Determination of Protein-Lipid Profiles in Hydrolysates Obtained from Trout Byproduct
Determination of Protein-Lipid Profiles in Hydrolysates Obtained from Trout Byproduct
Doğukan Ölmez1 and Gonca Alak2,*
1Graduate School of Natural and Applied Sciences, TR-25030 Erzurum, Turkey
2Department of Aquaculture, Faculty of Fisheries, Ataturk University, TR-25030 Erzurum, Turkey
ABSTRACT
The seafood processing sector contains too rich wastes in terms of protein. These wastes can be converted into different economic value products under controlled conditions. Under controlled conditions, byproducts have the potential to be converted into nutrients suitable for human consumption, as well as different products that have economic value. For this purpose, hydrolysates were prepared by enzymatic methods at different time periods using different trout byproducts. In this study, two different groups (viscera and muscles) of trout processing wastes were hydrolyzed by enzymatic method based on specific time periods (0, 30, 60, 90, 120 and 180 min) for this purpose. Protein profiles, molecular size and distribution changes of the obtained hydrolysates were calculated using SDS-PAGE and lipid profiles were calculated using high performance thin layer chromatography (HPTLC) method. As a result of the analyses, it was observed that there were 9 protein bands in the muscle and 7 protein bands in the visceral organs, and the volume increases were observed in protein amounts in the hydrolysates depending on time. In addition, as the lipid profile, cholesterol ester (CE), triacylglycerol (TAG), free fatty acids (FFA), cholesterol (CHOL), monoacylglycerol (MAG), diacylglycerol (DAG) and phospholipid (PL) values were determined (p <0.05). After 90 min of hydrolysis in muscle, both FFA and MAG-DAG started to collapse, the TAG ratio in total fat content increased relatively.
Article Information
Received 31 July 2018
Revised 01 September 2018
Accepted 05 October 2018
Available online 22 March 2019
Authors’ Contribution
GA, and DÖ conceived and designed the study, performed the laboratory analysis and wrote the paper. Both of them discussed and edited the manuscript.
Key words
Byproducts, Hydrolysate, SDS-PAGE, Protein profile, Lipid profile.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.3.945.954
* Corresponding author: [email protected]
0030-9923/2019/0003-0945 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Using different methods to recycle considerably high amount of waste products and by-products from processing, and research and development studies to produce goods from recycled products into integration of the country’s economy have been gaining more importance in time in the world and in our country. By-products (bones, crusts, skin, offal-like solids and emulsions, suspended liquid wastes, etc.) are waste materials produced during the preparation process of the main product, and are grouped considering their usage areas (Kim and Venkatesan, 2014) (Fig. 1).
Diagram of of seafood by-product sources and its utilization (Kim and Venkatesan, 2014).
Fish hydrolysates, similar to fish meat, contain free amino acids and peptides other than proteins (Kristinsson and Rasco, 2000). Especially free amino acids serve in the physiological functions as well as the characteristic taste of seafood (Wu et al., 2017). These products, which contain the same valuable protein as the fish muscle, are a good alternative for the recovery and exchange of the present protein and have a wide range of applications in a wide range of uses in enzymes and technology (Muzaifa et al., 2012). Hydrolysis with enzyme is widely used to improve and enhance the functional and nutritional properties of food proteins from different sources. This is specific for selective hydrolysis as well as for peptide bonds lying close to certain residues of amino acid (Khantaphant et al., 2008).
Many enzymes are used in the hydrolysis of fish proteins, and with the use of these proteolytic enzymes, fish protein hydrolysates can be converted into food components with novel and/or improved properties, along with peptides under controlled conditions (Souissi et al., 2007).
