regarding divalent (Ca+2 and Mg+2) and trivalent cation (Fe+3) that stimulated PG activity in banana and Ca+2, Mg+2 and Zn+2 in mango (Gayathri and Niar, 2014; Prassana et al., 2006). These studies revealed that divalent ions may stimulate enzyme activity due to stabilization of negatively charged carboxyl groups present in pectin structure (Pathak and Sanwal, 1998). Complete inhibition of PG activity in Jamaica cherry by Hg+1 and Pb+2 (Gayathari et al., 2007) was in accordance with present study.
Effect of PG on clarification of apple juice
Microbial pectinolytic enzymes have potential applications in various industries like textile, paper, food and beverage industries (Garg et al., 2016). PG has already been reported from several plants and their widely use in industrial applications. Throughout the world after orange juice, clarified apple juice is amongst the most consumed fruit juices (Kahle et al., 2005). Pectinolytic enzymes are gaining increasing interest to be used at commercial level for extraction of juices from various fruits and their clarification as well. Apples are perishable and delicate fruit and the juice produced has a high nutritional value hence the polygalacturonase extracted from grapes was applied to unripe apple juice to study its potential in juice clarification.
In the present study, purified PG from grapes skin enhanced the percentage yield and transmittance while it decreased the viscosity and turbidity of apple juice after clarification as shown in Table II. Color of juice is a sensory attribute which was very light as absorbance of juice at 440nm reduced from 1.42 to 0.28 after clarification. Minor changes in yield can have a great impact on cost of juice production at commercial level. The yield was improved up to 77%, transmittance of juice increased up to 95% and total soluble solids (°Brix) raised up to 14.8 (Figs. 9, 10). Reduction in percentage viscosity of juice was found to be 45.5% and turbidity value reduced to 3.9 NTU while total phenolic content improved up to 13.82 mg GAE/ml and DPPH scavenging activity increased up to 77% (Figs. 11, 12). Hence, nutraceutical potential and organoleptic features of apple juice were improved.
Previous findings on enzyme based juice clarification have fascinating results which is reported for lemon juice clarification by enzymatic treatment (Maktouf et al., 2014). Lemon juice after extraction has cloudy, viscous sol like appearance with high gelling power which is due to pectin polymer. The enzymatic treatment of the juice degrades the colloidal particles. PG enables the reduction in viscosity and turbidity of lemon juice up to 77% and 47%, respectively by depolymerization of insoluble pectin. Moreover, comparable results to present study obtained regarding reduction in viscosity after clarification which increased juice yield and minimized membrane clogging, a common hurdle observed during filtration procedures (de Carvalho et al., 2008; Chaudhri and Suneetha, 2012). Storage of juice did not affect the appearance of enzyme treated clarified juices. Notable haze formation was not observed even after 2 months of storage at room temperature (Gupta and Singh, 2004). Interestingly, raw fruits treated with pectinolytic enzyme aids in conspicuous release of phenolic content from the fruits (Sharma et al., 2013). Phenolic components act as potent antioxidant which helps to improve and maintain general health by limiting the risk of debilitating diseases like heart disease and different malignancies (Miller and Rice-Evans, 1997). Phenolic content, its composition or structure demonstrate crucial role for improving antioxidant activity (Bhanja et al., 2008). It was also found that 15% increase in phenolic content of drinks after enzyme treatment suggested that dragon fruit beverage is much better than the untreated beverage in terms of improved antioxidant capacity (Aliaa et al., 2010). Total soluble solids also tend to increase by enzyme based juice clarification. Yousaf and Ibrahim (1994) noted that total soluble solid content of soursop juice after enzyme clarification was improved from 6.8 to 7.3 °Brix after one hour incubation time. However, increasing incubation time did not raise total soluble solids of juice. Enzyme treatment has sophisticated effect on juice clarity. A similar behavior was observed in banana juice clarification regarding fluctuation in temperature, incubation time and enzyme concentration (Lee et al., 2006).
