Effect of Bio-Priming, Organic and Inorganic Nitrogen Sources and Beneficial Microorganisms on Growth and Biochemical Traits of Wheat
Effect of Bio-Priming, Organic and Inorganic Nitrogen Sources and Beneficial Microorganisms on Growth and Biochemical Traits of Wheat
Abid Khan*, Mukhtar Alam and Yousaf Jamal
Department of Agriculture, University of Swabi, Khyber Pakhtunkhwa, Pakistan
Abstract | Major sources of replenishing crop nutrients in soil are organic, inorganic and bio-fertilizers. The current experiments were conducted on “Effect of bio-priming, organic and inorganic nitrogen sources and beneficial microorganisms on growth and biochemical traits of wheat” for two years at Agricultural Research Station Buner, Khyber Pakhtunkhwa, Pakistan. The experiments were laid out using three factors factorial Randomized Complete Block Design (RCBD) with three replications. Bio-aab was used as a source of (BM) culture. Microbes present in this culture were species of photosynthetic bacteria (Rhodopseudomonas palustris, Rhodobacter spaeroides), lactic acid bacteria (Lactobacillus plantarum, Lactobacillus casei, Streptococcus lactis), yeasts (Saccharomyces cerevisae, Candida utilis), actinomycetes (Streptomyces albus, Streptomyces griesus) and fermenting fungi (Aspergillus oryzae, Mucor heimalis). Farm yard manure (FYM) and poultry manure (PM) were used as organic sources of nitrogen. Bio-primed (seeds soaked in 10% BM aqueous solution for 30 minutes just before sowing) and unprimed wheat seeds were tested under various nitrogen sources (NS) with and without BM. Nitrogen sources were NS1 (control), NS2 (full dose from Urea), NS3 (full dose from FYM), NS4 (full dose from PM), NS5 (half dose from Urea + half dose from FYM), NS6 (half dose from urea + half dose from PM) and NS7 (half dose from FYM + half dose from PM). Nitrogen @ 120 kg ha-1 was quantified from each combination except NS1. Seed bio-priming significantly increased emergence (9.6%), delayed heading (2.1%) and maturity (2.4%), increased leaves and leaf area tiller-1 (6.8 and 4.1%), increased LAI (9.6%), increased height (3.5%), enhanced crop growth rate (CGR) (8.6%) and increased chlorophyll a and b (9.8% and 8.5%). BM application significantly increased emergence (6.1%), delayed heading (2.3%) and maturity (3.04%), increased leaves and leaf area tiller-1 (9.1 and 14.1%), increased LAI (36.4%), increased height (8.65%), enhanced CGR (27.8%) and increased chlorophyll a and b (29% and 27.9%). Significantly earlier emergence (12 days), more leaves tiller-1 (4.9), delayed heading (114.6), greater leaf area tiller-1 (31.8 cm2), higher LAI (0.99), taller plants (89 cm), higher CGR (8.76 g m-2 day-1), delayed maturity (171 days) and more chlorophyll a and b (1.95 and 0.64 µg ml-1) were achieved while using NS6 as compared to other nitrogen sources. Significantly more leaves and leaf area tiller-1 (5.2 and 34.3 cm2), higher LAI (1.18), taller plants (93.8 cm), higher CGR (10.27 g m-2 day-1) and greater chlorophyll a and b (2.30 and 0.75 µg ml-1) were recorded while using NS6+BM as compared to same treatment without BM. Performance of the above-mentioned parameters were significantly higher during subsequent year of study. It is concluded that seed bio-priming and NS6+BM should be used for better performance of wheat.
Received | January 22, 2020; Accepted | April 29, 2020; Published | June 01, 2020
*Correspondence | Abid Khan, Department of Agriculture, University of Swabi, Khyber Pakhtunkhwa, Pakistan; Email: akbuneri@gmail.com
Citation | Khan, A., M. Alam and Y. Jamal. 2020. Effect of bio-priming, organic and inorganic nitrogen sources and beneficial microorganisms on growth and biochemical traits of wheat. Sarhad Journal of Agriculture, 36(2): 685-701.
DOI | http://dx.doi.org/10.17582/journal.sja/2020/36.2.685.701
Keywords | Bio-priming, Beneficial microorganisms, Nitrogen sources, Organic farming, Sustainable agriculture
Introduction
Plant nutrients status in soil is the key factor of success or collapse of a crop production system. To ensure food supply for increasing population, optimum nutrients availability to plants is required to boost crop yields. Major sources of restocking crop nutrients in agricultural soils are organic, inorganic and bio-fertilizers (Masarirambi et al., 2012). A single source of crop nutrients cannot fulfill total requirements of nutrients for sustainable crop production (Korsaeth et al., 2002). Utilization of excessive chemical fertilizers create environmental issues as well as need energy for manufacturing (Shaxson, 2006). Furthermore, use of inorganic fertilizers is imbalanced and not useful in preserving soil health which is desired for sustainable crop production. The ever-increasing prices of inorganic fertilizers and pollution developing from their application led to a new interest towards some alternative measures of fertilization such as integrated plant nutrient management, particularly use of organic plant nutrients (Korsaeth et al., 2002). Consequently, alternative agricultural systems such as usage of Effective Microorganisms (EM) in integration with organic materials and limited inorganic fertilizers were being worked out. EM culture contains photosynthetic bacteria (Rhodopseudomonas palustris and Rhodobacter spaeroides), Lactic acid bacteria (Lactobacillus plantarum, Lactobacillus casei, and Streptococcus lactis), Yeasts (Saccharomyces cerevisae and Candida utilis), Actinomycetes (Streptomyces albus and Streptomyces griesus) and Fermenting fungi (Aspergillus oryzae and Mucor heimalis). EM-Technology was developed by Prof. Dr. Terou Higa in 1980s, at Ryukyus University, Japan (Higa and Wididana, 1991a; Higa, 1991) which was familiarized in Pakistan with the name of Beneficial Microorganisms (BM) technology by the University of Agriculture, Faisalabad in 1990. BM enhanced crop health and production by means of boosting photosynthesis, producing enzymes and hormones, speeding up the process of decomposition, suppressing diseases related to soil and enhancing soil quality (Zaman and Chang, 2004; Kurepin et al., 2014). BM treated compost produced maximum values of N, P, K, Fe, and Zn while untreated produced lowest values of the respective nutrients (Sahain et al., 2007). Combined use of BM and organic materials as soil amendment stimulated mineralization of nutrients, and its application with fresh organic matter was more beneficial (Fatunbi and Ncube, 2009). Research works done on BM had shown that plant performance in BM added treatments was better than traditional farming (Javaid, 2009).
Bio-Priming (BP) is a biological management approach in which seeds are soaked in bacterial aqueous solution for a specific period of time for bacterial imbibition into the seed (Abuamsha et al., 2011). Seed bio-priming boosted germination and initial phase of plant development (Singh et al., 2016). Grain yield was significantly increased because of bio-priming of maize seeds with different strains of Azotobacter and Azospirillum (Sharifi, 2011). Drought tolerance in wheat plants was increased through bio-priming with two strains of bacteria including Azospirillum brasilense and Bacillus amyloliquefaciens (Kasim et al., 2013). Bio-priming enhanced various plant growth promoting activities which had been reported by many researchers (Saber et al., 2012).