Hydrolysate production and industrial use of these hydrolysates have increased rapidly in recent years. The molecular weight of the hydrolyzed protein is the most important factor in the use of these hydrolysates rich in amino acid content as functional materials. Different techniques are used to obtain a desired hydrolysate or peptide fraction in a desired molecular size and functional specification, and different methods are utilized in the detection of these proteins (Jeon et al., 1999; Ekici and Akyüz, 2003; Ayana and Turhan, 2010). It has been stated that the SDS-PAGE technique is very suitable for distinguishing meat proteins and for the determination of meat types in some meat mixtures and products (Ekici and Akyüz, 2003; Aslam, et al., 2018). Thin layer chromatography is a simple, inexpensive, precise, fast and practical method to determine the number of substances in a mixture by separating them from each other, whether to detect the target substance is in the mixture and to control the purity of the product. It is a frequently used method due to its high solvent strength and fast obtained results. Another chromatographic method is high performance thin layer chromatography (HPTLC), which has many advantages compared to the conventional thin layer chromatography (TLC) with various validation parameters such as high sensitivity, precision and accuracy. In the development of HPTLC, the development of plate technology played an active role and made important differences with traditional TLC plates. In addition, the particle size is reduced to narrow the distribution, which leads to effective separation. Reducing the layer thickness, increases the number of samples to be analyzed at one analysis by about five times, provides a controlled application, and prevents user error and system deformations. Similarly, isolating the tank from environmental influences (with humidity and temperature control units) provides a significant advantage for standardization. The recording of both the chromatogram and the UV spectra with the computer helps to identify the UV library as well as the Rf value of the substances. By using the peak area and/or height in the obtained chromatogram, the quantity can be determined as compared with the standard solutions (Türkmen et al., 2008).
The aim of this study is to obtain protein hydrolysate from trout by-products using alcalase enzyme and to examine the changes in protein and lipid profiles of prepared hydrolysates depending on time and by product type.
Materials and Methods
Processing byproduct
Daily fillet (market size trouts used in making fillets) prepared at Atatürk University Faculty of Fisheries, Freshwater Fish Application and Research Center Processing Unit was obtained under sterilized conditions.
Protein hydrolysis from fisheries products byproducts
The supplied trout fillet byproduct (muscle and visceral organs, approximately 2 kg) was washed 3-4 times with drinking water and separated into 200-gram pieces. Tissue samples (muscle and visceral organs) weighing 200 g were grinded for 2 min with water at low speed for 1 min and then left in the water bath at 90°C ±10°C for 20±10 min. The samples were further homogenized for 2 min and the optimum temperature and pH were adjusted with NaOH. After the mixture was rested for 30 min, Alcalase enzyme (1.2 AU/100 g protein) was added. The mixture was then allowed to settle with 1 N NaOH at pH = 7.5 at five selected time periods (30 min, 60 min, 90 min, 120 min and 180 min). The samples were then incubated at 80°C for 20 min and subjected to cooling treatment for enzyme inactivation. At the end of the cooling process, the samples were centrifuged at 15000 g for 20 min at 4°C. The middle part (liquid form) between the fat layer (upper part) and the residue (bottom part) of the ependorf tubes was evaluated as hydrolysate (Benhabiles et al., 2012; Muzaifa et al., 2012; Alimentar, 2013; Ibarra et al., 2013). Samples were stored at -20°C until the time of analysis.
Protein analysis method
Total quantities of protein were calculated by the Lowry method (Lowry et al., 1951). In this method, copperions form a complex in the alkaline environment with protein peptide bonds and the copper is reduced. Some amino acids (Tyr, Trp and Cys) in the reduced medium reduce the Folin-Phenol reagent and cause color formation. The intensity of the resulting color is directly proportional to the protein concentration and is measured spectrophotometrically at 660 nm.
Protein profile
To determine the hydrolysate proteins, the samples were taken from at -20°C storage and thawed the previous day. Protein profile analysis was performed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method (Laemmli, 1970). On 100 μlhydrolyzate taken from Eppendorf scrapers, 900 μl electrophoresis sample buffer was added. After thoroughly mixing the tubes and incubating at 100°C for 1 min, SDS-PAGE was performed by adapting the Laemmli method to the TetraCellBioRad system. For staining the proteins in the gel, samples were incubated for 2 h in Oriole’s solution and densitometric analyses of protein bands were performed (BioRad Oriole user manual).