Response surface methodology (RSM) is a valuable statistical tool helpful in demonstration of impact of numerous independent and response variables (Box and Draper, 1987). To assess the relationship between experimental aspects and observed results, response surface methodology was employed and to estimate statistical significance of the model, Fisher’s test for analysis of variance (ANOVA) was performed. The model was actually significant according to ANOVA results. Small p-value depicted that PG treatment on various juice clarification parameters were statistically significant up to 99% confidence level with p-value <0.05. Incubation time, enzyme concentration and temperature were described as three independent variables and eleven combinations were used. The collaborative effect of time and incubation temperature on various parameters of apple juice using PG purified from grapes skin was indicated in response surface curves and it showed that the temperature up to 50°C has positive effect on juice clarification. Similarly, treatment time appeared to have positive effect on juice clarification
Table III.- ANOVA results of apple juice parameters based on response surface methodology (RSM).
|
Source |
df |
SS |
MS |
F-Value |
P-Value |
|
Juice yield% (R2 = 80.92%) |
|||||
|
Model |
2 |
3782.0 |
1891.0 |
19.08 |
0.001 |
|
Residuals |
9 |
892.0 |
99.1 |
||
|
Total |
11 |
4674.0 |
|||
|
% Transmittance (R2 = 85.28%) |
|||||
|
Model |
2 |
5370 |
2685 |
26.08 |
0.000 |
|
Residuals |
9 |
927 |
103 |
||
|
Total |
11 |
6297 |
|||
|
% Viscosity (R2 = 93.30%) |
|||||
|
Model |
2 |
2847.5 |
1423.8 |
62.62 |
0.000 |
|
Residuals |
9 |
204.6 |
22.7 |
||
|
Total |
11 |
3052.2 |
|||
|
Total phenolic content (R2 = 89.13%) |
|||||
|
Model |
2 |
225.17 |
112.59 |
36.90 |
0.000 |
|
Residuals |
9 |
27.46 |
3.05 |
||
|
Total |
11 |
252.63 |
|||
|
% DPPH antioxidant (R2 = 85.22% ) |
|||||
|
Model |
2 |
2.927 |
1.463 |
2.45 |
0.002 |
|
Residuals |
9 |
5.382 |
0.598 |
||
|
Total |
11 |
8.309 |
|||
|
Total soluble solids (°Brix) (R2 = 88.59%) |
|||||
|
Model |
2 |
16.927 |
8.463 |
34.94 |
0.000 |
|
Residuals |
9 |
2.180 |
0.242 |
||
|
Total |
11 |
19.107 |
|||
|
Turbidity (NTU) (R2 = 83.16% ) |
|||||
|
Model |
2 |
1024.7 |
512.3 |
22.22 |
0.000 |
|
Residuals |
9 |
207.6 |
23.1 |
||
|
Total |
11 |
1232.2 |
|||
up to one hour. PG enzyme concentration up to 5 U/ml had positive effect while low enzyme concentration found to be less effective for juice clarification purpose. The analysis of variance (ANOVA) was done using Minitab software which showed that the response surface models developed for all response variables were adequate (Table III). Interaction of time and enzyme concentration was found to be significant. Previous studies for optimum conditions of enzyme based juice clarification have nearly similar results. Under optimized conditions of enzyme concentration 1.5 U/ml, temperature 40°C and incubation time of 30 min for mango juice clarification by PG purified from Aspergillus awamori MTCC 9166, response plots and contour plots were established based on regression coefficient values which showed that 60% viscosity reduction of mango juice was achieved after clarification (Anuradha et al., 2016). Sapodilla juice was clarified by PG purified from Streptomyces lydicus and response surface methodology (RSM) was employed to establish optimum conditions of juice clarification (Jacob et al., 2008). Diwan and Shukla (2005) optimized conditions for clarification of guava juice employing 2% purified enzyme for 20 h incubation time. Optimum conditions for pectinase were established to achieve maximum sweet lime juice yield after clarification (Rai et al., 2004).