Wheat is cultivated in the whole country as the main staple food item of Pakistan. Wheat yield and quality is affected by various management practices performed during a year (Wiatrak et al., 2006). To increase per hectare production of wheat is of great magnitude because average yield of our country and especially Khyber Pakhtunkhwa is very low. Important factors for increasing wheat productivity include farm management practices and adoption of recent technology (Tariq et al., 2014). The use of organic manures (David et al., 2005), microbial biomass of soil, composition of soil microorganisms (Filip, 1998), combination of different decomposable organic wastes and nitrogen fertilizers (Ouedraogo et al., 2006) and N management (Torbert et al., 2001) are credited to improve agriculture sustainably. Thus, appropriate nitrogen source (organic, inorganic or their combinations) along with BM application needed to be hypothesized to test their effects on improving wheat productivity.
Objectives of the experiments
The aim of this research work was the assessment of seed bio-priming and nitrogen from various sources and their combinations with and without BM application for evaluating growth and biochemical traits of wheat.
Materials and Methods
Field experiments were conducted at Agricultural Research Station, Buner, Khyber Pakhtunkhwa, Pakistan during Rabi season of 2015-16 and 2016-17 (Nov-May each year) for two years successively to assess the effect of bio-priming and various organic and inorganic nitrogen sources with and without BM on growth and biochemical traits of wheat. Environmental conditions like temperature and rainfall for the years 2015, 2016 and 2017 are displayed in Figures 1, 2 and 3 respectively. Experiments were arranged using RCBD with 3 replications having subplot size of 9 m2 which comprised of 6 rows each of 5 meters length with row to row distance of 30 cm. Winter wheat variety Pirsabak-2015 was used in the experiments. Sowing was done on 12th November each year on the same piece of land. Beneficial microorganisms (BM) culture was obtained from The University of Agriculture, Faisalabad, Pakistan. Farm yard manure (FYM) and poultry manure (PM) were arranged locally from the nearby farmer’s field and poultry farm, respectively. Representative samples of experimental site, FYM and PM were analyzed for their nutritional status. Phosphorus and potassium (90:60 kg ha-1 P2O5:K2O) were applied as single dose during final seed bed preparation before sowing and kept constant for the whole experiment. In case of seed bio-priming, seeds were soaked in 10% BM aqueous solution for 30 minutes just before start of sowing each year. Bio-primed and unprimed wheat seeds were tested under various nitrogen sources with and without BM application. In case of BM application, 10% solution of BM was used. Nitrogen sources were NS1 (Control), NS2 (full dose from Urea), NS3 (full dose from FYM), NS4 (full dose from PM), NS5 (half dose from Urea + half dose from FYM), NS6 (half dose from Urea + half dose from PM) and NS7 (half dose from FYM + half dose from PM). Nitrogen @ 120 kg ha-1 was quantified from each combination except NS1 and applied once after final seed bed preparation prior to sowing. FYM and PM quantification at the mentioned rate was done after lab analysis of the samples. Standard agronomic practices were carried out throughout the crop growing period. Nutritional composition of FYM and PM and soil status of the experimental site are given below.
| Nutrient/property | FYM | PM | Soil |
| N | 0.64% | 2% | 0.028% |
| P | 0.5% | 1.1% |
8.75 mg kg-1 |
| K | 1.5% | 3% |
80 mg kg-1 |
| PH | - | - | 8.3 |
| EC | - | - |
0.2 dSm-1 |
| OM | - | - | 0.4% |
| Sand | - | - | 50% |
| Silt | - | - | 40% |
| Clay | - | - | 10% |
| Textural class | - | - | Loam |
Measurements and procedures of data recording
Days to emergence were noted down from sowing till 75% emergence. Leaves per tiller were noted at heading stage by randomly selecting five plants and average was worked out. Tillers m-2 were also recorded at heading stage at two different locations each of 1 m2. Tillers were counted in each selected location and their averages were worked out. Days to heading were noted down from sowing till 50% plants produced spikes. Leaf area at heading stage was calculated using the formula given by Quarrie and Jones (1979).
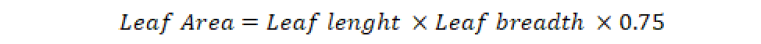
Leaf area index (LAI) was recorded by the following formula, Where LA stands for leaf area and GA for ground area covered by the measured leaf area.
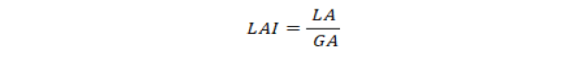
Height (cm) was measured at maturity from base of the wheat plant to apex of the spike. Maturity in term of days was considered from sowing till harvesting. CGR (gm-2 day-1) was measured from tillering stage till physiological maturity at 30 days interval according to the formula described by Gardner et al. (1985).
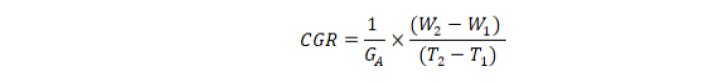
Where;
GA stand for ground area, W1 for sample dry weight at 1st interval (T1) and W2 for sample dry weight at 2nd interval (T2).
Samples of fresh leaves were collected at boot stage. Method described by Linchtenthaler and Wellburn (1983) having the following equations was used for measuring Chlorophyll a and b.
Chl a (µg ml-1) = 12.21 A663 - 2.81 A646
Chl b (µg ml-1) = 20.13 A646- 5.03 A663
Statistical analysis
The data collected were analyzed statistically using three factors factorial RCBD. LSD test at 5% probability was used for measuring significant differences among treatments (Gomez and Gomez, 1983).
Results and Discussion
Bio-priming (BP)
Data on days to emergence, 50% heading and maturity, leaves and leaf area per tiller, LAI, height, crop growth rate, and Chlorophyll a and b of wheat affected by bio-priming over two consecutive years are given in Table 1. During 2015-16, seed bio-priming significantly accelerated emergence from 13.7 to 12.6 days, enhanced number of leaves tiller-1 from 4.4 to 4.6, delayed 50% heading from 110.5 to 113 days, enlarged leaf area from 24 to 24.8 cm2, enhanced LAI from 0.58 to 0.62, increased plant height from 78.9 to 81.9 cm, increased CGR from 6.05 to 6.43 g m-2 day-1, delayed maturity from 163 to 167 days and increased chlorophyll a from 1.33 to 1.42 and chlorophyll b from 0.43 to 0.46 µg ml-1.
During 2016-17, seed bio-priming significantly accelerated emergence from 13.2 to 11.7 days, enhanced number of leaves tiller-1 from 4.5 to 5.0, delayed 50% heading from 111.7 to 113.8 days, enlarged leaf area from 25.2 to 26.3 cm2, enhanced LAI from 0.66 to 0.73, increased plant height from 82.3 to 85 cm, increased CGR from 6.92 to 7.66 g m-2 day-1, delayed maturity from 165 to 169 days and increased chlorophyll a from 1.53 to 1.71 and chlorophyll b from 0.50 to 0.56 µg ml-1.
Pooled data over two years revealed that 9.6% earliness in emergence, 6.8% increase in leaves tiller-1, 2.1% delay in 50% heading, 4.1% broadened leaf area, 9.6% increase in LAI, 3.5% increase in plant height, 8.6% increase in crop growth rate, 2.4% delay in maturity, 9.8% increase in chlorophyll a and 8.5% increase in chlorophyll b were noticed as a result of seed bio-priming.
Interaction between year and bio-priming (Y×BP) for all the above parameters was found non-significant.
