Influence of IBA Concentrations on the rooting Response of Olive Cultivars in Air-Layering
Research Article
Influence of IBA Concentrations on the rooting Response of Olive Cultivars in Air-Layering
Riaz Alam1*, Muhammad Sajid2 and Durri Shahwar3
1Horticultural Research Institute, National Agricultural Research Centre, Islamabad, Pakistan; 2Department of Horticulture, The University of Agriculture, Peshawar, Pakistan; 3Department of Agriculture, University of Swabi, KP, Pakistan.
Abstract | The research trial to evaluate the “Influence of IBA concentrations on the rooting response of olive cultivar in air-layering” was conducted at Olive Model Farm, Sangbhatti, Mardan-Pakistan during the year 2015 using Randomized Complete Block Design (RCBD). Air layering was practiced in cultivars: Frontoio, Manzanilla, Pendolino, Ottobratica and Picual during monsoon season. The de-barked portions at the terminal sides were applied with 0, 1000, 2000, 3000 and 4000 ppm IBA solution with the help of dropper. The daughter saplings of cultivar Manzanilla produced through air-layering by the application of IBA in various concentrations, produced early rooting (36.00 days), high rooting percentage (77.47%), maximum number of roots (6.38) per layering, root length (7.03 cm), root weight (2.62 g) and more number of re-sprouts (5.57), followed by number of days to root appearance (38.80), rooting percentage (65.73%), number of roots (5.69) and root length (6.14 cm) produced by the saplings of cultivar Frontoio. The layering treated with 2000 ppm IBA solution took less days to root appearance (35.13), accomplished 72.00% rooting success, generated 5.85 number of roots per layering with 6.65 cm length, 2.47 g root weight and 4.89 number of re-sprouts and 7.00 cm shoot length while the untreated saplings took maximum days to root appearance (50.40) and lowest rooting percentage (47.07%), and minimum number of roots (3.50) per layering, root length (3.93 cm), root thickness (2.28 mm), root weight (1.46 g), number of re-sprouts (3.21) per layering and shoot length (4.81 cm) were produced in daughter saplings treated with 4000 ppm IBA solution. It is concluded that the layerings of cultivars Frontoio, Manzanilla and Picual treated with 2000 ppm IBA showed better results for the studied attributes while Ottobratica and Pendolino responded efficiently to all these attributes, when treated with 1000 and 3000 ppm IBA solution respectively.
Received | April 23, 2021; Accepted | September 30, 2021; Published | December 09, 2021
*Correspondence | Riaz Alam, Horticultural Research Institute, National Agricultural Research Centre, Islamabad, Pakistan; Email: riazalam@parc.gov.pk
Citation | Alam, R., M. Sajid and D. Shahwar. 2022. Influence of IBA Concentrations on the rooting response of olive cultivars in air-layering. Sarhad Journal of Agriculture, 38(1): 238-248.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.1.238.248
Keywords | Olive cultivars, IBA concentrations, Air-layering, Rooting, Survival
Introduction
Olive is successfully grown in areas having mean annual temperature 15-20 °C, with a minimum of 4 °C and maximum of 40 °C but temperature below 4 °C can damage the olive trees (Loannis, 2009). Therefore, areas having long warm summer and mild winter are suitable for the production of quality fruit of olive. Pakistan is located in the same region have varied climatic and soil factors, due to which all sort of temperate, subtropical and tropical zone fruit trees can be grown. Olive being a sub-tropical plant is grown successfully in the subtropical mountainous region of Khyber Pakhtunkhwa and Balochistan (Baloch, 1994). The existence of wild olive (Oleo cuspidate), locally known as Zytoon in Urdu, Showan in Pushto, kow in Punjabi, Sindhi and Saraiki and Khat in Brahavi, indicates it’s adaptation in sub-continent of South Asia, including Pakistan.
Propagation of olive through sexual mean is usually not recommended, because seedlings are not true to type and take lot of time to bear fruits. Asexual means of propagation are mostly used to propagate olives commercially and the propagation methods include: cutting, air-layering, budding and grafting. Air-layering is usually done in the months of July and August as better rooting in olives induced when layered during humid conditions in monsoon (Rehman et al., 2013). Branches of 5-10 mm in diameter are selected from well developed trees, free from pests and diseases for air-layering and bark of 2-3 cm is removed, covered with soil or any other rooting medium and then wrapped with polythene film. When a good ball of roots develops, the branch is then cut off below the wrapped position after which it is generally placed in media to become firmly established before being set out permanently (Wilson, 1920).