Conclusion
Enzymes possessing distinguished biochemical, physic-chemical features with economical production employed in downstream applications have always been attractive in research projects. A well maintained upstream process technology is required for the production of microbial pectinolytic enzymes which alters overall cost. No such upstream technology is required for extraction of enzymes from plants. The benefit of enzyme extraction from plant source is that plants itself behaves as bioreactor, thereby, making easy harvest of enzymes (Mukharjee, 2013). Enzyme based juice clarification possess several benefits over mechanical methods. Under mechanical extraction, pectin present in fruit pulp has unpleasant effect on juice recovery due to reduced filtration. Pectinolytic enzymes are better choice to overcome recovery losses and have become part of modern fruit juice processing industries claiming improvement in juice recovery, total soluble solids, clarity, total phenolic content and antioxidant activity and significant decline in juice viscosity and turbidity.
In present study, purified PG from grapes had pH optima 4.5 and stability in pH range 3.5 to 5.5; hence the potential of acidic grape polygalacturonase in apple juice clarification was studied under optimized conditions of incubation time, enzyme concentration and temperature. For better understanding of juice clarification results, box plots and response plots based on RSM were developed which demonstrated that the operating variables (enzyme concentration, temperature and incubation time) had affected the overall quality of apple juice by achieving significant results with low p-value (less than 0.05). Considering the significance of PG enzyme, experiments are in progress for its biotechnological applications and to improve the production and its stability by recombinant DNA technology.
Statement of conflict of interest
There is no conflict of interests among the authors.
References
Ajayi, A.A., Aina, O. and Olasehinde, G.I., 2011. Extraction and clarification of apple juice with polygalacturonase obtained from apple (Malus domestica) fruits deteriorated by Aspergillus niger. Int. J. Biol. Chem. Sci., 5: 1047-1053. https://doi.org/10.4314/ijbcs.v5i3.72206
Ali, Z.M. and Brady, C.J., 1982. Purification and characterization of the polygalacturonases of tomato fruits. Australian J. Pl. Physiol., 9: 155-169.
Aliaa, A.R.N., Mazlina, M.S. and Taip, F.S., 2010. Impact of commercial pectolytic enzymes on selected properties of white dragon fruit juice. J. Inst. Eng. Malays., 71: 25-31.
Antranikian, G., 2009. Extremophiles and biotechnology. In: Encyclopedia of life sciences (ELS). John Wiley & Sons, Ltd., Chichester. https://doi.org/10.1002/9780470015902.a0000391.pub2
Anuradha, K., Padma, P.N., Venkateshwar, S. and Reddy, G., 2016. Mango juice clarification with polygalacturonase produced by Aspergillus awamori MTCC 9166-Optimization of conditions. Int. Fd. Res. J., 23: 147-151.
Askar, A., 1998. Enzymes in fruit juice processing. Fruit Process., 7: 273-276.
Béguin, P., 1983. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Analyt. Biochem., 131: 333-336. https://doi.org/10.1016/0003-2697(83)90178-1
Bhanja, T., Rout, S., Banerjee, R. and Bhattacharyya, B.C., 2008. Studies on the performance of a new bioreactor for improving antioxidant potential of rice. LWT-Fd. Sci. Technol., 41: 1459-1465.
Box, G.E. and Draper, N.R., 1987. Empirical model-building and response surfaces. John Wiley & Sons.
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem., 72: 248-254. https://doi.org/10.1016/0003-2697(76)90527-3
Brand-Williams, W., Cuvelier, M.E. and Berset, C., 1995. Use of a free radical method to evaluate antioxidant activity. LWT-Fd. Sci. Technol., 28: 25-30.
Buchert, J., Koponen, J.M., Suutarinen, M., Mustranta, A., Lille, M., Torronen, R. and Poutanen, K., 2005. Effect of enzyme-aided pressing on anthocyanin yield and profiles in bilberry and blackcurrant juices. J. Sci. Fd. Agric., 85: 2548-2556. https://doi.org/10.1002/jsfa.2284
Chan, Jr. H.T. and Tam, S.Y.T., 1982. Partial separation and characterization of papaya endo- and exo-polygalacturonase. J. Fd. Sci., 47: 1478-1483. https://doi.org/10.1111/j.1365-2621.1982.tb04965.x
Chaplin, M.F. and Bucke, C., 1990. Enzyme technology. Cambridge University Press, pp. 12.