Earliness in emergence, increase in leaves tiller-1, delayed 50% heading, broadened leaf area, higher LAI and plants, greater crop growth rate, delayed maturity and increase in chlorophyll as a result of bio-priming of seed might be due to biological management approach which boosted initial phase of plant establishment via improved germination of seed and protection before seedling emergence (Singh et al., 2016). Our outcomes also agreed with Bennett et al. (2009) who conveyed earliness in emergence of carrot and onion as a result of bio-priming with BM. Our findings agreed with Singh et al. (2016) who conveyed 28.1% more leaves with seed bio-priming which had been reported by Maureen (2016) as an effective mechanism for allocating beneficial microbes into the soil where they colonized in emerging roots and protected seedling against soil borne diseases and pests. Delayed 50% heading and maturity could be due to better and vigorous establishment of early phase of plant development (Singh et al., 2016) and supplementation of organic source with BM which might had resulted more nutrient availability and robust crop growth (Li et al., 2003). Greater leaf area, LAI and plant height might be due to more favourable environment for the growth, development and health of plants because of BM inoculation which resulted vigorous growth of crop (Margit, 2015). Our results also agree with Moeinzadeh et al. (2010) who described that seed bio-priming has the potential for promoting fast and even germination along with better plant growth. Bio-priming of seed with BM also boosted crop growth rate as compared to without bio-priming because of increase in many agro-morphological characters of wheat plants (Saber et al., 2012). Our findings showing more leaves, greater leaf area and chlorophyll in bio-primed crops were similar with those of Rawat et al. (2012) and Priya et al. (2016). Our results agreed with Mirshekari et al. (2012) who revealed that bio-priming of barley seed with a group of beneficial microorganisms improved grain yield.
Beneficial microorganisms (BM)
Data on days to emergence, 50% heading and maturity, leaves and leaf area per tiller, LAI, height, crop growth rate, Chlorophyll a and b of wheat affected by beneficial microorganisms for two consecutive years are given in Table 2. During 2015-16, BM application significantly accelerated emergence from 13.5 to 12.8 days, enhanced number of leaves tiller-1 from 4.3 to 4.6, delayed 50% heading from 110.5 to 113 days, enlarged leaf area from 23 to 25.7 cm2, enhanced LAI from 0.52 to 0.68, increased plant height from 77.80 to 82.90 cm, increased CGR from 5.70 to 6.78 g m-2 day-1, delayed maturity from 162 to 168 days and increased chlorophyll a from 1.25 to 1.49 and chlorophyll b from 0.41 to 0.49 µg ml-1.
During 2016-17 BM application significantly accelerated emergence from 13 to 12 days, enhanced number of leaves tiller-1 from 4.5 to 5.0, delayed 50% heading from 111.5 to 114 days, enlarged leaf area from 23.90 to 27.70 cm2, enhanced LAI from 0.58 to 0.81, increased plant height from 79.4 to 88 cm, increased CGR from 6.18 to 8.40 g m-2 day-1, delayed maturity from 165 to 170 days, increased chlorophyll a from 1.36 to 1.89 and chlorophyll b from 0.44 to 0.62 µg ml-1.
Pooled analysis of data over two years revealed significant differences and 6.1% earliness in emergence, 9.1% increase in leaves tiller-1, 2.3% delay in 50% heading, 14.1% broadened leaf area, 36.4% increase in LAI, 8.65% increase in plant height, 27.8% increase in crop growth rate, 3.04% delay in maturity, 29% increase in chlorophyll a and 27.9% increase in chlorophyll b were noticed because of BM application.
Interaction between year and beneficial microorganisms (Y×BM) was found non-significant for days to emergence, 50% heading and maturity and significant for leaves per tiller, leaf area, LAI, plant height, CGR and Chlorophyll a and b.
BM application encouraged earlier emergence, increased leaves tiller-1, delayed 50% heading, broadened leaf area, increased LAI, plant height and CGR, delayed maturity and increased chlorophyll a and b contents of wheat crop. These findings agreed with Ali et al. (2013) who conveyed that effective microorganisms had been used for improving germination of various crops. Our findings are also in line with Gorski and Kleiber (2010) who revealed more number of inflorescences and leaves in case of gerberas with application of effective microorganisms which might be due to availability and solubility of nutrients from the applied substrate because of BM application. Delayed heading and maturity, higher leaf area and LAI, taller plants and higher crop growth rate could be due to supplementation of organic sources with BM which increased decomposition of organic wastes and nutrients discharge for plant uptake that resulted higher nutrient availability and favorable soil conditions for growth, development and health of plants and extended the growing period due to vigorous growth of crop (Margit, 2015; Javaid and Bajwa, 2011; Iqtedar et al., 2006; Mbouobda et al., 2014). Higher chlorophyll a and b might be due to greater N-mineralization of organic sources with BM application that improved chlorophyll contents in shoots through greater N availability and uptake (Houles et al., 2007). Application of beneficial microorganisms in soil plant ecosystem improved plant growth, yield and quality of soil and crops (Mowa and Maass, 2012; Hu and Qi, 2013).
Nitrogen sources (NS)
Effect of using different nitrogen sources on days to emergence, 50% heading and maturity, leaves and leaf area tiller-1 and LAI of wheat over two consecutive years are given in Table 3. Non-significant differences were observed regarding days to emergence during 2015-16 and 2016-17 individually, however, based on mean data over two years, significantly earlier emergence (12 days) was recorded by NS6 and it was found statistically at par with NS5. Delayed emergence (13.3 days) was observed in NS3 and it was found statistically at par with NS1 and all other nitrogen sources utilized during the trial except NS6.
Leaves tiller-1 significantly differed during 2015-16 and varied from 3.9 for NS1 to 4.8 for NS6 while during 2016-17, it varied from 3.9 to 5.1 for the same treatments respectively. Based on pooled data over two years, more leaves per tiller (4.9) was attained by NS6 that was found statistically at par (4.9) with NS5, while lesser leaves (3.9) were noticed by NS1.
Table 1: Days to emergence, leaves tiller-1, days to 50% heading, leaf area tiller-1 (cm2), leaf area index, plant height (cm), CGR (g m-2 day-1), days to maturity, Chl a (µg ml-1) and Chl b (µg ml-1) of wheat as affected by bio-priming (BP) over two consecutive years.
| Parameters | BP |
Year (Y) |
LSD at P≤0.05 |
|||
| 2015-16 | 2016-17 | Mean | Y×BP | BP | ||
|
Y×BP |
||||||
| Days to emergence | Primed | 12.6 | 11.7 | 12.2 b | ns | 0.4458 |
| Unprimed | 13.7 | 13.2 | 13.5 a | |||
|
Leaves tiller-1 |
Primed | 4.6 | 5.0 | 4.8 a | ns | 0.0834 |
| Unprimed | 4.4 | 4.6 | 4.5 b | |||
| Days to 50% heading | Primed | 113.0 | 113.8 | 113.4 a | ns | 1.5958 |
| Unprimed | 110.5 | 111.7 | 111.1 b | |||
|
Leaf area tiller-1 (cm2) |
Primed | 24.8 | 26.3 | 25.6 a | ns | 0.5165 |
| Unprimed | 24.0 | 25.2 | 24.6 b | |||
| Leaf area index | Primed | 0.62 | 0.73 | 0.68 a | ns | 0.0207 |
| Unprimed | 0.58 | 0.66 | 0.62 b | |||
| Plant height (cm) | Primed | 81.9 | 85.0 | 83.4 a | ns | 1.6630 |
| Unprimed | 78.9 | 82.3 | 80.6 b | |||
|
CGR (g m-2 day-1) |
Primed | 6.43 | 7.66 | 7.04 a | ns | 0.2346 |
| Unprimed | 6.05 | 6.92 | 6.48 b | |||
| Days to maturity | Primed | 167 | 169 | 168 a | ns | 2.6088 |
| Unprimed | 163 | 165 | 164 b | |||
|
Chl a (µg ml-1) |
Primed | 1.42 | 1.71 | 1.57 a | ns | 0.0548 |
| Unprimed | 1.33 | 1.53 | 1.43 b | |||
|
Chl b (µg ml-1) |
Primed | 0.46 | 0.56 | 0.51 a | ns | 0.0185 |
| Unprimed | 0.43 | 0.50 | 0.47 b | |||
Means in each category showing different letter(s) are significantly different applying LSD test at P≤0.05
Table 2: Days to emergence, leaves tiller-1, days to 50% heading, leaf area tiller-1 (cm2), leaf area index, plant height (cm), CGR (g m-2 day-1), days to maturity, Chl a (µg ml-1) and Chl b (µg ml-1) of wheat as affected by beneficial ,icroorganisms (BM) over two consecutive years.
