Physicochemical Analysis of Residual Water from Treated Olive Fruits (Olea europaea)
Research Article
Physicochemical Analysis of Residual Water from Treated Olive Fruits (Olea europaea)
Ali Muhammad*, Muhammad Ayub
Department of Food Science and Technology, The University of Agriculture Peshawar, Pakistan.
Abstract | Olive fruit is the most important part of the olive tree which is popular for their medicinal values. Due to its bitter taste the fruit is unable to eat directly because the fruit contain high level of bitter glucoside such as oleourepean. Aim of this research work was to reduce the bitterness level of olive fruit by different treatment such as water, salt and lye solution which were coded as RW1, RW2 and RW3 respectively. During treatment process the bitter glucoside level such as oleourepean leached out from the fruits to the solvents by osmosis process which alters the composition of olive fruit. Phenolic compounds, flavonoids and anthocyanins which are the major components of olive fruits degrades significantly during four weeks of treatment process. Results showed that during brine treatment, fermentation take place which significantly convert the oleourepean into simple sugar and decreased during treatment process. Lye solution penetrates inside the msesocarp and than endocarp during four weeks of treatment process. The lye treated olive fruits were treated afterwards into brine solution for the next week and so on which gradually remove the bitter taste of lye solution and got salted taste during four week of treatment process. The residual water of treated olive fruits were changed on weekly basis and their residual water were analyzed physicochemically. Result showed that data is significantly different from each other at alpha (α) level ≤ 0.05. Physicochemical analysis showed that decrease was observed in ascorbic acid (15.18 to 7.21), pH (4.56 to 3.9) and TSS (5 to 2.5). Standard deviation showed the least variation in data. Results show that on nutritional basis RW1 was found best followed by RW2.
Editor | Tahir Sarwar, The University of Agriculture, Peshawar, Pakistan.
Received | January 21, 2015; Accepted | September 03, 2015; Published | September 27, 2015
*Correspondence | Ali Muhammad, The University of Agricult was found ure Peshawar, Pakistan; E-mail | [email protected]
Citation | Muhammad, A. and M. Ayub. 2015. Physicochemical analysis of residual water from treated olive fruits (Olea europaea). Sarhad Journal of Agriculture, 31(3): 159-164.
DOI | http://dx.doi.org/10.17582/journal.sja/2015/31.3.159.164
Keywords | Salt, Lye, Olive fruit, Water, Bitterness, Oleourepean, Sugar/acid ratio and overall acceptability
Introduction
Oleourepean is the major cause of bitterness in olive fruit which make them unable to eat freshly, so the fruit must be processed by different methods like fresh water, lye and salt treatment during research period (McEachern and Stein, 1997).
Citric acids, oxalic, succinic and malic are organic acids which are widely present in olive fruit, similarly it also contain free fatty acids of high level. The sugar content decrease when the fruit become mature. Decrease also occurs in protein content which ranges 1.5 and 2.2% by weight of the fruit. The pectic substances which cement the cells were hydrolyzed by enzyme pectinolytic which affect the texture of the fruit (Marsilio, 2006).
The composition of olive fruit from this variety contain 1.5-2.0% N-compound, oil 13-28%, 45-55% water, 5-8% fibre, C-compound 18-40% and 1-2% Ash. The fruit have high medicinal value and were used for curing of heart problem, as anti cancer and antioxidant (Kastorini et al., 2010).
Oleuropein is the major bioactive compound of Olea europaea, widely known as the olive tree and present in high amount in unprocessed olive fruit and leaves. During maturation of fruit or as a results of olive processing (such as oil production), chemical and enzyme reactions occur which reduce the concentration of oleuropein and raise the concentration of hydroxytyrosol which is the principal degradation product of oleuropein. The oleuropein molecule consists of three structural subunits: a polyphenol, namely 4-(2-hydroxyethyl) benzene-1, 2-diol which is also known as hydroxytyrosol (HT), a secoiridoid called elenolic acid and a glucose molecule. Oleuropein possesses beneficial pharmacological effects such as cardiprotective effect antioxidant (Andreadou et al., 2006), anti-inflammatory (Visioli et al., 1998), inhibition of platelet aggregation (Petroni et al., 1995), anti-atherogenic (Manna et al., 2004; Visioli and Galli, 2001) activity, anti-cancer (Hamdi and Castellon, 2005), anti-microbial properties (Bisignano et al., 1999) and neuroprotective effect (Bazoti et al., 2006).
