Efficient Decolorization of Water and Oil-Soluble Azo Dyes by Enterococcus avium Treated with HP-β-CD
Efficient Decolorization of Water and Oil-Soluble Azo Dyes by Enterococcus avium Treated with HP-β-CD
Peng Song1,2, Wei Feng2, Haiying Shi2, Jinsheng Zhao3, Renmin Liu3 and Wei Xu2,*
1Lab of Enzyme and Applied Microbiology, College of Bioengineering, Tianjin University of Science and Technology, Tianjin 300457, China
2School of Life Sciences, Liaocheng University, Liaocheng 252000, China
3College of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252000, China
ABSTRACT
The industrial effluents containing azo dyes will contaminate the environment without decolorization. Bacterial decolorization generally demonstrates good color removal effects. In this study, the capability of Enterococcus avium to decolorize both water and oil-soluble azo dyes were investigated. The bacteria was incubated under static conditions in the presence of 50mg/L Amaranth or 5 mg/L Sudan I in LB media at 37°C for 10 h, and reduction of the dyes was monitored. The preferable pH for the decolorization of Amaranth by ts17 strain was between pH 6.0-9.0, and with the optimum pH at 7.0. The promotion effect of NaCl on the decoloration of Amaranth up to 5% (w/w) content, and the inhibition action appeared at 7% NaCl. The maximum decolorization concentration of the Amaranth reached 1450mg/L. Mg2+ and Fe3+ could accelerate Amaranth decolorization, and Cu2+, Zn2+, Co2+ showed inhibition of Amaranth decolorization. Compared with the traditional method dissolving oil-soluble dye in DMSO, HP-β-CD was used to monitor the decolorization rate of the sudan I and appropriate molar ratio of HP-β-CD to dye (nC:nS=9:1) effectively improved the decolorization rate from 85.45% to 99.45% after 8 reaction.
Article Information
Received 21 August 2018
Revised 19 November 2018
Accepted 14 December 2018
Available online 22 February 2019
Authors’ Contribution
WF designed the study and purchased materials. PS and HS performed the experimental work, analyzed the data and wrote the article. JS, RL and WX helped perform the analysis with constructive discussion.
Key words
Azo dyes, Decolorization, Enterococcus avium, HP-β-CD.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.2.675.680
* Corresponding author: xuwei@lcu.edu.cn
0030-9923/2019/0002-0675 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Azo dyes represent a major group of dyes causing environmental concern because of their color, bio-recalcitrance, and potential toxicity to animal and human (Pierce et al., 2003; Forgacs et al., 2004). They reduce the amount of sunlight to photosynthetic organisms resulting in decreased oxygen levels in aquatic ecosystems (Yogesh et al., 2013). The presence of dyes or their degradation products in water even at very low concentrations can be toxic and sometimes carcinogenic, mutagenic, or teratogenic to various organisms, including human being (Novotny et al., 2006; Hai et al., 2007). Development of an efficient dye degradation technology requires application of suitable strains and its use under favorable conditions to realize its degradation potential (Novotny and Nenov, 2004). Most studies on azo dye biodegradation have focused on bacteria and fungi, where bacteria are found to be more efficient (Elisangela et al., 2009; Vijaykumar et al., 2007; Aftab et al., 2011).
Cyclodextrins (CDs) are cyclic (α-1, 4)-linked oligosaccharides with different numbers of α- D -glucopyranose units (Larsen et al., 1998). Cyclodextrins have a toroidal or cone shape due to the lack of freely rotating bonds between glucopyranose units and possess a hydrophilic outer surface and a hydrophobic inner cavity in which hydrophobic small guest molecules can reside. In addition to this hydrophobic interaction, other interactions such as Van der Waals forces, electrostatic interactions, and hydrogen bonding interactions can also be driving forces for complex formation of guest molecules with CDs (Szejtli, 1998) .This property provides CDs with the capacity to increase the aqueous solubility of hydrophobic drugs (Loftsson and Brewster, 1996; Stella and Rajewsk, 1997). But it is rarely used to improve the solubility of oil soluble dyes. Amongst cyclodextrins, hydroxypropyl-β-cyclodextrin (HP-β-CD) possesses greater ability to accommodate several lipophilic molecules (Miro et al., 2006).