Chaudhri, A. and Suneetha, V., 2012. Microbially derived pectinases: A review. IOSR J. Pharm. biol. Sci., 2:1-5.
Chinedu, S.N., Dayo-Odukoya, O.P. and Iheagwam, F.N., 2017. Partial purification and kinetic properties of polygalacturonase from Solanum macrocarpum L. fruit. Biotechnology, 16: 27-33. https://doi.org/10.3923/biotech.2017.27.33
Chun, J.P. and Huber, D.J., 1998. Polygalacturonase mediated solubilization and depolymerization of pectic polymers in tomato fruit cell walls: Regulation by pH and ionic conditions. Pl. Physiol., 117: 1293-1299. https://doi.org/10.1104/pp.117.4.1293
de Carvalho, L.M.J., De Castro, I.M. and da Silva, C.A.B., 2008. A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro-and ultra-filtration. J. Fd. Engin., 87: 447-454. https://doi.org/10.1016/j.jfoodeng.2007.12.015
de Roos, A., Grassin, C., Herweijer, M., Kragh, K.M., Poulsen, C.H., Soe, J.B., Sorensen, J.F. and Wilms, J., 2007. Industrial enzymes: Enzymes in food applications. In: Enzymes in industry: Production and applications (ed. W. Aehle). Wiley-VCH, Germany, pp. 123-131.
Diwan, A. and Shukla, S.S., 2005. Process development for the production of clarified guava juice. J. Fd. Sci. Technol., 42: 245-249.
Emmons, C.L. and Peterson, D.M., 2001. Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci., 41: 1676-1681. https://doi.org/10.2135/cropsci2001.1676
Garg, G., Singh, A., Kaur, A., Singh, R., Kaur, J. and Mahajan, R., 2016. Microbial pectinases: An eco-friendly tool of nature for industries. 3 Biotech, 6: 47.
Gayathri, T. and Nair, A.S., 2014. Isolation, purification and characterisation of polygalacturonase from ripened banana (Musa acuminata cv. Kadali). Int. J. Fd. Sci. Technol., 49: 429-434. https://doi.org/10.1111/ijfs.12319
Gayathri, T. and Nair, A.S., 2015. Purification and characterization of polygalacturonase from ripened fruits of Musa acuminata cultivar from Kerala (Musa acuminata cv. Palayankodan). J. Fd. Measure. Character., 9: 233-239.
Gayathri, T., Mohan, T.K. and Murugan, K., 2007. Purification and characterization of polygalacturonase-3 from Jamaica cherry (Muntingiacalabura Linn). J. Pl. Biochem. Biotechnol., 16: 127-130. https://doi.org/10.1007/BF03321987
Gupta, R. and Singh, S., 2004. Apple juice clarification using fungal pectinolytic enzymes and gelatin. Indian J. Biotechnol., 3: 573-576.
Hemalatha, R., Kumar, A., Prakash, O., Supriya, A., Chauhan, A.S. and Kudachikar, V.B., 2018. Development and quality evaluation of ready to serve (RTS) beverage from cape gooseberry (Physalis peruviana L.). Beverages, 4: 42. https://doi.org/10.3390/beverages4020042
Hyson, D.A., 2015. A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Adv. Nutri., 6: 37-51. https://doi.org/10.3945/an.114.005728
Jach, G., Soezer, N., Schullehner, K., Lalla, B., Welters, P. and Mueller, A., 2010. Phytomining of plant enzymes for biotechnological use of fats and oils. Eur. J. Lipid Sci. Technol., 112: 75-86. https://doi.org/10.1002/ejlt.200900100
Jacob, N., Sukumaran, R.K. and Prema, P., 2008. Optimization of enzymatic clarification of sapodilla juice: A statistical perspective. Appl. Biochem. Biotechnol., 151: 353-363.