| Parameters | BM |
Year (Y) |
LSD at P≤0.05 |
|||
| 2015-16 | 2016-17 | Mean | Y×BM | BM | ||
| Y×BM | ||||||
| Days to emergence | With BM | 12.8 | 12.0 | 12.4 b | ns | 0.4458 |
| Without BM | 13.5 | 13.0 | 13.2 a | |||
|
Leaves tiller-1 |
With BM | 4.6 b | 5.0 a | 4.8 a | 0.1179 | 0.0834 |
| Without BM | 4.3 c | 4.5 b | 4.4 b | |||
| Days to 50% heading | With BM | 113.0 | 114.0 | 113.5 a | ns | 1.5958 |
| Without BM | 110.5 | 111.5 | 111.0 b | |||
|
Leaf area tiller-1 (cm2) |
With BM | 25.7 b | 27.7 a | 26.7 a | 0.7304 | 0.5165 |
| Without BM | 23.0 d | 23.9 c | 23.4 b | |||
| Leaf area index | With BM | 0.68 b | 0.81 a | 0.75 a | 0.0293 | 0.0207 |
| Without BM | 0.52 d | 0.58 c | 0.55 b | |||
| Plant height (cm) | With BM | 82.9 b | 88.0 a | 85.4 a | 2.3518 | 1.6630 |
| Without BM | 77.8 c | 79.4 c | 78.6 b | |||
|
CGR (g m-2 day-1) |
With BM | 6.78 b | 8.40 a | 7.59 a | 0.3318 | 0.2346 |
| Without BM | 5.70 d | 6.18 c | 5.94 b | |||
| Days to maturity | With BM | 168 | 170 | 169 a | ns | 2.6088 |
| Without BM | 162 | 165 | 164 b | |||
|
Chl a (µg ml-1) |
With BM | 1.49 b | 1.89 a | 1.69 a | 0.0775 | 0.0548 |
| Without BM | 1.25 d | 1.36 c | 1.31 b | |||
|
Chl b (µg ml-1) |
With BM | 0.49 b | 0.62 a | 0.55 a | 0.0261 | 0.0185 |
| Without BM | 0.41 d | 0.44 c | 0.43 b | |||
Means in each category showing different letter(s) are significantly different applying LSD test at P≤0.05
Table 3: Days to emergence, leaves tiller-1, days to 50% heading, leaf area tiller-1 (cm2) and leaf area index of wheat as affected by different nitrogen Sources (NS) over two consecutive years.
| Particulars | NS |
Year (Y) |
LSD at P≤0.05 |
||||
| 2015-16 | 2016-17 | Mean | Y×NS | Y | NS | ||
|
Y×NS |
|||||||
| Days to emergence | NS1 | 13.4 | 12.9 | 13.2 a | ns | 0.4458 | 0.8340 |
| NS2 | 13.2 | 12.6 | 12.9 a | ||||
| NS3 | 13.7 | 12.9 | 13.3 a | ||||
| NS4 | 13.3 | 12.5 | 12.9 a | ||||
| NS5 | 12.9 | 12.2 | 12.5 ab | ||||
| NS6 | 12.3 | 11.7 | 12.0 b | ||||
| NS7 | 13.5 | 12.6 | 13.0 a | ||||
| Mean | 13.2 a | 12.5 b | |||||
|
Leaves tiller-1 |
NS1 | 3.9 | 3.9 | 3.9 c | ns | 0.0834 | 0.1559 |
| NS2 | 4.4 | 4.8 | 4.6 b | ||||
| NS3 | 4.4 | 4.8 | 4.6 b | ||||
| NS4 | 4.6 | 4.9 | 4.7 b | ||||
| NS5 | 4.7 | 5.1 | 4.9 a | ||||
| NS6 | 4.8 | 5.1 | 4.9 a | ||||
| NS7 | 4.5 | 4.9 | 4.7 b | ||||
| Mean | 4.5 b | 4.8 a | |||||
| Days to 50% heading | NS1 | 108.4 | 107.8 | 108.1 b | ns | ns | 2.9854 |
| NS2 | 111.5 | 112.6 | 112.0 a | ||||
| NS3 | 110.9 | 112.5 | 111.7 a | ||||
| NS4 | 112.6 | 113.4 | 113.0 a | ||||
| NS5 | 113.2 | 114.3 | 113.7 a | ||||
| NS6 | 113.9 | 115.3 | 114.6 a | ||||
| NS7 | 111.6 | 113.6 | 112.6 a | ||||
| Mean | 111.7 | 112.8 | |||||
|
Leaf area tiller-1 (cm2) |
NS1 | 12.8 g | 12.1 g | 12.5 f | 0.3665 | 0.5165 | 0.9663 |
| NS2 | 24.4 e | 26.3 d | 25.4 d | ||||
| NS3 | 22.6 f | 25.7 de | 24.2 e | ||||
| NS4 | 25.7 e | 28.1 c | 26.9 c | ||||
| NS5 | 28.7 bc | 29.8 b | 29.2 b | ||||
| NS6 | 31.7 a | 31.8 a | 31.8 a | ||||
| NS7 | 24.6 e | 26.6 d | 25.6 d | ||||
| Mean | 24.4 b | 25.8 a | |||||
| Leaf area index | NS1 | 0.27 h | 0.24 h | 0.26 f | 0.0549 | 0.0207 | 0.0388 |
| NS2 | 0.53 fg | 0.61 e | 0.57 e | ||||
| NS3 | 0.50 g | 0.60 e | 0.55 e | ||||
| NS4 | 0.64 de | 0.77 c | 0.70 c | ||||
| NS5 | 0.76 c | 0.88 b | 0.82 b | ||||
| NS6 | 0.91 b | 1.07 a | 0.99 a | ||||
| NS7 | 0.59 ef | 0.69 d | 0.64 d | ||||
| Mean | 0.60 b | 0.69 a | |||||
Means in each category showing different letter(s) are significantly different applying LSD test at P≤0.05
Table 4: Plant height (cm), CGR (g m-2 day-1), days to maturity, Chl a (µg ml-1) and Chl b (µg ml-1) of wheat as affected by different nitrogen Sources (NS) over two consecutive years.