Antioxidant and antimicrobial properties of the fruit are mainly due due to Tocopherols and phenolic compounds are minor constituents of the fruit (Armstrong et al., 1997). Table olives are processed by different methods such as brine is used for green olive fruit which is immature, black colour fruit in fresh water and lye solution is used for black ripe olive (Sabatini et al., 2009). Environmental conditions such as climatic changes and maturity index of olive fruits can affect the plant phenolic and nutritional quality (Romero et al., 2004). Residual water is a waste which is full of nutrition but unfortunately after curing and treatment process of olive fruits, these water were discarded. Utilization of residual water in various products instead of normal water, it would be beneficial for human beings to overcome some of the deficiencies and also can be used as animal feed for preparation of their diet.
Material and Methods
The fruits which brought from CCRI Nowshera district were thoroughly washed with potable water to remove dirt, dust, pesticide residues and surface microbial load in the Laboratories of Food Science and Technology and after preparatory operation the samples were kept in large size plastic jars and steeliness steel containers.
Physicochemical analysis
All the residual water was analyzed for pH, TSS, %acidity and ascorbic acid by the According to the standard method of AOAC (2000) the physicochemical analysis were carried out during research period.
pH of the samples was determined by using Inolab Digital pH meter 720 according to the manual instruction of apparatus it was standardized by using buffer solutions of known pH (4 and 7) then 10 ml of sample was taken in a clean beaker and probe was directly dipped into the sample to record the pH value.
Total soluble solids were determined by the method of AOAC (2000) using hand Refractometer at room temperature. Ascorbic acid content was determined by Titrimetric method as reported in AOAC (2000).
Take 50 mg of 2, 6-dichlorophenol indophenol dye and 42 mg of sodium bicarbonate were weighed accurately, dissolved in hot distilled water and volume was made up to 250ml with distilled water. Standard ascorbic acid 50 mg was taken in 250 ml of volumetric flask and the volume was made up with 0.4% oxalic acid. 2 ml of this ascorbic acid solution was titrated against dye solution until light pink color was obtained which persisted for 15 seconds.
The sample (10g) was taken and volume was made up to the 100 ml by 0.4% oxalic acid. Then 10 ml of this sample were taken and titrated against dye solution until light pink color appeared, which persisted for 15 seconds. Three consecutive readings were taken for each sample. The ascorbic acid (mg/100g) was calculated by using the following formula:
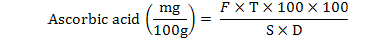
Where,
F = Factor from standardization = used dye of ml solution acid ascorbic standard of ml
T = ml of dye used for sample
S = ml of diluted sample taken for titration
D = ml of sample taken for dilution
Statistical analysis
The Data were analyzed by Statistix® version 1.8 which is statistical software package and according to method as described by Steel et al. (1997) the means were separated by LSD.
Results and Discussion
The fruits were destined manually and were kept for one week in fresh water in a different solution for in one week and the solution were removed from the container on weekly basis. The residual water which was removed from the containers was taken for analysis during four weeks of treatment. The samples were given different code such as RW1, RW2 and RW3 respectively for olive fruit with water, olive fruit with salt and olive fruit with lye solution and were subjected for analysis during the research period. Result showed that decrease in the mean values was observed as 4.56, 5 and 10.8 for pH while the mean values were recorded as 5, 4.6 and 3.8 respectively for TSS. Similarly the mean values of the samples were 0.268, 0.281 and 0.082 were observed in % acidity of the samples. Similarly 18.66, 16.37 and 46.34 for sugar/acid ratios and 15.18, 15.12 and 15.15 were observed in ascorbic acid content. Table 1 and Figure 1 showed brief explanation of the depth olives fruits samples their results during storage period. Comparison of acidity and pH during 1st week of treatment are showed in Figure 1.