Jayani, R.S., Saxena, S. and Gupta, R., 2005. Microbial pectinolytic enzymes: A review. Process Biochem., 40: 2931-2944. https://doi.org/10.1016/j.procbio.2005.03.026
Kahle, K., Kraus, M. and Richling, E., 2005. Polyphenol profiles of apple juices. Mol. Nutri. Fd. Res., 49: 797-806. https://doi.org/10.1002/mnfr.200500064
Kashyap, D.R., Vohra, P.K., Chopra, S. and Tewari, R., 2001. Applications of pectinases in the commercial sector: A review. Bioresour. Technol., 77: 215-227. https://doi.org/10.1016/S0960-8524(00)00118-8
Kausar, S., Asad, S., Kiran, C. and Khan, T., 2017. An allometric growth estimation of grapes (Vitis vinifera L.) from high mountainous region of Gilgit-Baltistan. Am. J. biol. environ. Stat., 3: 81-84.
Khan, A.W., Shafiq, T. and Ahmad, M., 2008. Physical and biochemical changes in commonly grown grapes (Vitis vinifera) in Pakistan at different maturity levels. Pakistan J. Sci., 60: 3-4.
Kornberg., A., 2009. Why purify enzymes? Meth. Enzymol., 182: 1-5. https://doi.org/10.1016/0076-6879(90)82003-K
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680. https://doi.org/10.1038/227680a0
Lee, W.C., Yusof, S., Hamid, N.S.A. and Baharin, B.S., 2006. Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (RSM). J. Fd. Engin., 73: 55-63. https://doi.org/10.1016/j.jfoodeng.2005.01.005
Maktouf, S., Neifar, M., Drira, S.J., Baklouti, S., Fendri, M. and Châabouni, S.E., 2014. Lemon juice clarification using fungal pectinolytic enzymes coupled to membrane ultrafiltration. Fd. Bioprod. Process., 92: 14-19. https://doi.org/10.1016/j.fbp.2013.07.003
Miller, G.L., 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analyt. Chem., 31: 426-428. https://doi.org/10.1021/ac60147a030
Miller, N.J. and Rice-Evans, C.A., 1997. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Fd. Chem., 60: 331-337. https://doi.org/10.1016/S0308-8146(96)00339-1
Muchuweti, M., Moyo, E. and Mushipe, S., 2005. Some properties of the polygalacturonase from four Zimbabwean wild fruits (Uapaca kirkiana, Zizphus mauritiana, Tamarindus indica and Berchemia discolor fruits). Fd. Chem., 90: 655-661. https://doi.org/10.1016/j.foodchem.2004.04.026
Mukherjee, A., 2013. Extraction, partial purification and characterization of several carbohydrases (polygalacturonase, sucrase and β-galactosidase) from the shrub, Calotropis gigantea. Int. J. Biol. Pharm. All. Sci., 2: 1535-1561.
Nakkeeran, E., Umesh-Kumar, S. and Subramanian, R., 2011. Aspergillus carbonarius polygalacturonases purified by integrated membrane process and affinity precipitation for apple juice production. Bioresour. Technol., 102: 3293-3297. https://doi.org/10.1016/j.biortech.2010.10.048
Nogata, Y., Ohta, H. and Voragen, A.G.J., 1993. Polygalacturonase in strawberry fruit. Phytochemistry, 34: 617-620. https://doi.org/10.1016/0031-9422(93)85327-N
Pathak, N. and Sanwal, G.G., 1998. Multiple forms of polygalacturonase from banana fruits. Phytochemistry, 48: 249-255. https://doi.org/10.1016/S0031-9422(98)00005-3
Pedrolli, D.B., Monteiro, A.C., Gomes, E. and Carmona, E.C., 2009. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J., 3: 9-18. https://doi.org/10.2174/1874070700903010009
Prasanna, V., Prabha, T.N. and Tharanathan, R.N., 2006. Multiple forms of polygalacturonase from mango (Mangifera indica L. cv Alphonso) fruit. Fd. Chem., 95: 30-36. https://doi.org/10.1016/j.foodchem.2004.12.014
Pressey, R. and Avants, J.K., 1973. Separation and characterization of endopolygalacturonase and exopolygalacturonase from peaches. Pl. Physiol., 52: 252-256. https://doi.org/10.1104/pp.52.3.252