| Parameters | NS |
Year (Y) |
LSD at P≤0.05 |
||||
| 2015-16 | 2016-17 | Mean | Y×NS | Y | NS | ||
|
Y×NS |
|||||||
| Plant height (cm) | NS1 | 72.3 | 70.2 | 71.2 d | ns | 1.6630 | 3.1111 |
| NS2 | 78.7 | 83.1 | 80.9 c | ||||
| NS3 | 78.9 | 83.2 | 81.0 c | ||||
| NS4 | 81.8 | 85.6 | 83.7 bc | ||||
| NS5 | 84.0 | 88.6 | 86.3 ab | ||||
| NS6 | 87.0 | 90.9 | 89.0 a | ||||
| NS7 | 80.1 | 84.1 | 82.1 c | ||||
| Mean | 80.4 b | 83.7 a | |||||
|
CGR (g m-2 day-1) |
NS1 | 4.53 i | 4.05 i | 4.29 f | 0.6207 | 0.2346 | 0.4389 |
| NS2 | 5.17 h | 6.61 ef | 5.89 e | ||||
| NS3 | 5.56 gh | 7.20 de | 6.38 d | ||||
| NS4 | 6.59 ef | 7.91 bc | 7.25 c | ||||
| NS5 | 7.52 cd | 8.43 b | 7.97 b | ||||
| NS6 | 8.24 b | 9.28 a | 8.76 a | ||||
| NS7 | 6.06 fg | 7.55 cd | 6.80 d | ||||
| Mean | 6.24 b | 7.29 a | |||||
| Days to maturity | NS1 | 156 | 159 | 158 c | ns | ns | 4.8807 |
| NS2 | 164 | 166 | 165 b | ||||
| NS3 | 164 | 168 | 166 ab | ||||
| NS4 | 167 | 169 | 168 ab | ||||
| NS5 | 169 | 170 | 169 ab | ||||
| NS6 | 170 | 171 | 171 a | ||||
| NS7 | 165 | 169 | 167 ab | ||||
| Mean | 165 | 167 | |||||
|
Chl a (µg ml-1) |
NS1 | 0.95 h | 0.86 h | 0.91 f | 0.1450 | 0.0548 | 0.1025 |
| NS2 | 1.14 g | 1.46 e | 1.30 e | ||||
| NS3 | 1.23 fg | 1.62 d | 1.43 d | ||||
| NS4 | 1.46 e | 1.76 bcd | 1.61 c | ||||
| NS5 | 1.66 d | 1.88 b | 1.77 b | ||||
| NS6 | 1.82 bc | 2.08 a | 1.95 a | ||||
| NS7 | 1.34 ef | 1.70 cd | 1.52 cd | ||||
| Mean | 1.37 b | 1.62 a | |||||
|
Chl b (µg ml-1) |
NS1 | 0.33 jk | 0.29 k | 0.31 f | 0.0489 | 0.0185 | 0.0346 |
| NS2 | 0.37 ij | 0.49 ef | 0.43 e | ||||
| NS3 | 0.40 hi | 0.53 de | 0.46 de | ||||
| NS4 | 0.48 fg | 0.57 bcd | 0.52 c | ||||
| NS5 | 0.54 d | 0.61 b | 0.57 b | ||||
| NS6 | 0.59 bc | 0.68 a | 0.64 a | ||||
| NS7 | 0.44 gh | 0.55 cd | 0.49 cd | ||||
| Mean | 0.45 b | 0.53 a | |||||
Means in each category showing different letter(s) are significantly different applying LSD test at P≤0.05
Days to 50% heading were found non-significant during 2015-16, while during 2016-17, significant differences were noted by various nitrogen sources and were found in the range of 107.8 for NS1 to 115.3 days for NS6. Based on pooled data over two years, more days to 50% heading (114.6 days) were recorded by NS6 that was found statistically at par with other nitrogen sources except NS1.
Leaf area varied significantly from 12.8 cm2 recorded for NS1 to 31.7 cm2 for NS6 during 2015-16, while it varied from 12.1 to 31.8 cm2 for the same treatments respectively during 2016-17. Pooled data over two years revealed that broadened leaf area of 31.8 cm2 was achieved by NS6 as compared to other nitrogen sources showing an enhancement of 154.4% over control.
Leaf area index significantly varied from 0.27 for NS1 to 0.91 for NS6 during 2015-16, while it varied from 0.24 to 1.07 for the same treatments respectively during 2016-17. Combined analysis of data over 2015-16 and 2016-17 showed that higher LAI of 0.99 was recorded by NS6 as compared to other nitrogen sources used in the trial.
Interaction between year and various nitrogen sources (Y×NS) was found non-significant for emergence, heading and leaves per tiller and significant for leaf area and LAI.
Similarly, effect of using various nitrogen sources on plant height, crop growth rate, days to maturity, and Chlorophyll a and b of wheat over two consecutive years are given in Table 4. Various nitrogen sources affected plant height significantly during both years. It varied from 72.3 for NS1 to 87 cm for NS6 during 2015-16 while it varied from 70.2 to 90.9 cm for the same treatments respectively during 2016-17. Based on pooled data over two years, higher plants (89 cm) were observed by NS6 that was found at par with plant height (86.3 cm) noted for NS5. Smaller plants (71.2 cm) were recorded by NS1.
Crop growth rate significantly varied from 4.53 for NS1 to 8.24 g m-2 day-1 for NS6 during 2015-16, while it was 4.05 to 9.28 g m-2 day-1 for the same treatments respectively during 2016-17. Based on pooled data over 2015-16 and 2016-17, higher CGR of 8.76 g m-2 day-1 was attained by NS6 as compared to other nitrogen sources used during the trial.
Days to maturity varied significantly from 156 for NS1 to 170 for NS6 during 2015-16, while during 2016-17, it was found in the range of 159 for NS1 to 171 for NS6. Based on pooled data over two years, more days to maturity (171) were attained by NS6 that was found at par with other nitrogen sources utilized in the trial except 165 days recorded for NS2 and 158 for NS1.
Chlorophyll a content differed significantly from 0.95 for NS1 to 1.82 µg ml-1 for NS6 during 2015-16, while it was from 0.86 to 2.08 µg ml-1 for the same treatments respectively during 2016-17. Based on pooled data over two years, higher chlorophyll a content of 1.95 µg ml-1 was attained by NS6 as compared to other nitrogen sources used in the trial.
Chlorophyll b content during 2015-16 varied significantly from 0.33 for NS1 to 0.59 µg ml-1 for NS6 while for the same treatments it was from 0.29 to 0.68 µg ml-1 respectively during 2016-17. Based on pooled data over 2015-16 and 2016-17, higher chlorophyll b content of 0.64 µg ml-1 was attained by NS6 when compared with other nitrogen sources.
Interaction between year and various nitrogen sources (Y×NS) was found non-significant for plant height and maturity and significant for CGR and Chlorophyll a and b.
Significant differences in days to emergence, heading and maturity, leaves and leaf area per tiller, LAI, plant height, CGR and chlorophyll a and b regarding various nitrogen sources combined over two years may be attributed to varied mineralization capability and nutrient availability from each source which resulted favorable conditions and outcomes in case of using NS6 as compared to other nitrogen sources. Our findings agreed with Khan et al. (2008) who described that earlier emergence was noted where N was applied from organic source as compared to inorganic source. More leaves tiller-1 may be attributed to the application of PM in wheat as similar results had been reported by Channabasanagowda et al. (2008). Delayed heading and maturity of wheat may be attributed to the collective utilization of PM and inorganic N fertilizer in wheat (Manna et al., 2005; Rehman et al., 2010). These achievements are consistent with Yadvinder et al. (2009) who described that combined use of PM with mineral N increased nutrients concentration, uptake and wheat yield. Taller plants and higher CGR of wheat may be ascribed to combined use of Urea and PM that improved soil quality and nutrient availability that corresponds to the findings of Abbasi and Tahir (2012) who illustrated that combined application of FYM, PM and Urea improved yield, soil properties and N use efficiency and uptake. Our results of higher chlorophyll a and b were in line with Ofosu and Leitch (2009) who revealed that usage of organic manures increased leaves chlorophyll in barley. Our results were also similar with Fernendez et al. (2010) and Abbasi and Khizar (2012) who reported that N mineralization, where N being an integral part of chlorophyll molecule, significantly increased with combined use of Urea and PM as compared to individual application of PM. Similarly, Amir et al. (2010) itemized that integrated application of manure and N-fertilizer increased total chlorophyll contents and yield of potato.