Table 1: Physicochemical analysis of residual water on 1st week
|
Treatments |
pH |
TSS (0brix) |
% acidity |
Sugar/acid ratio |
Ascorbic acid (mg/100g) |
|
RW1 |
4.56± 0.021 |
5± 0.153 |
0.268± 0.005 |
18.66± 0.142 |
15.18± 0.020 |
|
RW2 |
5± 0.015 |
4.6± 0.100 |
0.281± 0.002 |
16.37± 0.021 |
15.12± 0.021 |
|
RW3 |
10.8± 0.208 |
3.8± 0.153 |
0.082± 0.006 |
46.34± 0.038 |
15.15± 0.026 |
Table 2: Physicochemical analysis of residual water on 2nd week
|
Treatments |
pH |
TSS (0brix) |
% acidity |
Sugar/acid ratio |
Ascorbic acid (mg/100g) |
|
RW1 |
4.2± 0.100 |
3.4± 0.100 |
0.253± 0.002 |
13.44± 0.021 |
14.45± 0.026 |
|
RW2 |
4.8± 0.058 |
3.9± 0.058 |
0.272± 0.004 |
14.34± 0.020 |
13.32± 0.015 |
|
RW3 |
10.7± 0.153 |
3.6± 0.015 |
0.061± 0.005 |
59.02± 0.025 |
13.11± 0.010 |
The mean values of pH during four weeks of treatments for RW1, RW2 and RW3 are showed in Table.2 that was 4.2, 4.8 and 10.7 respectively. The mean values for TSS were 3.4, 3.9 and 3.6 respectively and 0.253, 0.272 and 0.061 for % acidity, 13.44, 14.34 and 59.02 for sugar/acid ratios and 14.45, 13.32 and 13.11 for ascorbic acid content of the samples during four weeks of treatments as shown in Table 2. Figure 2 showed brief explanation of the depth olives fruits samples their results during storage period.
Mean values of pH for treated samples RW1, RW2 and RW3were 4.1, 4.6 and 11.38, for TSS the mean values were 3.01, 3.5 and 3.11, the mean value of acidity of the samples were 0.246, 0.255 and 0.031, the mean values 12.24, 13.73 and 100.32 were observed for sugar/acid ratio and 13.15, 12.87 and 12.14 were observed for ascorbic acid respectively as shown in Table 3. Figure 3 showed brief explanation of the depth olives fruits samples their results during storage period.
Table 3: Physicochemical analysis of residual of water on 3rd week
|
Treatments |
pH |
TSS (0brix) |
% acidity |
Sugar/ acid ratio |
Ascorbic acid (mg/100g) |
|
RW1 |
4.1± 0.015 |
3.01± 0.010 |
0.246± 0.003 |
12.24± 0.031 |
13.15±0.017 |
|
RW2 |
4.6± 0.100 |
3.5± 0.100 |
0.255± 0.004 |
13.73± 0.015 |
12.87±0.223 |
|
RW3 |
11.38± 0.015 |
3.11± 0.012 |
0.031± 0.002 |
100.32± 0.237 |
12.14±0.010 |
Table 4: Physicochemical analysis of residual of water on 4th week
|
Treatments |
pH |
TSS (0brix) |
% acidity |
Sugar/ acid ratio |
Ascorbic acid (mg/100g) |
|
RW1 |
3.9± 0.030 |
2.5± 0.025 |
0.241± 0.002 |
10.37± 0.029 |
10.01±0.025 |
|
RW2 |
4.4± 0.038 |
3.11± 0.021 |
0.247± 0.004 |
12.59± 0.065 |
8.42±0.015 |
|
RW3 |
11.5± .333 |
2.7± 0.038 |
0.021± 0.005 |
128.57± 0.377 |
7.21±0.010 |
The mean values of pH for RW1, RW2 and RW3 were 3.9, 4.4 and 11.5, for TSS the mean values were 2.5, 3.11 and 2.7 for % acidity the mean values were 0.241, 0.247 and 0.021 respectively during storage period of the treated samples. Sugar/acid ratios were 10.37, 12.59 and 128.57 while mean values such as 10.01, 8.42 and 7.21 were observed in ascorbic acid content of the samples as shown in Table 4. Figure 4 showed brief explanation of the depth olives fruits samples their results during storage period. Polyphenols and
Figure 1: Physicochemical analysis of residual water on 1st week
Figure 2: Physicochemical analysis of residual water on 2nd week
Figure 3: Physicochemical analysis of residual of water on 3rd week
Figure 4: Physicochemical analysis of residual of water on 4th week
other nutrients which are very beneficial to human health are transferred to solvent by osmosis process and were continually discarded during each step. The experiments was done in order to reduce its bitterness and further it will be used for product development but unfortunately due to the nutrients losses and changing behavior of bioactive substances the processed olive fruits were not used for product developments.