Adventitious root formation has many practical implications in horticulture and there is a lot of commercial interest because of many plant species, those are difficult to root (Davies et al., 1994; Kovar and Kuchenbuch, 1994). The auxin, Indole-3-Acetic Acid (IAA) was the first plant hormone to be used to stimulate rooting of cuttings (Cooper, 1935) but synthetic auxin Indole-3-Butyric Acid (IBA) was even more effective than IAA (Zimmerman and Wilcoxon, 1935) and is now used commercially worldwide for rooting in many plant species (Hartmann et al., 1990). Indole-3-butyric acid was long regarded as a synthetic auxin, but recently has been shown to occur naturally in several plant species (Ludwig-Muller et al., 1993), and often more effective in root initiation, making it important in commercial propagation (De Klerk et al., 1999). In Arabidopsis, IBA induced adventitious roots and was widely used to generate roots on shoot explants (Marton and Browse, 1991).
Keeping in view the importance of IBA in rooting, the present study was undertaken to find out the appropriate concentration of IBA for rooting of olive cultivars through air-layering.
Materials and Methods
Influence of IBA concentrations on the rooting response of olive cultivar in air-layering was evaluated during 2015 at Olive Model Farm Sangbhatti, situated in Mardan, Khyber Pakhtunkhwa-Pakistan (Altitude: 375 m; Latitude: 34°16ʹ21.32ʺN; Longitude: 72°18ʹ06.33ʺ). The region has an average annual rainfall of around 600 mm, mainly occurs in July-August and the dry season lasts from May-June. Annual rainfall during 2015 was 630 mm. Mean minimal temperature ranged from 2° C in the coldest month (December-January) to 25 °C in the hottest month (June-July), while the mean maximum temperatures for the same months varied from 18 to 38 °C.
Air layering was practiced in one year old healthy shoots at all the aspects of the canopy in healthy and productive mother plants of olive cultivars: Frontoio, Manzanilla, Pendolino, Ottobratica and Picual during monsoon season. The layerings were treated with
0 (control), 1000, 2000, 3000 and 4000 ppm IBA solutions with the help of dropper. Fifty layering were practiced for each 25 treatments replicated three times with total of 3750 layerings in the trial.
The moist medium of silt, saw dust and garden soil (1:1:1 by volume) was placed around the 2-3 cm debarked area of the branch for rooting and wrapped with poly-ethylene film to conserve the moisture at the root zone. After the roots grown outside the ball of medium, the daughter saplings of each treatment were detached from the mother plants and planted on its own roots for growth and development, in polythene bags containing media of silt, garden soil and compost in equal proportion by volume.
Attributes studied
To investigate the “Influence of IBA concentrations on the rooting response of olive cultivars in air-layering” data on following attributes were recorded and analyzed accordingly:
Days to root appearance
Days between air-layering and root appearance in the layered portion were counted for each treatment and average value for days to root appearance was worked out.
Rooting percentage (%)
Rooting percentage was computed by the formula;
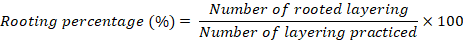
Number of roots layering-1
Five layering for each treatment were detached randomly from the mother plants, roots were exposed by washing with water, counted the number of roots in each layering and computed their averages.
Root length (cm)
Five rooted layers were detached randomly in each treatment, roots were exposed by washing with water and root length from the point of induction to the tip of the longer root was measured and calculated their average values.
Root thickness (mm)
Root thickness of the exposed roots were measured with the help of vernier caliper and averaged for five samples taken randomly for each treatment.
Root weight (g)
Root weight for five randomly taken air-layering were determined with the help of electronic balance and computed their average values.
Number of re-sprouts plant-1
The number of re-sprouted shoots in five randomly taken saplings for each treatment were counted and averaged.
Shoot length (cm)
The length of the re-sprouted shoots was measured and averaged for randomly taken five saplings in each treatment.
Statistical Procedure
The experiment was laid out in Randomized Complete Block Design (RCBD) with two factors factorial arrangements replicated three times. The data were analyzed according to factorial analysis using Statistix-8.1 software. If the data were found significant, these were subjected to Least Significant Difference Test (LSD, P ≤ 0.05), for mean comparison (Steel et al., 1997).
Results and Discussion
Days to root appearance
Olive cultivars responded variously for number of days taken for root appearance in air-layering when treated with different concentrations of IBA. The Statistical analysis of data (P ≤ 0.05) revealed that number of days to root appearance of olive cultivars through air-layering was significantly influenced by cultivars, IBA concentrations and their interaction.
Maximum number of days to root appearance (48.40) was taken by the layers of cultivar Pendolino for roots development and appearance, followed by 42.93 numbers of days to root appearance, recorded in layered branches of cultivar Picual. The layers of Manzanilla produced early rooting and took minimum days (36.00) for root development and appearance. The untreated layerings took more days to root appearance (50.40) in comparison with the layerings treated with 2000 ppm IBA solution, which took 35.13 days and statistically at par with the treatment of 3000 ppm that produced and developed roots within 35.93 days (Table 1).