Utilization of nitrogen sources with and without BM
Data on days to emergence and 50% heading, leaves and leaf area per tiller and LAI based on pooled means over two consecutive years regarding comparison of different nitrogen sources with and without BM are furnished in Table 5. Different N-sources with and without BM had no effect on days to emergence and 50% heading, while leaves and leaf area per tiller and LAI were affected significantly. More leaves per tiller (5.2) were noted by NS6 + BM that was found at par with NS5 + BM (5.1) and NS4 + BM (5.0) as compared to the same treatments without BM where lesser leaves per tiller (4.7), (4.6) and (4.5) were observed respectively. Higher leaf area (34.3 cm2) was noted by NS6 + BM as compared to the same treatments (29.3 cm2) without BM. Higher LAI of 1.18 was observed by NS6 + BM as compared to 0.80 by same treatments without BM.
Similarly, data on plant height, crop growth rate, days to maturity and Chlorophyll a and b based on pooled mean over two consecutive years regarding comparison of different nitrogen sources with and without BM are given in Table 6. Different nitrogen sources with and without BM had significantly affected plant height, CGR and Chlorophyll a and b, while days to maturity were not affected significantly. Among various nitrogen sources, NS6 + BM developed taller plants (93.8 cm) that was found at par with NS5 + BM (90.7 cm) as compared to the same treatments without BM where shorter plants of 84.2 and 81.9 cm were observed, respectively. Similarly, NS6 + BM recorded higher CGR of 10.27 g m-2 day-1 as compared to the same treatment without BM (7.25 g m-2 day-1). Higher chlorophyll a contents of 2.30 µg ml-1 were recorded by NS6 + BM as compared to the same treatment without BM (1.60 µg ml-1). Similarly, NS6 + BM recorded higher chlorophyll b contents of 0.75 µg ml-1 as compared to the same treatment without BM (0.52 µg ml-1).
Table 5: Days to emergence, leaves tiller-1, days to 50% heading, leaf area tiller-1 (cm2) and leaf area index of wheat as affected by different nitrogen sources (NS) with and without beneficial microorganisms (BM) over two consecutive years.
| NS | BM | Days to emergence |
Leaves tiller-1 |
Days to 50% heading |
Leaf area tiller-1 (cm2) |
Leaf area index |
| NS1 | With | 12.7 | 3.9 h | 109.3 | 14.2 k | 0.29 j |
| NS2 | With | 12.5 | 4.8 cde | 113.3 | 26.1 fg | 0.60 fg |
| NS3 | With | 12.9 | 4.8 de | 113.3 | 25.8 gh | 0.61 f |
| NS4 | With | 12.4 | 5.0 abc | 114.4 | 28.8 cd | 0.83 c |
| NS5 | With | 12.2 | 5.1 ab | 114.9 | 30.6 b | 0.97 b |
| NS6 | With | 11.6 | 5.2 a | 115.6 | 34.3 a | 1.18 a |
| NS7 | With | 12.7 | 4.9 bcd | 113.8 | 27.3 ef | 0.74 d |
| NS1 | Without | 13.7 | 3.9 h | 107.0 | 10.8 l | 0.22 k |
| NS2 | Without | 13.3 | 4.4 g | 110.8 | 24.6 hi | 0.55 gh |
| NS3 | Without | 13.7 | 4.4 g | 110.2 | 22.5 j | 0.49 i |
| NS4 | Without | 13.3 | 4.5 fg | 111.6 | 25.1 ghi | 0.58 fgh |
| NS5 | Without | 12.9 | 4.6 efg | 112.5 | 27.9 de | 0.67 e |
| NS6 | Without | 12.3 | 4.7 ef | 113.6 | 29.3 bc | 0.80 c |
| NS7 | Without | 13.4 | 4.5 fg | 111.4 | 23.9 ij | 0.53 hi |
| LSD at P≤0.05 | ns | 0.2205 | ns | 1.3665 | 0.0549 |
Means in each category showing different letter(s) are significantly different applying LSD test at P≤0.05
Table 6: Plant height (cm), CGR (g m-2 day-1), days to maturity, Chl a (µg ml-1) and Chl b (µg ml-1) of wheat as affected by different Nitrogen Sources (NS) with and without beneficial microorganisms (BM) over two consecutive years.
| NS | BM | Plant height (cm) |
CGR (g m-2 day-1) |
Days to maturity |
Chl a (µg ml-1) |
Chl b (µg ml-1) |
| NS1 | With | 70.8 g | 4.31 i | 160 | 0.92 i | 0.32 j |
| NS2 | With | 83.9 cd | 6.38 fg | 168 | 1.41 fg | 0.47 fg |
| NS3 | With | 84.6 cd | 7.10 de | 168 | 1.60 de | 0.52 de |
| NS4 | With | 88.0 bc | 8.18 c | 171 | 1.82 c | 0.59 c |
| NS5 | With | 90.7 ab | 9.27 b | 172 | 2.06 b | 0.67 b |
| NS6 | With | 93.8 a | 10.27 a | 173 | 2.30 a | 0.75 a |
| NS7 | With | 86.3 bc | 7.63 cd | 169 | 1.71 cd | 0.56 cd |
| NS1 | Without | 71.6 g | 4.27 i | 155 | 0.90 i | 0.30 j |
| NS2 | Without | 77.8 ef | 5.39 h | 162 | 1.19 h | 0.39 i |
| NS3 | Without | 77.5 f | 5.66 h | 163 | 1.25 h | 0.41 hi |
| NS4 | Without | 79.3 ef | 6.33 fg | 165 | 1.40 fg | 0.45 fgh |
| NS5 | Without | 81.9 de | 6.68 ef | 167 | 1.48 ef | 0.48 ef |
| NS6 | Without | 84.2 cd | 7.25 de | 168 | 1.60 de | 0.52 de |
| NS7 | Without | 77.8 ef | 5.98 gh | 165 | 1.32 gh | 0.43 ghi |
| LSD at P≤0.05 | 4.3998 | 0.6207 | ns | 0.1450 | 0.0489 |
Means in each category showing different letter(s) are significantly different applying LSD test at P≤0.05
Interaction among year, nitrogen sources and beneficial microorganisms (Y×NS×BM) was found non-significant for days to emergence, 50% heading and maturity, leaves tiller-1 and plant height and significant for leaf area, LAI, CGR, Chlorophyll a and b. Significant interactions had been shown from Figures 4 to 8 respectively.
More leaves tiller-1, delayed 50% heading and maturity, higher leaf area and LAI, taller plants, excellent CGR and greater chlorophyll a and b achieved by NS6 + BM as compared to the same treatment without BM may be ascribed to stimulating nutrient cycling and plant growth because of BM application as reported by Shaheen et al. (2017). Belated heading and maturity, higher leaf area and LAI were linked with improved vegetative growth along with better utilization of nitrogen and availability and solubility of nutrients from the applied substrate as a result of BM application (Gorski and Kleiber, 2010; Sahain et al., 2007; Deldon, 2001). Taller plants, higher CGR and greater chlorophyll a and b of wheat were linked with quick decomposition of organic wastes which had boosted nutrients release from organic sources with BM utilization that ensured sufficient uptake of nutrients for growth and development (Ahmed et al., 2012; Lack et al., 2013). The lone use of inorganic fertilizer has been reported to lack the ability to sustain productivity under continuous intensive cropping system (Benbi et al., 1998). Higher rate of decomposition, mineralization and accelerated nutrients release from organic sources due to BM application (Mbouobda et al., 2014) might had caused higher performance of all the studied parameters.