SDS-PAGE analysis
The plate with a flat glass plate (10 x 8.5 cm) and plastic strips with a thickness of 1.5 mm were assembled to form a plate sandwich. First, the resolving gel was filled into gaps between the plates using a 10-ml syringe until the top edge was 2.5 cm thick without forming air bubbles. Following the pouring of the gel, a flat polymerization layer was formed using a thin layer of butanol on the gel surface with a 1 ml syringe. After leaving to polymerize for at least 2 h, the water was removed with a filter paper and the chemical composition stacking gel was prepared with the help of an injector without forming air bubbles. Then, immediately, 1.5 mm thick finger plates were placed and allowed to rest for at least 3 h for polymerization. At the end of this period, the finger plates were removed and the wells to which the samples were applied were washed 3 times with tris-glycine electrode buffer (pH 8.3) to remove polymerization residues, and after the washing procedure, the wells were filled with the same buffer and 15 μl sample was applied to the wells (Laemmli, 1970). Electrophoresis was performed for about 90 min at 20 mA/gel constant current mode. At the end of the period, the gel was left in the oriole solution for 2 h and photos were taken with a gel imaging system (Bio Rad Gel Doc XR).
Commercially available 500 μl marker mix was used (Precision Plus Protein™ All Blue Prestained Protein Standard) as the protein standard. No: 1610373). Accordingly, 15 μlwas added to sample wells and electrophoresis was carried out for about 90 min at 20 mA/gel constant current. After electrophoresis, the homogenate samples were stained with coomassiebrillant blue R250 and photographed on a Biorad Gel Doc XR gel imaging system and analyzed with the ImageLab 4.1 gel analysis program.
Molecular weight calculation is based on the known molecular weight of standard proteins and the determination of molecular weight of the sample protein with the aid of a semi-logarithmic graph in relation to the migration distances of the gel. The Rf value for each standard protein was calculated and the following formula was used:
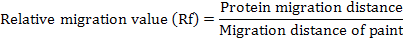
The molecular weights of the photographed tissue proteins were automatically calculated according to the principle mentioned above Bio Rad Image Lab. Ver. 5.2.1. program.
Determination of lipid profile
The procedure was performed using 20 x 10 cm Silica Gel 60 F254 high performance thin layer chromatography (HPTLC) plate. After a mixture of 900 μl n-hexane/iso-propanol (2:1 (v/v)) was added to the hydrolysate (450 μl) samples, the mixture was vortexed at high speed (Hara and Radin, 1978), incubated for 10 min and vortexed again. This process was repeated two more times. The samples were then centrifuged at 5000g for 10 min 4°C and the supernatant (hexane phase) was loaded onto the HPTLC plates. Lipid classes loaded to the plates were run in the hexane: diethylether: formic acid mixture (80:20:2 (v/v/v)) for 7 cm, and dried at the room temperature. The dried plates were sprayed with 3% CuSO4 in 8% H3PO4 and the lipid bands were made visible by burning at 180oC for about 10 min. After photographing the HPTLC plates with the Epson Perfection V700 photoreceptor, the area covered by the lipid bands of each sample was determined using Phoretix 1D (TL120) software and expressed as % of the total mixture (Kaynar et al., 2013).
Statistical analysis
The data obtained in the research were analyzed using the SPSS software package program and the Duncan test was applied to the differences between the groups and the significance levels were determined as p <0.05.
Results
Protein profile
As a result of SDS-PAGE analysis, total protein profiles of muscle and visceral organs were determined volumetrically (Figs. 2, 3). Control (the muscle and visceral organ protein extract obtained without enzymatic treatment, 0th min) and the protein electrophoretic profile of the time-dependent application are given in Tables I and II. The time-dependent statistical changes in the amount of protein in the hydrolysates obtained from muscles and visceral organs are shown in Table III.
Different protein models were observed between control and experimental hydrolysates. After SDS-PAGE analysis of muscle protein hydrolysate, 13 major bands with molecular weights ranging from 10 kDa to 188 kDa were detected. If the first 30 min of heat application, the number of protein bands that can be detected decreased to 9. Again, during the first 30 min, the amount of protein decreased drastically while it progressively increased in the further minutes. This increase continued until the 120th min, after which it started to decrease slightly.