Table 1: Days to root appearance, rooting percentage (%), number of roots and root length (cm) of olive cultivars as affected by different concentration of IBA in air-layering.
|
Cultivars (Cv) |
Parameters |
|||
|
Days to root appearance |
Rooting percentage (%) |
Number of roots |
Root length (cm) |
|
|
Frontoio |
38.80c |
65.73b |
5.69b |
6.14b |
|
Manzanilla |
36.00d |
77.47a |
6.38a |
7.03a |
|
Ottobratica |
41.00bc |
55.33c |
4.81c |
4.83c |
|
Pendolino |
48.40a |
48.27d |
3.38d |
3.83d |
|
Picual |
42.93b |
57.73c |
4.99c |
4.33cd |
|
LSD (α = 0.05) |
2.4536 |
3.7840 |
0.3210 |
0.5883 |
|
IBA concentrations (IBA) |
||||
|
0 (Control) |
50.40a |
47.07d |
5.04c |
4.62c |
|
1000 ppm |
40.60c |
63.87b |
5.56ab |
5.65b |
|
2000 ppm |
35.13d |
72.00a |
5.85a |
6.65a |
|
3000 ppm |
35.93d |
66.00b |
5.29bc |
5.31b |
|
4000 ppm |
45.07b |
55.60c |
3.50d |
3.93d |
|
LSD (α = 0.05) |
2.4536 |
3.7840 |
0.3210 |
0.5883 |
|
Interaction of olive cultivars and IBA concentrations (Cv×IBA) |
||||
|
Significance levels |
*(Figure 1) |
*(Figure 2) |
*(Figure 3) |
*(Figure 4) |
Mean followed by similar letter(s) in column do not differ significantly from one another.
* = Significant at P ≤ 0.05
A significant variation was recorded for the interaction of olive cultivars and IBA concentrations regarding number of days to root appearance through air-layerage. The data revealed that untreated layerings of cultivar Pendolino took more number of days to root appearance. On other side the layering of Manzanilla, treated with 2000 ppm IBA required less number of days to root appearance (Figure 1).
The cultivars greatly affect the days to rooting in olives (Rehman et al., 2013), early rooting in Manzanilla might be linked with the genetic potential and response of the cultivar to rooting through air-layering, also the activity of cambium tissues, synthesis, accumulation and utilization of photosynthate and growth promoters influenced rooting (Sharma and Srivastav, 2004). Furthermore, the days to rooting were different in olive cultivars (Kareem et al., 2013) and optimum concentration of IBA stimulated rooting in olive (Gautum and Chauhan, 1990) also it played a vital role in numerous aspects of growth and development, including vascular development and root initiation (Davies, 1995).
Rooting percentage (%)
Statistical analysis of the data revealed that cultivars, IBA concentration and their interaction had significantly affected (P ≤ 0.05) the rooting percentage in air-layering of olive cultivars.
The mean data showed that high rooting percentage (77.47%) was obtained in the IBA treated air-layerings of cultivar Manzanilla, followed by rooting percentage (65.73%) noted in layerings of cultivar Frontoio, the means of both were significantly varied from each other and rest of the cultivars, while less rooting percentage (48.27%) was achieved by the layerings of Pendolino.
Rooting percentage was increased from control to 2000 ppm IBA treatment and then started decline as the concentration further enhanced till 4000 ppm. The highest rooting percentage (72.00%) was recorded in saplings produced through air-layering, treated with 2000 ppm IBA solution followed by the 66.00% rooting attained by the saplings, treated with 3000 ppm and their means were statistically dissimilar from each. The lowest rooting percentage (47.07%) was achieved in saplings of the control treatment (Table 1).
The interaction between cultivars and IBA concentration in the air-layering indicated that high percentage of rooting was attained by the layerings of Manzanilla, treated with 2000 ppm IBA solution, while low percentage of rooting was recorded in the untreated saplings of Pendolino (Figure 2).
Root development and survival in air-layering largely depend on operation time and optimal use of growth regulators (Vyas, 1938), many endogenous and exogenous factors like genetic makeup, physiological influences, environment and status of the mother plant (Nemeth, 1986). Olive cultivars differ widely in their ability to root; some produce roots easily, whereas others are very difficult to root (Sutter, 2005).
The rooting compounds promoted the root growth (John, 2004) and air-layering at appropriate time supported by the optimal concentration of IBA enhanced rooting. Similarly, the rooting attributes of litchi were improved by the application of 2500 ppm of IBA through air-layering (Rahman et al., 2000).