Year effect on various parameters
Significant variation of year was noted regarding days to emergence, leaves per tiller, leaf area, LAI, height, CGR, Chlorophyll a and b of wheat which had been illustrated from Figures 9 to 16 respectively, while no significant effect of year was seen on days to 50% heading and maturity.
Earlier emergence, more leaves tiller-1, higher leaf area and LAI, taller plants, greater crop growth rate and higher chlorophyll a and b contents of wheat in case of the following year as compared to the preceding year would probably be due to the carry-over effect of nutrient mineralization, availability and cycling due to BM application (Shaheen et al., 2017; Atilla, 2013) as BM accelerated organic matter decomposition which in turn speeded up nitrogen availability from organic sources (Zydlik and Zydlik, 2008) that resulted greater crop growth of wheat. Such results had also been illustrated by Kurepin et al. (2014) who conveyed that better performance of these parameters might be due to accelerated decomposition of organic matter because of BM application to release plant essential nutrients. It also might be due to carry over effect as total N content of organic materials cannot be fully mineralized within one growing season (Hartz et al., 2000; Crew and Peoples, 2005; Hartz and Johnstone, 2006).
Conclusions and Recommendations
The results obtained in the studies denoted distinctive advantages of seed bio-priming and integration of organic and inorganic N-sources and beneficial microorganisms. It was concluded from our findings that seed bio-priming and application of half of the required N from Urea and half from PM with BM application resulted in higher growth and improved chlorophyll content in wheat. Therefore, it is recommended to practise seed bio-priming and apply the required nitrogen as half from Urea and half from PM with BM inoculation (NS6 + BM) continuously for better performance of wheat.
Acknowledgments
The authors are thankful to the Director, Agricultural Research Station, Buner for providing space, labors and assistance.
Novelty Statement
This research work has focused on utilization of beneficial microorganisms (BM) as an innovative technique. BM are primary decomposers which help in releasing nitrogen and other essential nutrients and products from organic materials that might stimulate plant growth and development. The current research work denoted distinctive advantages of practising seed bio-priming and applying the required nitrogen as half from urea and half from poultry manure with BM inoculation. It will enable the farmers to efficiently utilize their organic sources of plant nutrients for enhancing crop productivity and ensuring food security.
Author’s Contributions
Abid Khan is the main author who conducted the experiments for writing his Ph.D. thesis and collected and analysed data along with tabulation and compilation of data and also prepared draft of this manuscript. Mukhtar Alam and Yousaf Jamal proposed the idea and designed and supervised the research work as well as made corrections in the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbasi, M.K. and A. Khizar. 2012. Microbial biomass carbon and nitrogen transformations in a loam soil amended with organic–inorganic N sources and their effect on growth and N-uptake in maize. Ecol. Eng., 39: 123-132. https://doi.org/10.1016/j.ecoleng.2011.12.027
Abbasi, M.K. and M.M. Tahir. 2012. Economizing nitrogen fertilizer in wheat through combinations with organic manures in Kashmir, Pakistan. Agron. J., 104(1): 169-177. https://doi.org/10.2134/agronj2011.0264
Abuamsha, R., M. Salman and R. Ehlers. 2011. Effect of seed priming with Serratia plymuthica and Pseudomonas chlororaphis to control Leptosphaeria maculans in different oilseed rape cultivars. Eur. J. Plant Pathol., 130: 287-295. https://doi.org/10.1007/s10658-011-9753-y
Ali, M., W. Gebreselassie and T. Nardos. 2013. Effect of effective microorganisms (EM) seed treatment and types of potting mix on the emergence and growth of coffee (Coffea arabica L.) Seedlings. Int. J. Agric. Res., 8:34-41. https://doi.org/10.3923/ijar.2013.34.41
Amir, A.N., R.H.S.H. Mohammed, F. Faezeh, T.D. Mohammed and R. Alireze. 2010. Effect of integrated management of nitrogen fertilizer and cattle manure on the leaf chlorophyll, yield and tuber glycoalkaloids of agria potato. Commun. Soil Sci. Plant Anal., 43(6): 912-923. https://doi.org/10.1080/00103624.2012.653027
Benbi, D.K., C.R. Biswas, S.S. Bawa and K. Kumar. 1998. Influence of farmyard manure, inorganic fertilizers and weed control practices on some soil physical properties in a long-term experiment. Soil Use Mgmt., 14: 52-54. https://doi.org/10.1111/j.1475-2743.1998.tb00610.x
Bennett, A.J., M. Andrew and J.M. Whipps. 2009. Performance of carrot and onion seed primed with beneficial microorganisms in glasshouse and field trials. Biol. Control., 51: 417-426. https://doi.org/10.1016/j.biocontrol.2009.08.001
Crew, T.E. and M.B. Peoples. 2005. Can the synchrony of nitrogen supply and the crop demand be improved in legume and fertilizer-based agro-ecosystems? A review. Nutr. Cycl. Agroecosyst., 72: 101-120. https://doi.org/10.1007/s10705-004-6480-1
David, C., M.H. Jeuffroy, F. Laurent, M. Mangin and J.M. Meynard. 2005. The assessment of AZODYN-ORG model for managing nitrogen fertilization of organic winter wheat. Eur. J. Agron., 2: 225-242. https://doi.org/10.1016/j.eja.2004.08.002
Deldon, A.V., 2001. Yield and growth components of potato and wheat under organic N management. Agron. J. 93: 1370-1385. https://doi.org/10.2134/agronj2001.1370
Fernandez, L.F., V.V. Reyes, S.C. Artinez, H.G. Salomon, M.J. Yanez, R.J.M. Ceballos and L. Dendooven. 2010. Effect of different nitrogen sources on plant characteristics and yield of common bean (Phaseolus vulgaris L.). Biores. Technol., 101: 396-403. https://doi.org/10.1016/j.biortech.2009.07.058
Gardner, F.P., R.B. Pearce and R.L. Mitchell. 1985. Physiology of crop plants. 1st Edition. p 327. Iowa State Univ. Press. Ames. USA
Gomez, K.A. and A.A. Gomez. 1983. Statistical Procedures for Agricultural Research. 2nd Edition. An Int. Rice Res. Inst. Book. Inc. pp. 316-356.
Gorski, R. and T. Kleiber. 2010. Effect of effective microorganisms on nutrient contents in substrate and development and yielding of rose and gerbera. Ecol. Chem. Eng., 17(4): 505-513.
Hartz, T.K. and P.R. Johnstone. 2006. Nitrogen availability from high nitrogen containing organic fertilizers. Hort. Technol., 16: 39-42. https://doi.org/10.21273/HORTTECH.16.1.0039
Hartz, T.K., J.P. Mitchell and C. Giannini. 2000. Nitrogen and carbon mineralization dynamic of manures and composts. Hortic. Sci. 35: 209-212. https://doi.org/10.21273/HORTSCI.35.2.209
Higa, T., and G.N. Wididana. 1991a. The concept and theories of effective microorganisms. In: S.B. Parr, Hornick and C.E. Whitman (ed.). Proc. First Int. Conf. Kyusei Nat. Farm. U.S. Dep. Agric., Washington. D.C., USA. pp. 118-124.