In the present work, the dye decolorization efficiency of strain ts17 isolated from activated sludge is studied. Amaranth and sudan (Table I) were chosen to study the influence of different conditions on the decolorization ability of the bacteria and its ability of water and oil-soluble dye decorloring. Previously, it was demonstrated that water-soluble azo dyes can freely move in medium, pass through cell walls more easily than water-insoluble azo dyes, and are degraded by azoreductases in several other bacteria (Chen, 2006). Although a few reports described the ability of some bacteria to decorlor both water and oil-soluble azo dyes (Chen et al., 2009). Furthermore, no report regarding either the decorlorization of both azo dyes by Enterococcus avium or its application to improve the decoloration rate of oil soluble dyes with HP-β-CD has been published.
Table I.- Chemical structures of water-soluble azo dye Amaranth and oil-soluble azo dye Sudan I used in this study.
Materials and methods
Chemicals and media
The dyes such as Amaranth and Sudan I used in this study were supplied by Aladdin chemical, China. The other chemicals used were of analytical grade.
The basal culture medium used for the dye decolourization studies were LB medium with azo dyes. Regarding the influence of culture conditions on dye decolorization, some basic conditions were changed and others remained unchanged.
Isolation and cultivation of microbes
The activated sludge, which was obtained from a plant sewage treatment located at Shandong Province, eastern China, was used as a source for the isolation of bacterial strains. The colony showing maximum decolourization zone named ts17 was transferred to the broth containing the same dye (50 mgL−1) to test the decolourization potential of the bacterium in static liquid medium at 37°C and drawn growth curve of bacterium ts17 on the same condition.
16S rDNA sequencing
Extraction of genomic DNA was performed according to the method of Sambrook and Russell. PCR amplification of the 16S rRNA gene was performed using the following two primers: 27F (5’-AGAGTTTGATCMTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT-3’).
The purified gene fragment was sequenced by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd. Phylogenetic neighbours were identified using GenBank (http://www.ncbi.nlm.nih.gov/blast/), and multiple alignments were generated using CLUSTAL X. A phylogenetic tree was constructed from the evolutionary distance data with the neighbour-joining, maximum-likelihood PHYLIP (phylogeny inference package, version 3.5c. Department of Genetics, University of Washington, Seattle) and maximum-parsimony methods using MEGA 5.0, and bootstrap values were calculated based on 1,000 replicates.
Decolorization of amaranth by growing cells
In order to study the effect of initial dye concentration on dye decolorization potential and tolerance of the strain ts17, the isolate was cultivated for more than 4 days and was amended with different concentrations (50-2000mg/L) of Amaranth at concentration gradient 50mg/L. To study the effect of initial pH on dye decolorization, the strain ts17 was cultivated for 24 h at varying initial pH (5-10) and was supplemented with 50 mg/L of Amaranth. The effect of salt concentration on dye decolorization was studied by using different concentrations of sodium chloride (0%, 1%, 3%, 5% and 7%) in the medium with 50 mg/L of Amaranth. To inspect the influence of various metal ions such as Mg2+ (MgCl2), Co2+(CoCl2), Zn2+ (ZnSO4), Mn2+ (MnCl2), Fe3+ (FeCl3)and Cu2+ (CuSO4) on decolorization activity, experiments were carried out in presence of these metals at concentration of 2 mM. Metal ions from stock solutions were added to the cell suspensions and incubated for 15 min, and then flasks were added with the dyes.
In the process of detecting decolorization rate affected by various kinds of factors, supernatants were analysed at 520 nm on spectrophotometer every 2 h. The decolorization rate (DR) was calculated by using the formula (Shekhar et al., 2012):
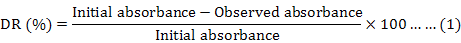
Decolorization of Sudan I by dtrain ts17
In order to study the decolorizing ability and decoloring conditions of oil-soluble azo dyes by strain ts17, the bacteria were incubated in the presence of 5 mg/L Sudan I which was dissolved in DMSO broth at 37°C without agitation., and reduction of the dyes was monitored.