Number of roots layering-1
The number of roots produced by the saplings of olive cultivars through air-layering was significantly affected by cultivars, IBA concentrations and their interaction at P ≤ 0.05.
Olive cultivars vary in rooting potential, more roots (6.38) were generated in the IBA treated layerings of Manzanilla, followed by the number of roots layering-1 (5.69) produced by the saplings of cultivar Frontoio and were statistically different from each other and rest of the cultivars, while less number of roots layering-1 (3.38) was noted in the plants, developed through air-layering in Pendolino.
Application of IBA at optimal dose triggered the intensity of adventitious rooting, more number of roots layering-1 (5.85) was recorded in the air-layering, treated with 2000 ppm IBA solution, followed by 5.56 number of roots layering-1 induced by the application of 1000 ppm IBA solution, while less number of roots (3.50) were generated in the layering treated with 4000 ppm IBA (Table 1).
Response of olive cultivars to IBA concentrations as interaction was not similar; Manzanilla, Frontoio and Picual produced more roots, when layerings were treated with 2000 ppm IBA solution, while more roots were generated in the layerings of Ottobratica and Pendolino, treated with 1000 and 3000 ppm IBA solution respectively. The highest number of roots was observed in the layering of Manzanilla treated with 2000 ppm IBA solution, while few roots were produced by sapling in control treatment of Pendolino (Figure 3).
More roots in Manzanilla and Frontoio might be due the genetic potential of these cultivars, supported by the application of optimal dose of IBA, also early and high rooting percentage was observed in these cultivars which provided additional opportunity to the layerings for the production of more roots. Adventitious root induction is influenced by number of external and internal factors (Gyana, 2006) and application of exogenous auxins play a vital role in the rooting capacity, whereas most of the commercial propagation is practiced with the application of IBA (Kotis et al., 2009). Rooting in ‘Domat’ olive was significantly stimulated by the application of 1000 mg L-1 IBA (Murat and Elmas, 2008), also the rooting response and number of roots in olive cultivars were significantly different (Ahmad et al., 2014). Furthermore, the results of Awan et al. (2003) are in partial support of the present results, who reported maximum rooting in Frontoio, compared with other cultivars under trial. IBA increased both the percentage and number of roots by enhancing the cell wall elasticity that accelerated cell division and stimulated percentage and number of roots upto certain level (Rahman et al., 2000). The IBA significantly increased the production of roots and application of IBA at 2000 ppm might have triggered the cell division and root primordia formation. The differentiation of phloem ray parenchyma cells into root primordia depends upon the concentration of auxin (Sabatini et al., 1999). Further, the differentiating cells required the most appropriate auxin to become competent to respond to the organogenic signal (Blakesley and Chaldecott, 1997). The IBA enhanced the cell division of first root initials in Avocado (Wynne and McDonald, 2002).
Root length (cm)
Significant variations (P ≤ 0.05) among olive cultivars, IBA concentrations and their interaction, regarding root length were observed in the daughter saplings produced through air-layering.
Olive cultivars respond variously to root related attributes. The lengthy roots (7.03 cm) were produced in the IBA treated layering of cultivar Manzanilla, followed by root length (6.14 cm) noted in saplings of cultivar Frontoio. The minimum root length (3.83 cm) was produced by plants of Pendolino.
Indole butyric acid has great influence on adventitious root production in olive. The lengthy roots (6.65 cm) were produced in the layering treated with 2000 ppm IBA solution, followed by 5.65 and 5.31 cm long roots generated by the application of 1000 and 3000 ppm IBA solution respectively. The minimum root length (3.93 cm) was noted in the layering treated with 4000 ppm solution (Table 1).
The interaction effect in terms of root length was significantly different for various olive cultivars and IBA concentrations. The longest roots were observed in the layerings of Manzanilla treated with 2000 ppm IBA solution and short were produced in the layering of Pendolino, treated with 4000 ppm IBA solution (Figure 4).
Production of lengthy root by saplings of cultivar Manzanilla and Frontoio might be due to the fact that both cultivars generated more and early rooting hence got enough time for root growth and development, lead to increased root length as compared to other cultivars under trail. The increase in root length by optimal IBA levels might be due to fact that rooting hormone significantly increased the roots related attributes of olive and also can be linked with the effect of growth regulator (IBA) on the metabolites translocation and carbohydrates metabolism (Siddiqui and Hussain, 2007). Application of IBA fastens the callus formation in the air-layered portion and makes way to callus portion to initiate roots and increase the other rooting attributes (Cerveny and Gibson, 2005).
Root thickness (mm)
The cultivars, IBA concentrations, and interaction of cultivars and IBA concentrations significantly influenced the root thickness of air-layered saplings produced by olive cultivars.