Table 5: pH of residual water during 30 days of storage period
|
Treatments |
1st week |
2nd week |
3rd week |
4th week |
|
RW1 |
4.56± 0.02 |
4.2± 0.10 |
4.1± 0.02 |
3.9±0.03 |
|
RW2 |
5± 0.015 |
4.8± 0.06 |
4.6± 0.10 |
4.4±0.038 |
|
RW3 |
10.8± 0.21 |
10.7±0.15 |
11.4± 0.02 |
11.5±0.33 |
Table 6: TSS of residual water during 30 days of storage period
|
Treatments |
1st week |
2nd week |
3rd week |
4th week |
|
RW1 |
5±0.153 |
3.4±0.10 |
3.01±0.010 |
2.5±0.025 |
|
RW2 |
4.6±0.10 |
3.9±0.06 |
3.5±0.100 |
3.11±0.021 |
|
RW3 |
3.8±0.15 |
3.6±0.02 |
3.11±0.012 |
2.7±0.038 |
The stability of vitamin C is mainly affecting by pH and the oxidation processes of vitamin C is occurs due to high pH. The pH increased slowly whereas titratable acidity decreased during the research period of the treatments. The decrease in acidity and rise in pH indicate formation of organic acids in the samples during osmosis process. After 7 weeks storage time the total soluble solids started to decrease as reported by Rivas et al. (2006). Due to the presence of the microorganisms the fruit juice become deteriorates and as a result change in total soluble solids occurs. Studying a range of fruit and vegetables, during thermal processing, the flavonoids and ascorbic acid significantly loss as determined by the authors during storage in orange juice (Esteve et al., 2005).
Conclusions
For reduction of bitterness of olive fruit, various treatments such as water, brine and lye treatments were given to olive fruits. On nutritional basis RW1 was found best followed by RW2 and RW3 respectively during treated residual water which was taken on weekly basis. Osmosis process during storage occurs which diffuses the nutrients from fruits to solvents. So during this process leached out nutrients was avoided by consumers and only used the treated fruits. From the research work it was concluded that residual water is also a source of nutrients which can be used for nourishment of baby foods. Incorporation of the residual water in other products preparation instead of usual drinking water can enhance their nutritional value. Olive fruits that treated with fresh water were found best followed by brine treated olive fruits.
It is recommended for further study, that glass jars should be used for all the treatment process for reduction of bitterness of olive fruits. Shrinkage is one the problem in this process, so wide and open containers should be used to avoid this problem.
Authors’ Contribution
This paper is a part of first author’s (Ali Muhammad) PhD research work which was carried out under the supervision of Prof. Dr. Muhammad Ayub in the laboratories of Department of Food Science and Technology, The University of Agriculture Peshawar, Pakistan.
References
- AOAC. 2000. Official Methods of Analysis 17th Edition, Association of Official Agric. Chem. Washigton D.C.
- Armstrong, N., Paganga, G., Brunev, E and N.Miller. 1997. Reference values for α- tocopherol and β-carotene in the Whitehall II study. Free Radical Research. 27: 207–219. http://dx.doi.org/10.3109/10715769709097853
- Andreadou, I., E. K. Iliodromitis, E. Mikros, M. Constantinou, A. Agalias, P. Magiatis, A. L. Skaltsounis, E. Kamber, A. TsantiliKakoulidou, D. T. Kremastinos. 2006. The olive constituent oleuropein exhibits anti-ischemic, antioxidative and hypolipidemic effects in anesthetized rabbits. J. Nutr. 136: 2213–2219.