The time-course of Sudan I decolorization by ts17 was monitored using high-performance liquid chromatography (HPLC). The peak area was used to calculate the concentration of Sudan I. Reduction of Sudan I was determined by monitoring the disappearance of the absorption peak for the dye at 500 nm (Chen et al., 2009). The average of the same measurements group was used as initial or monitored peak area. The decolorization rate was calculated by using the following formula:
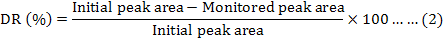
In addition, we also explored the decolorization of Sudan I by the presence of different molar ratio of HP-β-CD. We mixed different molar ratio of HP-β-CD to Sudan I at 3:1, 6:1, 9:1, 12:1 to medium before inoculation, respectively.
Results and discussion
Decolorizing bacteria
Comparison of the near full-length 16S rRNA gene sequence (1,512 bp) from strain 17 with other bacteria showed that it is most closely affiliated to the genus Enterococcus (Fig. 1). The highest 16S rRNA gene sequence similarity of 99 % was found with Enterococcus avium (GenBank accession number KP735772).
Decolourization of amarnath
When dye decolorization was conducted at shaking condition (120 rpm), no decolorization ability was found for strain ts17. This result demonstrated that the static condition was necessary for the decolorization of ts17, as it is also the case for most of the microbial species for the decolorization of azo dyes. The general mechanism for the azo dye degradation is through reduction of azo bond by azoreductase (Chacko and Subramaniam, 2011). It has been suggested that the azo bond cleavage is catalyzed by azoreductase, which is very sensitive to oxygen in the medium (Nikolova and Nenov, 2004). After decolourization of Amaranth by the bacterium, there was no visible dye adsorbed to the biomass and no colouration in the methanol extract of the biomass was found. This indicates that the observed dye decolourization is mainly due to the biological activity of the bacterium rather than adsorption or biosorption.
Ability of ts17 for decoloring Amaranth was checked at higher dye concentrations. The dye was also decolorized when dye concentration reached 1450 mg/L. Longer time is required for decolorization when dye concentration is increased.
Bacterial growth curve of ts17 is shown in Figure 2, which indicates 0-4 h of lag phase, 4-10 h of logarithmic phase and 12 h of stationary phase.
Effect of pH
As shown in Figure 3, the decolorization rate of the incubation are 14.99%, 94.57%, 96.21%, 93.67%, 91.95% and 63.93% respectively, corresponding to pH at 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0 after 8 h incubation. The preferable pH for the decolorization of Amaranth azo dye by ts17 strain was between pH 6.0-9.0, and with optimum pH of 7.0.
Effect of NaCl concentration
The concentrations of NaCl have impacts on the decolorization of Amaranth by strain ts17. After 6 h of incubation, the decolorization rate of the incubation are 54.07%, 84.10 %, 96.82%, 72.26% and 32.35%, respectively, corresponding to 0%, 1%, 3%, 5% and 7% NaCl solutions (Fig. 4), indicating the promotion effect of NaCl on the decoloration up to 5% (w/w) content, and the inhibition action appeared at 7% NaCl.
Effect of heavy metal ions
As shown in Figure 5, the decolorization rate of Amaranth was 100% and 99.08% in the presence of Mg2+ and Fe3+, respectively, compared with that of the control sample of 84.10% after 6 h. Fe3+ can obviously improve the decolorization rate in the lag phase of bacteria reflected in bacterial growth curve. Moreover, we detected that decolorization rate increased from 18.36% to 100% between 4 h and 6 h with Mg2+. Mg2+ can obviously improve the decolorization in the logarithmic phase of bacteria, and the contributions of decolorization rate account for 80%. Mg2+ has been reported to enhance the enzyme activity of azoreductase from Rhodobacter sphaeroides (Yan et al., 2004). On the other hand, other metal ions including Cu2+, Zn2+ and Co2+ have inhibitory effect on the decolorization
process, and the decolorization rate was only 7.10%, 15.89% and 12.46%, respectively after 8 h of process at a concentration of 2 mM, which is much lower than that of the control samples of 97.04%. Shekhar et al. (2012) reported a strong negative effect of Zn2+ and Co2+ at 5 mM concentration. In another study, less than 30 % residual enzyme activity for Zn2+ was found in the range from 5 to 50 mM (Yang et al., 2013). Mn2+ did not show any major effect on decolorization of Amaranth. When all metal ions were added, decolorization rate reached 60% in 2 h and remained unchanged afterwards.