Pressey, R. and Avants, J.K., 1976. Pear polygalacturonases. Phytochemistry, 15: 1349-1351. https://doi.org/10.1016/S0031-9422(00)97116-4
Rai, P., Majumdar, G.C., Dasgupta, S. and De, S., 2004. Optimizing pectinase usage in pretreatment of mosambi juice for clarification by response surface methodology. J. Fd. Engin., 64: 397-403. https://doi.org/10.1016/j.jfoodeng.2003.11.008
Ramadan, M.F. and Moersel, J.T., 2007. Impact of enzymatic treatment on chemical composition, physicochemical properties and radical scavenging activity of goldenberry (Physalis peruviana L.) juice. J. Sci. Fd. Agric., 87: 452-460. https://doi.org/10.1002/jsfa.2728
Rehman, H.U., Aman, A., Silipo, A., Qader, S.A.U., Molinaro, A. and Ansari, A., 2013. Degradation of complex carbohydrate: Immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Fd. Chem., 139: 1081-1086. https://doi.org/10.1016/j.foodchem.2013.01.069
Sakai, T., Sakamoto, T., Hallaert, J. and Van-Damme, E.J., 1993. Pectin, pectinase, protopectinase: Production, properties and applications. Adv. appl. Microbiol., 39: 213-294. https://doi.org/10.1016/S0065-2164(08)70597-5
Sharma, N., Rathore, M. and Sharma, M., 2013. Microbial pectinase: Sources, characterization and applications. Rev. environ. Sci. Biotechnol., 12: 45-60. https://doi.org/10.1007/s11157-012-9276-9
Sin, H.N., Yusof, S., Hamid, N.S.A. and Rahman, R.A., 2006. Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Fd. Engin., 73: 313-319. https://doi.org/10.1016/j.jfoodeng.2005.01.031
Singh, P. and Dwivedi, U.N., 2008. Purification and characterisation of multiple forms of polygalacturonase from mango (Mangifera indica cv. Dashehari) fruit. Fd. Chem., 111: 345-349. https://doi.org/10.1016/j.foodchem.2008.03.072
Soares, M.M.C.N., da Silva, R., Carmona, E.C. and Gomes, E., 2001. Pectinolytic enzyme production by Bacillus species and their potential application on juice extraction. World J. Microbiol. Biotechnol., 17: 79-82. https://doi.org/10.1023/A:1016667930174
Tapre, A.R. and Jain, R.K., 2014. Pectinases: Enzymes for fruit processing industry. Int. Fd. Res. J., 21: 447-453.
Tochi, B.N., Wang, Z., Xu, S.Y. and Zhang, W., 2009. The influence of a pectinase and pectinase/hemicellulases enzyme preparations on percentage pineapple juice recovery, particulates and sensory attributes. Pakistan J. Nutri., 8: 1184-1189. https://doi.org/10.3923/pjn.2009.1184.1189
Urlaub, R., 2002. Modern use of enzymes in fruit processing. Fruit Process., 8: 360-361.
Voordouw, G., Milo, C. and Roche, R.S., 1976. Role of bound calcium ions in thermostable, proteolytic enzymes. Separation of intrinsic and calcium ion contributions to the kinetic thermal stability. Biochem. J., 15: 3716-3723. https://doi.org/10.1021/bi00662a012
Wakabayashi, K. and Huber, D.J., 2001. Purification and catalytic properties of polygalacturonase isoforms from ripe avocado (Persea americana) fruit mesocarp. Physiol. Plant., 113: 210-216. https://doi.org/10.1034/j.1399-3054.2001.1130208.x
Yang, J., Luo, H., Li, J., Wang, K., Cheng, H., Bai, Y., Yuan, T., Fan, Y. and Yao, B., 2011. Cloning, expression and characterization of an acidic endo-polygalacturonase from Bispora sp. MEY-1 and its potential application in juice clarification. Process Biochem., 46: 272-277. https://doi.org/10.1016/j.procbio.2010.08.022
Yusof, S. and Ibrahim, N., 1994. Quality of soursop juice after pectinase enzyme treatment. Fd. Chem., 51: 83-88. https://doi.org/10.1016/0308-8146(94)90052-3
To share on other social networks, click on any share button. What are these?