- Bazoti, F.N., J. Bergquist, K. Markides, A. Tsarbopoulos. 2006. Noncovalent interaction between amyloid-b-peptide (1–40) and oleuropein studied by electrospray ionization mass spectrometry. J. Am. Soc. Mass. Spectrom. 17: 568–575. http://dx.doi.org/10.1016/j.jasms.2005.11.016
- Bisignano, G., A. Tomaino, R. Lo Cascio, G. Crisafi, N. Uccella, A. Saija. 1999. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 51:971–974. http://dx.doi.org/10.1211/0022357991773258
- Esteve, M.J., A. Frigola, C. Rodrigo, D. Rodrigo. 2005. Effect of storage period under variable conditions on the chemical and physical composition and colour of Spanish refrigerated orange juices. Food and Chemical Toxicology. 43(9):1413–1422. http://dx.doi.org/10.1016/j.fct.2005.03.016
- McEachern, G. R. and Stein, L. A. 1997. Growing Olives in Texas Gardens. Extension Horticulturists Texas A & M University College Station, Texas 77843-2134.
- Hamdi, H.K., R. Castellon. 2005. Oleuropein, a non-toxic olive iridoid, is an antitumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 334: 769–778. http://dx.doi.org/10.1016/j.bbrc.2005.06.161
- Kastorini, C. M., H. J. Milionis, J. A. Goudevenos and D. B. Panagiotakos. 2010. Mediterranean diet and coronary heart disease: is obesity a link? Asystematic review. Nutrition, Metabolism, and Cardiovascular Diseases, 20: 536–551. http://dx.doi.org/10.1016/j.numecd.2010.04.006
- Marsilio, V. 2006. The use of LAB starters during table olive fermentation. In: Proceedings of the 2nd International Seminar Olivebioteq, 5–10 November 2006, Marsala-Mazara del Vallo, Italy, Seminars and invited lectures, pp. 221–233.
- Manna, C., V. Migliardi, P. Golino, A. Scognmiglio, P. Galletti, M. Chiariello, V. Zappia. 2004. Oleuropein prevents oxidative myocardial injury by ischemia and reperfusion. J. Nutr. Biochem. 15: 461–468. http://dx.doi.org/10.1016/j.jnutbio.2003.12.010
- Petroni, A., M. Blasevich, M. Salami, N. Papini, G. F. Montedoro, C. Galli. 1995. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb.Res. 78: 151–160. http://dx.doi.org/10.1016/0049-3848(95)00043-7
- Romero, C., M. Brenes, K. Yousfi, P. Garcia, A. Garcia and A. Garrido. 2004. Effect of cultivar and processing method on the contents of polyphenols in table olives. J. Agric. Food Chem. 52: 479-484. http://dx.doi.org/10.1021/jf030525l
- Rivas, A., D. Rodrigo, A. Martinez, G. V. B. Canovas and M. Rodrigo. 2006. Effect of PEF and heat pasteurization on the physicochemical characteristics of blended orange and carrot juice. Food science and technology 39: 1163-1170.
- Sabatini, N., E. Perri and V. Marsilio. 2009. An investigation on molecular partition of aroma compounds in fruit matrix and brine medium of fermented table olives. Innovative Food Sci. and Emerging Tech. 10: 621–626. http://dx.doi.org/10.1016/j.ifset.2009.05.001
- Steel, R.G.D., J. H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics - a biometrical approach (3rd edition). McGraw Hill Book Co. Inc., New York, USA.
- Visioli, F., C. Galli. 2001. Antiatherogenic components of olive oil. Curr. Atheroscler. Rep. 3: 64–67. http://dx.doi.org/10.1007/s11883-001-0012-0
- Visioli, F., S. Bellosta, C. Galli. 1998. Oleuropein, the bitter principles of olives, enhances nitric oxide production by mouse macrophages. Life Sci. 62: 541–546. http://dx.doi.org/10.1016/S0024-3205(97)01150-8
To share on other social networks, click on any share button. What are these?