Decolourization of Sudan Ι dyes
It was found that ts17 also has ability of decolorizing oil solution azo dye such as Sudan I and decolorization rates of 69.09%, 74.55%, and 85.45% at 4 h, 6 h and 8 h when dissolved in DMSO, respectively (Fig. 6). These data were higher than that of control which showed decolorization rate of 32.12%, 59.39%, 75.15%, respectively at the same time period.
In the cell suspensions of ts17, the Sudan I dye and HP-β-CD are simultaneously added to the solution in the range of 3-9 mole ratios (Fig. 6). The addition of the HP-β-CD had a positive effect on the decolorization rate of ts17 strain on the Sudan I dye, and with the increasing concentration of HP-β-CD, decolorization rates of Sudan I increased. However, further increase in the molar ratio of HP-β-CD in Sudan I decreased the decolorization rate. So, the treatment of Sudan I with 9 times mole ratio of HP-β-CD increased the decolorization rate comparable to the dye dissolved in DMSO.
So, in brief the isolated strain ts17 has a high ability to decolorize azo dye Amaranth. The ts17 cultured in LB medium under static condition containing Amaranth (50 mg/L) decolored the dyes more than 99% at 37°C, pH7.0, with 3% NaCl after 8 h. The maximum decolorization concentration of the dye reached 1450mg/L. Mg2+ and Fe3+ accelerated dye decolorization and Cu2+, Zn2+, Co2+ on the other hand inhibited dye decolorization. The strain ts17 was also able to completely reduce the oil-soluble dyes Sudan I. It was also shown that the appropriate concentration of HP-β-CD mixed with dye (nC:nS=9:1) effectively improved the decolorization rate from 85.45% to 99.45% after 8h.
Conclusion
In conclusion, Enterococcus avium is capable of efficiently decorloring monoazo water-soluble Amaranth and monoazo oil-soluble Sudan I treated with HP-β-CD. Our further investigations will be focused on the mechanism by which the HP-β-CD played and the degradation of aromatic amines caused by azo dye reduction.
Acknowledgments
This work was supported by the Natural Science Foundation of Liaocheng University (Project No. 318011610).
Statement of conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article
References
Aftab, U., Khan, M.R., Mahfooz, M., Ali, M., Aslam, S.H. and Rehman, A, 2011. Decolourization and degradation of textile azo dyes by corynebacterium sp.isolated from industrial effluent. Pakistan J. Zool., 43: 1-8.
Chacko, J. and Subramaniam, K., 2011. Enzymatic degradation of azo dyes - A review. Int. J. environ. Sci., 1: 1250-1260.