Root thickness is cultivar dependent attribute, the air-layered saplings of cultivar Pendolino developed thicker roots (3.69 mm), followed by the root thickness (2.84 mm) noted in the saplings of cultivar Manzanilla, while minimum root thickness (2.34 mm) was recorded in the plants of Ottobratica, produced through air-layering.
The root related attributes are directly associated with proper application of Indole-3 butyric acid. The air-layerings treated with 2000 ppm IBA solution developed maximum root thickness (3.34 mm), followed by root thickness (3.12 mm) in daughter saplings of olive cultivars when treated with 1000 ppm IBA solution. Minimum root thickness
(2.28 mm) was recorded in layerings, treated with 4000 ppm IBA solution (Table 2).
Table 2: Root thickness (mm), root weight (g), number of re-sprouts and shoots length (cm) of olive cultivars as affected by different concentration of IBA in layering.
|
Cultivars (Cv) |
Parameters |
|||
|
Root thickness (mm) |
Root weight (g) |
Number of re- sprouts |
Shoot height (cm) |
|
|
Frontoio |
2.50c |
1.84bc |
4.44b |
7.55a |
|
Manzanilla |
2.84b |
2.62a |
5.57a |
6.49b |
|
Ottobratica |
2.34d |
1.88b |
2.61d |
5.91c |
|
Pendolino |
3.69a |
1.70c |
4.01c |
5.43d |
|
Picual |
2.52c |
1.98b |
4.61b |
5.33d |
|
LSD (α = 0.05) |
0.0961 |
0.1407 |
0.3241 |
0.3608 |
|
IBA concentration (IBA) |
||||
|
O (Control) |
2.47d |
1.76d |
3.99c |
6.17c |
|
1000 ppm |
3.12b |
2.25b |
4.73ab |
6.55b |
|
2000 ppm |
3.34a |
2.47a |
4.89a |
7.00a |
|
3000 ppm |
2.69c |
1.94c |
4.41b |
6.18bc |
|
4000 ppm |
2.28e |
1.46e |
3.21d |
4.81d |
|
LSD (α = 0.05) |
0.0961 |
0.1407 |
0.3241 |
0.3608 |
|
Interaction of olive cultivars and IBA concentration (Cv × IBA) |
||||
|
Significance levels |
*(Figure 5) |
*(Figure 6) |
*(Figure 7) |
*(Figure 8) |
Mean followed by similar letter(s) in column do not differ significantly from one another.
* = Significant at P ≤ 0.05
The interaction effect of cultivars and IBA concentrations in terms of root thickness varied for studied cultivars; the plants of Manzanilla, Frontoio and Picual produced thicker roots in air-layerings, treated with 2000 ppm IBA solution, while more root thickness was observed in saplings of Ottobratica and Pendolino when treated with 1000 and 3000 ppm IBA solution respectively. The plants of cultivar Pendolino produced more root thickness when treated with 3000 ppm IBA solution, while on other side less root thickness was noted in plants of Ottobratica, treated with 4000 ppm IBA solution (Figure 5).
The root thickness significantly varied in different olive cultivars; Pendolino categorized as hard to root produced few, short but thicker roots and might be associated with the genetic potential of the variety. The plant species those are difficult to root have many practical implications in propagation which is of commercial interest (Kovar and Kuchenbuch, 1994) but the use of IBA can stimulate rooting of mother stock (Cooper, 1935). Moreover, one of the key properties of IBA is the increased rate of cell division and enlargement; it also increases the activity of nutrient uptake which lead to the increase in root thickness (Siddiqui and Hussain, 2007), more root growth and development was recorded in the layers of litchi plants, treated with 2500 ppm IBA solution (Rahman et al., 2000).
Root weight (g)
Significant variations were observed at P ≤ 0.05 among cultivars, IBA concentrations and their interaction regarding root weight in the daughter saplings of olive cultivars produced through air-layering.
The mean data of Table 2 showed that heavy root weight (2.62 g) was produced by layerings of Manzanilla which is significantly different from rest of the cultivars followed by the root weight (1.98 g) developed by the daughter saplings of cultivar Picual, while less root weight (1.70 g) was generated by the saplings of Pendolino.
An increasing trend in root weight of the layerings was observed from 0 (control) to 2000 ppm treatment and then declined with the increase in IBA concentration. Heavy root weight (2.47 g) was recorded in the layerings, treated with 2000 ppm IBA solution, followed by root weight (2.25 g) induced by the daughter saplings, treated with 1000 ppm IBA solution, while less root weight (1.46 g) was produced by the layerings treated with 4000 ppm IBA solution.
The interaction between olive cultivars and IBA concentrations in air-layering indicated that heavy root weight was produced by daughter saplings of Manzanilla, treated with 2000 ppm while less root weight was recorded for the layerings of cultivar Picual when treated with 4000 ppm IBA solution (Figure 6).