Table I.- Volume and % values of muscle hydrolysate proteins.
Table II.- Volume and % values of visceral organs hydrolysate proteins.
Table III.- Time-dependent protein change ratio of muscle and visceral organ hydrolysates (Mean ± standard deviation).
|
Hydrolysate time (minute) |
Hydrolysate types |
|
|
Muscle* |
Viscera* |
|
|
0 |
4236575.00±1000a |
3862332.0000±1000a |
|
30 |
2460780.00±1000e |
5521056.0000±1000d |
|
60 |
3049305.00±1000d |
6237300.0000±1000c |
|
90 |
3587745.00±1000c |
7043814.0000±1000b |
|
120 |
4108440.00±1000a |
7437942.0000±1000a |
|
180 |
3811115.00±1000b |
7428388.0000±1000a |
*p<0.05 superscripts (a, b) indicate significant differences among different time within each tissue type treatment group in same column.
After SDS-PAGE analysis of visceral organ hydrolysates, 7 major bands with molecular weights ranging from 10 kDa to 190 kDa were detected. The amount of total protein after the first 30 min of heat treatment following the preparation of the hydrolysate began to increase, and this increase continued until the 120th min. After this point, the quantity remained constant. Based on the heating durations, both hydrolysates were determined to have volume decreases and increases in protein amounts.
Statistically, it was determined that time is effective in the amount of protein. For muscle tissue, the highest amount of protein was recorded at the 0th and 120th min. This ratio was recorded at the 120th and 180th min for visceral organs. In general, a time-dependent increase was observed in the protein matrix (p <0.05).
Lipid Profile
According to the HPTLC results of lipid classes in muscles and visceral organs, 7 class lipids were detected in both tissues. These are phospholipids (FL) (Tables IV, V) with cholesterol ester (CE), triacylglycerol (TAG), free fatty acids (FFA), cholesterol (CHOL), monoacylglycerol (MAG), diacylglycerol (DAG). Time-dependent increases
and decreases in phospholipids content were observed in visceral organs, but these differences were not statistically significant (p>0.05). On the other hand, time-dependent changes in muscle lipid groups were found to be significant (p<0.05).
Table IV.- Percentile values of muscle hydrolysate lipids (Mean ± standard deviation).
|
Muscle |
Hydrolysate time* |
|||||
|
Lipid profile |
0 min |
30 min |
60 min |
90 min |
120 min |
180 min |
|
CE* |
5.49±1.0c |
11.95±1.0ab |
12.40±1.0a |
11.85±1.0ab |
11.20±1.0ab |
10.09±1.0b |
|
TAG* |
42.61±1.0a |
22.33±1.0e |
25.12±1.0d |
30.11±1.0c |
30.29±1.0c |
32.37±1.0b |
|
FFA* |
14.10±1.0ab |
15.67±1.0a |
13.11±1.0b |
12.24±1.0bc |
12.75±1.0b |
10.53±1.0c |
|
CHOL* |
5.09±1.0b |
12.30±1.0a |
13.46±1.0a |
12.42±1.0a |
11.77±1.0a |
12.11±1.0a |
|
MAG-DAG* |
16.74±1.0b |
20.36±1.0a |
19.32±1.0a |
16.27±1.0b |
15.49±1.0b |
15.76±1.0b |
|
PL* |
15.98±1.0c |
17.40±1.0abc |
16.59±1.0bc |
17.11±1.0bc |
18.48±1.0ab |
19.13±1.0a |
*p<0.05; superscripts (a, b) indicate significant differences among different time within each tissue type treatment group in same column.