Chen, H., 2006. Recent advances in azo dye degrading enzyme research. Curr. Protein Pept. Sci., 7: 101-111. https://doi.org/10.2174/138920306776359786
Chen, H., Xu, H., Heinze, T.M. and Cerniglia, C.E., 2009. Decolorization of water and oil-soluble azo dyes by Lactobacillus acidophilus and Lactobacillus fermentum. J. Ind. Microbiol. Biotechnol., 36: 1459-1466. https://doi.org/10.1007/s10295-009-0633-9
Elisangela, F., Andrea, Z., Fabio, D.G., Cristiano R.M., Regina D.L. and Artur, C.P., 2009. Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. Int. Biodeterior. Biodegrad., 63: 280-288. https://doi.org/10.1016/j.ibiod.2008.10.003
Forgacs, E., Cserhati, T. and Oros, G., 2004. Removal of synthetic dyes from wastewaters: A review. Environ. Int., 30: 953-971. https://doi.org/10.1016/j.envint.2004.02.001
Hai, F., Yamamoto, K. and Fukushi, K., 2007. Hybrid treatment system for dye wastewater. Crit. Rev. environ. Sci. Technol., 37: 315-377. https://doi.org/10.1080/10643380601174723
Larsen, K.L., Duedahl-Olesen, L., Christensen, S.J.H., Mathiesen, F., Pedersen, L. and Zimmermann, W., 1998. Purification and characterization of a cyclodextrin glycosyltransferase from Paenibacillus sp. Carbohydr. Res., 310: 211-219. https://doi.org/10.1016/S0008-6215(98)00178-5
Loftsson, T. and Brewster, M.E., 1996. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci., 85: 1017-1025. https://doi.org/10.1021/js950534b
Miro, A., Quaglia, F., Giannini, L., Cappello, B. and Giannini, L., 2006. Drug/cyclodextrin solid systems in the design of hydrophilic matrices: A strategy to modulate drug delivery rate. Curr. Drug Deliv., 3: 373-378. https://doi.org/10.2174/156720106778558994
Nikolova, N. and Nenov, V., 2004. Azo dye Schwarz GRS bioconversion under various conditions. Water Air Soil Pollut., 4: 137-146. https://doi.org/10.1023/B:WAFO.0000044793.39140.c0
Novotny, C., Dias, N., Kapanen, A., Malachova, K., Vandrovcova, M., Itavaara, M. and Lima, N., 2006. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo and anthraquinone dyes. Chemosphere, 63: 1436-1442. https://doi.org/10.1016/j.chemosphere.2005.10.002
Novotny, C., Svobodova, K., Kasinath, A. and Erbanova, P., 2004. Biodegradation of synthetic dyes by Irpex lacteus under various growth conditions. Int. Biodeter. Biodegr., 54: 215-223. https://doi.org/10.1016/j.ibiod.2004.06.003
Pierce, C.I., Lloyd, J.R. and Bhattacharya, G.J.T., 2003. The removal of color from textile wastewater using whole bacterial cells: A review. Dyes Pigments, 58: 179-185. https://doi.org/10.1016/S0143-7208(03)00064-0
Shekhar, B.J., Shripad, N.S., Dayanand, C.K., Ranjit, G.G. and Jyoti, P.J., 2012. Biodecolorization of azo dye remazol orange by Pseudomonas aeruginosa BCH and toxicity (oxidative stress) reduction in Allium cepa root cells. Appl. Biochem. Biotechnol., 168: 1319-1334. https://doi.org/10.1007/s12010-012-9860-z
Stella, V.J. and Rajewski, R.A., 1997. Cyclodextrins: their future in drug formulation and delivery. Pharmaceut. Res., 14: 556-567. https://doi.org/10.1023/A:1012136608249
Szejtli, J., 1998. Introduction and general overview of cyclodextrin chemistry. Chem. Rev., 98: 1743-1753. https://doi.org/10.1021/cr970022c
Vijaykumar, M.H., Vaishampayan, P.A., Shouche, Y.S. and Karegoudar, T.B., 2007. Decolourization of naphthalene-containing sulfonated azo dyes by Kerstersia sp. strain VKY1. Enzyme Microb. Technol., 40: 204-211. https://doi.org/10.1016/j.enzmictec.2006.04.001
Yan, B., Zhou, J., Wang, J., Du, C., Hou, H., Song, Z. and Bao, Y., 2004. Expression and characteristics of the gene encoding azoreductase from Rhodobacter sphaeroides. AS1.1737. FEMS Microbiol. Lett., 236: 129-136. https://doi.org/10.1111/j.1574-6968.2004.tb09638.x
Yang, Y., Lu, L., Gao, F. and Zhao, Y., 2013. Characterization of an efficient catalytic and organic solvent-tolerant azoreductase toward methyl red from Shewanella oneidensis MR-1. Environ. Sci. Pollut. Res., 20: 3232-3239. https://doi.org/10.1007/s11356-012-1221-5
Yogesh, M.K., Pallavi, D.K. and Vijay, L.M., 2013. Effective bioremoval and detoxification of textile dye mixture by Alishewanella sp. KMK6. Appl. Microbiol. Biotechnol., 97: 881-889. https://doi.org/10.1007/s00253-012-3983-6
To share on other social networks, click on any share button. What are these?