The reason for increased root weight in daughter saplings of cultivar Manzanilla might be due the fact that response of this cultivar to rooting percentage, number of roots and root length was significantly better as compared to other cultivars under trail that might have led to the incremental biomass of the root and ultimately increased the root weight. The IBA significantly affected root weight as it triggers root initiation and growth, by increasing the rate of cell division, elongation and cell enlargement (Muller et al., 2005). The studies on the hydrolytic enzymes found during root formation, after IBA treatment in the mother stock revealed that the genes for endo-b-1, 4-glucanase were expressed in the area of adventitious root primordia formation and in the cortex, where softening of the cell walls was in progress in order to enable root emergence (Shoseyov et al., 1989), once the root emergence occur the growth and development progressed. Moreover, acid-induced cell wall loosening are thought to be responsible for expansions and are expressed in rapidly growing tissues (McQueen-Mason, 1995) that lead to add bio-mass in roots. The IBA is more effective in root growth and development (Tomar, 2016) and during anabolism; the cambium constantly produces cells that differentiate into the phloem towards the outside, and the xylem towards the inside of the organ. At wounding, an undifferentiated cell mass, or callus, is produced, primarily to seal and heal the wound, when particular hormone concentrations are present; these cells can differentiate into functioning root and adds mass to it.
Number of re-sprouts plant-1
Statistical analysis of the data showed that number of re-sprouts after detachment and transplantation was significantly affected by cultivars, IBA concentration as well as their interaction at P ≤ 0.05.
The number of re-sprouts is directly linked with cultivars and indirectly associated with the concentration of IBA, more number of re-sprouts (5.57) was noted in the daughter saplings of cultivar Manzanilla, followed by number of re-sprouts (4.61 and 4.44) developed by plants of cultivars Picual and Frontoio respectively, with statistically similar means, while less number of re-sprouts (2.61) was noted in the detached saplings of Ottobratica.
The number of re-sprouts increased in the daughter saplings from control treatment till 2000 ppm IBA concentration and then started decline. Maximum number of re-sprouts (4.89) was noted in saplings, treated with 2000 ppm IBA, followed by 4.73 number of
re-sprouts produced by the saplings treated with 1000 ppm IBA, while few (3.21) were produced in 4000 ppm IBA treatment (Table 2).
In the interaction more re-sprouts were produced by the detached daughter sapling of Manzanilla, treated with 2000 ppm IBA, while few were developed in the sapling of Ottobratica, treated with 4000 ppm IBA solution (Figure 7).
The number of re-sprouts in the detached saplings is associated with the activation of vegetative buds and is cultivar dependent attribute. The emergence of sprouts (shoots) also depends on the rooting success of cultivar that provides nutrients for shoot development. More re-sprouts produced by Manzanilla might be due to the development of vigorous root system through air-layering due to the continuous supply of nutrients through xylem intact that led to the increased number of shoots (Sharma and Srivastav, 2004).
Shoot length (cm)
Significant differences were noted (P ≤ 0.05) among cultivars, IBA concentrations and their interaction regarding shoot length developed by the detached daughter saplings produced through air-layering.
The mean data in Table 2 indicated that shoot length of the re-sprouts was significantly affected by olive cultivars. The maximum shoots length (7.55 cm) was produced by saplings of cultivar Frontoio, followed by (6.49 cm) shoot length recorded in the plants of Manzanilla, while less shoot length (5.33 cm) was noted in the daughter saplings of cultivar Picual.
Indole butyric acid promotes advantatious rooting that leads to enhance aerial vegetative growth. An increasing trend in shoot length of the re-sprouts was observed from control treatment till 2000 ppm IBA application and then started decline. The highest value of shoot length (7.00 cm) was noted in the daughter saplings obtained from the layerings, treated with 2000 ppm IBA solution, followed by shoot length (6.55 cm) developed by the saplings produced from layerings, treated with 1000 ppm IBA solution, while short shoots (4.81 cm) were produced in the daughter saplings generated from the layerings, treated with 4000 ppm IBA solution (Table 2).
Regarding the interaction, lengthy shoots were recorded in the saplings of Frontoio produced from the layers treated with 2000 ppm IBA, while the short shoot length was recorded in daughter saplings of Pendolino, produced from layers when treated with
4000 ppm IBA solution (Figure 8).
Shoot length is a triat linked directly with cultivars and indirectly associated with the application of IBA. Frontoio might have utilized the available resources efficiently for the development of long shoots, favoured by the conducive environmental conditions. Furthermore, auxin have augmenting role in roots development in air-layering, the exogenous application of IBA enhanced the root initiation and induction in short duration (Bhojvaid and Negi, 2003). Once roots developed it absorbed nutrients efficiently and ultimately triggered the vegetative growth including shoot height. The number and length of shoots were significantly influenced by the application of IBA in daughter plants of litchi produced through air-layerings (Sharma et al., 1990) and application of 2500 ppm IBA solution increased shoot length in saplings of litchi, developed in air-layering (Rahman et al., 2000).