Houles, V., M. Guerif and B. Mary. 2007. Elaboration of a nitrogen nutrition indicator for winter wheat based on leaf area index and chlorophyll content for making nitrogen recommendations. Eur. J. Agron., 27: 1-11. https://doi.org/10.1016/j.eja.2006.10.001
Hu, C., and Y. Qi. 2013. Long-term effective micro-organisms application promote growth and increase yields and nutrition of wheat in China. Eur. J. Agron., 46: 63-67. https://doi.org/10.1016/j.eja.2012.12.003
Iqtedar, H., K.M. Ayyaz and K.E. Ahmad. 2006. Bread wheat varieties as influenced by different nitrogen levels. J. Zhejiang Univ. Sci., 7: 70-78. https://doi.org/10.1631/jzus.2006.B0070
Javaid, A., 2009. Growth, nodulation and yield of black gram (Vigna mungo L.) as influenced by bio-fertilizers and soil amendments. Afr. J. Biotechnol., 8: 5711-5717. https://doi.org/10.5897/AJB09.793
Kasim, W.A., M.E. Osman, M.N. Omar, I.A. Abd El-Daim, S. Bejai and J. Meijer 2013. Control of drought stress in wheat using plant growth-promoting bacteria. J. Plant Growth Regul., 32: 122-130. https://doi.org/10.1007/s00344-012-9283-7
Korsaeth, A., T.M. Henriksen and L.R. Bakken. 2002. Temporal changes in mineralization and immobilization of N during degradation of plant material. Implications for the plant N supply and nitrogen losses. Soil Biol. Biochem., 34: 789-799. https://doi.org/10.1016/S0038-0717(02)00008-1
Kurepin, L.V., M. Zaman and R.P. Pharis. 2014. Photochromal basis for plant growth promoting action of naturally occurring bio-stimulants. J. Sci. Food. Agric., 94(9): 1715-1722. https://doi.org/10.1002/jsfa.6545
Li, H., Y. Han and Z. Cai. 2003. Nitrogen mineralization in paddy soils of the Taihu Region of China under anaerobic conditions, dynamics and model fitting. Geoderma. 115: 161-175. https://doi.org/10.1016/S0016-7061(02)00358-0
Linchtenthaler, H.K. and A.R. Wellburn. 1983. Determination of carotenoids and chlorophylls ‘a’ and ‘b’ of leaf extracts in different solvents. Biochem. Soc. Trans., 2: 591-592. https://doi.org/10.1042/bst0110591
Manna, M.C., A. Swarup, R.H. Wanjari, H.N. Ravankar, B. Mishra, M.N. Saha, Y.V. Singh, D.K. Sahi and P.A. Sarap. 2005. Long-term effect of fertilizer and manure application on soil organic carbon storage, soil quality and yield sustainability under sub-humid and semi-arid tropical India. Field Crops Res., 93: 264-280. https://doi.org/10.1016/j.fcr.2004.10.006
Margit, O., 2015. The influence of effective microorganisms on the growth and nitrate content of vegetable transplants. J. Adv. Agric. Technol., 2(1): 25-28. https://doi.org/10.12720/joaat.2.1.25-28
Maureen, O.C., 2016. Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl. Microbiol. Biotechnol., 100: 5729-5746. https://doi.org/10.1007/s00253-016-7590-9
Mbouobda, H.D., Fotso, C.A. Djeuani, M.O. Baliga and D.N. Omokolo. 2014. Comparative evaluation of enzyme activities and phenol content of Irish potato (Solanum tuberosum L.) grown under EM and IMO manures Bokashi. Int. J. Biol. Chem. Sci. 8(1): 157-166. https://doi.org/10.4314/ijbcs.v8i1.15
Moeinzadeh, A., F. Sharifzadeh, M. Ahmadzadeh and F.H. Tajabadi. 2010. Biopriming of sunflower (Helianthus annuus L.) seed with Pseudomonas fluorescens for improvement of seed invigoration and seedling growth. Aust. J. Crop Sci., 4(7): 564-570.
Mowa, E. and E. Maass. 2012. The effect of sulphuric acid and effective micro-organisms on the seed germination of Harpagophytum procumbens (devil’s claw). South Afr. J. Bot., 83: 193-199. https://doi.org/10.1016/j.sajb.2012.05.006
Ouedraogo, E., A. Mando and L. Stroosnijder. 2006. Effects of tillage, organic resources and nitrogen fertilizer on soil carbon dynamics and crop nitrogen uptake in semi-arid West Africa. Soil Tillage Res., 91: 57- 67. https://doi.org/10.1016/j.still.2005.11.004
Quarrie, S.A. and H.G. Jones. 1979. Genotype variation in leaf water potential, stomatal conductance and abscisic acid concentration in spring wheat subjected to artificial drought stress. Ann. Bot., 44: 323-332. https://doi.org/10.1093/oxfordjournals.aob.a085736
Rehman, S., S.K. Khalil, F. Muhammad, A. Rehman, A.Z. Khan, Amanullah, A.R. Saljoki, M. Zubair and I.H. Khalil. 2010. Phenology, leaf area index and grain yield of rainfed wheat influenced by organic and inorganic fertilizer. Pak. J. Bot., 42(5): 3671-3685.
Saber Z., H. Pirdashti, M. Esmaeili, A. Abbasian and A. Heidarzadeh. 2012. Response of wheat growth parameters to co-inoculation of plant growth promoting rhizobacteria (PGPR) and different levels of inorganic nitrogen and phosphorus. World Appl. Sci. J., 16 :213-219.
Sahain, M.F.M., E.Z.A. El-Motty, M.H. El-Shiekh and L.F. Hagagg. 2007. Effect of some Bio-stimulant on growth and fruiting of Anna apple trees in newly reclaimed areas. Res. J. Agric. Biol. Sci., 3(5): 422-429.
Shaheen S., M. Khan, M.J. Khan, S. Jilani, Z. Bibi, M. Munir and M. Kiran. 2017. effective Microorganisms (EM) co-applied with organic wastes and NPK stimulate the growth, yield and quality of spinach (Spinacia oleracea L). Sarhad J. Agric., 33(1): 30-41. https://doi.org/10.17582/journal.sja/2017.33.1.30.41
Shaxson, T.F., 2006. Re-thinking the conservation of carbon, water and soil. A different perspective. Agron. Sustain. Dev., 26: 9-19. https://doi.org/10.1051/agro:2005054
Singh, V., R.S. Upadhyay, B.K. Sarma and H.B. Singh. 2016. Seed bio-priming with Trichoderma asperellum effectively modulate plant growth promotion in pea. Int. J. Agric. Environ. Biotechnol., 9(3): 361-365. https://doi.org/10.5958/2230-732X.2016.00047.4
Torbert, H.A., K.N. Potter and J.E. Jr. Morrison. 2001. Tillage system, fertilizer nitrogen rate and timing effect on corn yields in the Texas Blackland Prairie. Agron. J., 93: 1119-1124. https://doi.org/10.2134/agronj2001.9351119x
Wiatrak, P.J., D.L. Wright and J.J. Marois. 2006. The impact of tillage and residual nitrogen on wheat. Soil Tillage Res., 91: 150-156. https://doi.org/10.1016/j.still.2005.11.015
Yadvinder, S., R.K. Gupta, H.S. Tind, S. Bijay, S. Varinderpal, S. Gurpreet, S. Jagmohan and J.K. Ladha. 2009. Poultry litter as a nitrogen and phosphorous source for the rice-wheat cropping system. Biol. Fertil. Soils., 45: 701-710. https://doi.org/10.1007/s00374-009-0373-z
Zaman, M. and S.X. Chang. 2004. Substrate type, temperature, and moisture content affect gross and net soil N mineralization and nitrification rates in agro-forestry systems. Boil. Fertil. Oils., 39(4): 269-279. https://doi.org/10.1007/s00374-003-0716-0