Conclusions and Recommendations
It is concluded that the daughter saplings of cultivars Frontoio, Manzanilla and Picual propagated through air-layering produced early rooting, high survival percentage and vigorous root system, when treated with 2000 ppm IBA solution while the layers of Ottobratica and Pendolino responded effectively to all the studied attributes when treated with 1000 and 3000 ppm IBA solution respectively.
Acknowledgements
I feel pleasure to thank supervisor of the study Dr. Muhammad Sajid, Associate Professor and Syed Tanveer Shah, Lecturer, Department of Horticulture for providing sincere help and cooperation.
Novelty Statement
Air-layering is considered a successful tool in olive propagation but the rooting percentage was low with conventional practice. The study helped in optimization of IBA concentration to be applied in air-layering for profuse rooting and plants survivability in promising olive cultivars.
Author’s Contribution
Riaz Alam: Conducted the research, collected andstatistically analyzed the data, review of literature and
manuscript write-up.
Muhammad Sajid: Planned, supervised and provided technical assistance.
Durri Shahwar: Helped in data compilation and data analysis.
Conflict of interest
There is no conflict of interest among the authors of the manuscript.
References
Ahmad, S., F. Wahid, M. Sajid, I. Hussain, S. Ahmed, N. Ahmad, K. Zeb, A.A. Awan and N. Ahmed. 2014. Propagation of olive cultivars through air layerage. IOSR J. Agric. Vet. Sci., 7: 121-125. https://doi.org/10.9790/2380-0721121125
Awan, A., A. Iqbal, M.J. Rahman and G. Idris. 2003. Response of hardwood cuttings to different growth media and basal injuries for propagation. Asian J. Pl. Sci., 2(12): 883-886. https://doi.org/10.3923/ajps.2003.883.886
Baloch, A. 1994. Hort. Phases of plant growth. National Book Foundation Islamabad. pp. 633.
Bhojvaid P.P., S. Negi. 2003. Propagation of Elaeocarpus ganitrus by air layering. Indian Forester, 129(10): 1185- 1191.
Blakesley, D. and M.A. Chaldecott. 1997. The role of endogenous auxin in root initiation. Plant Growth Reg., 13(1): 77-84. https://doi.org/10.1007/BF00207595
Cooper, W.C. 1935. Hormones in relation to root formation on stem cuttings. Plant Physiol., 10: 789-794. https://doi.org/10.1104/pp.10.4.789
Cerveny, C. and J. Gibson. 2005. Rooting hormones. Grower 101. Crop Cultivation, Pp. 36-44.
Davies, P.J. 1995. Plant hormones. Plant physiology, biochemistry and molecular biology. 2nd Ed. Kluwer Academic Publishers, Dordrecht-Boston-London. Pp: 833.
Davist, D. and B.E. Haissig.1994. Biology of adventitious root formation. Plenum Press, New York, pp. 358. https://doi.org/10.1007/978-1-4757-9492-2
De Klerk, G.J., W.V.D. Krieken and J.C.D. Jong. 1999. The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant, 35: 189-199. https://doi.org/10.1007/s11627-999-0076-z
Gautum, R. and W. Chauhan. 1990. Hydrogen peroxide and indole-3-butyric acid effects on root induction and development in cuttings of Olea europaea L. (cv. Frantoio and Gentile di Larino). Adv. Hort. Sci., 1:7-12.
Gyana, R.R. 2006. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Reg., 48(2): 111-117. https://doi.org/10.1007/s10725-005-5665-1
Hartmann, H.T., D.E. Kester and F.T. Davies. 1990. Plant propagation: principles and practices. Englewood Cliffs, NJ: Prentice-Hall. Pp. 246-247.
John, R.H. 2004. The basics of plant growth; (Part 3) root formation in cuttings. Article source. http:// ezinearticles.com/?expert=John_R._Haughton.
Kareem, A., M.J. Jaskani, B. Fatima and B. Sadia. 2013. Clonal multiplication of guava through softwood cuttings under mist conditions. Pak. J. Agric. Sci., 50: 23- 27.
Kotis, M., T.A. Yupsanis, T.D. Syros and A.S. Economou. 2009. Peroxidase, acid phosphatase, RNase and DNase activity and isoform patterns during in vitro rooting of Petunia hybrida microshoots. Biol. Plant., 53(3): 530-538. https://doi.org/10.1007/s10535-009-0096-x
Kovar, J.L. and R.O. Kuchenbuch. 1994. Commerical importance of adventitious rooting to agronomy. In: Davis TD, Haissig BE, eds. Biology of adventitious root formation. New York: Plenum Press. pp. 25-34. https://doi.org/10.1007/978-1-4757-9492-2_2
Loannis, T. 2009. CAB International, Olives, Climatic and Soil Conditions, pp. 51. https://doi.org/10.1079/9781845934583.0051
Ludwig-Muller, J. and E. Epstein. 1993. Indole-3-butyric acid in Arabidopsis thaliana. II. In vivo metabolism. Plant Growth Reg., 13: 189-195. https://doi.org/10.1007/BF00024261
Marton, L. and J. Browse. 1991. Facile transformation of Arabidopsis. Plant Cell Rep., 10: 235-239. https://doi.org/10.1007/BF00232565
McQueen-Mason, S. 1995. Expansins and cell wall expansion. J. Exp. Bot., 46: 1639-1650. https://doi.org/10.1093/jxb/46.11.1639
Muller, J.L., A. Vertocnik and C.D. Town. 2005. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J. Exp. Bot., 56(418): 2095-2105. https://doi.org/10.1093/jxb/eri208
Murat, I. and O. Elmas. 2008. Rooting of Olea europaea ‘Domat’ cuttings by auxin and salicylic acid treatments. Pak. J. Bot., 40(3): 1135-1141.
Nemeth, M. 1986. Virus, Mycoplasma and Rikkeetcia diseases of fruit tree. Martinus, Nijhoff. Publishers and Akademai Kiodo, Budapest. pp. 750.
Rahman, M.N., I. Hussaini, M. Imran, T. Jan and A.A. Awan. 2000. Effect of different concentrations of IBA on rooting of litchi (Litchi chinensis) in air-layering. Pak. J. Biol. Sci., 3:330-331. https://doi.org/10.3923/pjbs.2000.330.331
Rehman M., A.A. Awan, O. Khan and I. Haq. 2013. Response of olive cultivars to air-layering at various timing. Pak. J. Agric. Sci., 50(4): 555-558.
Sabatini, S., D. Beis, H. Wolkenfelt, J. Murfett, T. Guilfoyle, J. Malamy, P. Benfey, O. Leyser, N. Bechtold, P. Weisbeek and B. Scheres. 1999. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 99:463-472. https://doi.org/10.1016/S0092-8674(00)81535-4
Sharma, R.R. and M. Srivastav. 2004. Plant propagation and Nursery Management. 1st Ed. Int. Book Distributing Co, Lucknow. pp. 146-226.
Sharma, S.B., P.K. Ray and B.K. Singh. 1990. Note on rooting and survival of litchi layers. Indian J. Hort., 47: 174-176.
Shoseyov, L., E.G. Sutter, E. Epstein and O. Shoseyov. 1989. IBA induces b-1,3-glucanase activity in 20-d-old mung bean cuttings. Plant Growth Soc. Amer. Quart., 17: 92.
Siddiqui, M.I. and S.A. Hussain. 2007. Effect of indole butyric acid and types of cuttings on root initiation of Ficus Hawaii. Sarhad J. Agric., 23(4): 919-926.
Statistix_8. 2006. Statistix 8 user guide, version 2.0. http://www.um.es/jmpaz/tayga/IEGARH/Statistix-UserGuide-ver2_0.pdf.
Steel, R.G.D., J.H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics: A biological approach. McGraw-Hill. pp. 12.
Sutter, E.G. and J.D. Cohen. 1992. Measurement of indolebutyric acid in plant tissues by isotope dilution gas chromatography-mass spectrometry analysis. Plant Physiol., 99: 1719-1722. https://doi.org/10.1104/pp.99.4.1719
Tomar, A. 2016. Impact of seasonal changes on air layering and rooting hormone in Spondias pinnata (J. Koenig ex L. f.) Kurz. Trop. Plant Res., 3(1): 131-135.
Vyas, N.D. 1938. The litchi. U. P. Dept. Agric. Bull. 12: 10-12.
Wilson, P. 1920. Manual of tropical and subtropical fruits. MacMillan publishing Co., Inc. New York. pp. 321-323.
Wynne, J. and M.S. McDonald. 2002. Adventitious root formation in woody plant tissue: The influence of light and indole-3-butyric acid. In vitro Cell Dev. Biol., 38(2): 210-212. https://doi.org/10.1079/IVPIVP2001266
Zimmerman, P.W. and F. Wilcoxon. 1935. Several chemical growth substances which cause initiation of roots and other responses in plants. Contributions of the Boyce Thompson Institute. 7: 209-229.
To share on other social networks, click on any share button. What are these?