Table V.- Percentile values of visceral organ hydrolysate lipids (Mean ± standard deviation).
|
Viscera |
Hydrolysate time |
|||||
|
Lipid profile |
0 min |
30 min |
60 min |
90 min |
120 min |
180 min |
|
CE* |
3.75±1.0b |
6.77±1.0a |
5.55±1.0a |
6.00±1.0a |
6.65±1.0a |
6.49±1.0a |
|
TAG* |
39.24±1.0a |
26.19±1.0bc |
26.19±1.0bc |
28.05±1.0b |
26.28±1.0bc |
25.51±1.0c |
|
FFA* |
20.17±1.0c |
23.17±1.0ab |
24.64±1.0ab |
24.87±1.0a |
23.64±1.0ab |
22.78±1.0b |
|
CHOL* |
7.91±1.0b |
12.51±1.0a |
12.33±1.0a |
11.36±1.0a |
12.53±1.0a |
12.14±1.0a |
|
MAG-DAG* |
10.16±1.0b |
12.17±1.0ab |
12.45±1.0ab |
11.10±1.0ab |
11.28±1.0ab |
12.39±1.0a |
|
PLNS |
18.77±1.0a |
19.20±1.0a |
18.83±1.0a |
18.62±1.0a |
19.62±1.0a |
20.70±1.0a |
*p<0,05, NS, not significant. Lowercase superscripts (a, b) indicate significant differences among diffirent time within each etissue type treatment group in same column.
Discussion
Fisheries products are highly important food products regarding their unsaturated fatty acid and high protein contents. Protein hydrolysates are defined as proteins that can be chemically or enzymatically metabolized to free amino acids and/or peptides, providing a broad molecular weight range depending on the degree of hydrolysis, either higher or lower. Enzymatic hydrolysis of proteins can be carried out using endogenous enzymes (proteases found in fish) that are added to promote hydrolytic action or exogenous enzymes (Alimentar, 2013).
Usage of endogenous enzymes, process is prolonged and protein hydrolysis requires a longer control, which prevents obtaining products with uniform quality standards. For enzymatic hydrolysis with exogenous Table
enzymes, these can be selected according to the intended properties of the product. Hydrolysis should be carried out under optimum conditions, excessive pH and heat applications affect the nutritive qualities of hydrolysate (Alimentar, 2013).
In the study, band densities of the total protein profile decreased. Furthermore, there was no difference between the application groups in terms of the band intensities. In our hydrolysate time periods, it is thought that the decrease in band density will continue in case of an increase in the period of the application longer than 120 min. In our study results, the ratio of the protein in the total protein did not change substantially, and the degree of heat applied to the hemolysates was not as high as to counteract and cause denaturation of the proteins, suggesting that the increase in the amount of protein is due to water loss during the heating of the samples (Harper, 1959). The increase in lipid peroxidation supports the decrease in protein denaturation and the total protein volume.
The increase in band density according to electrophoresis results is thought to be related to the non-disruption of disulfide covalent bonds and the absence of protein denaturation due to the duration of hydrolysis. Polypeptides are expected form by the hydrolysis of major proteins found in the muscle before the hydrolytic process (miosin heavy chain: 200 kDa, actin: 45 kDa, etc.). Low-molecular-weight bands indicate a progressive hydrolysis of proteins in the soluble fraction, and that there is an association between the molecular weight and functional properties of the polypeptides. The higher degree of hydrolysis yields lower molecular weight polypeptides and thus their solubility increase (Pacheco-Aguilar et al., 2008). Similar to our study, Silva et al. (2014) reported that the hydrolysis period had an effect on the hydrolization of the proteins to peptides and amino acids. Bhaskar and Mahendrakar (2008) reported that fish protein hydrolysates being nutrient-rich dietary proteins are related with free amino acids being rich in terms of low molecular weight peptides.
Higher kDa bands can be a consequence of proteins that are larger than the starting material, or proteins that are not completely hydrolyzed by the enzyme in the raw material (Souissi et al., 2007). The absence of high-weight bands can also indicate protein loss (Ünlüsayın et al., 2011).
Enzymatic and chemical changes have been widely used to improve the functional properties of proteins. Peptides produced by enzyme hydrolysis have smaller molecular sizes and fewer secondary structures than the original proteins (Jeon et al., 1999; Kim et al., 2004). Most of the protein hydrolysates are characterized by their hydrolysis conditions and their starting materials. Protein hydrolysates are particularly rich in low molecular weight peptides containing free amino acids and di- and tri-peptides (Bhaskar and Mahendrakar 2008).
The molecular weight of the hydrolyzed protein is one of the most important factors in the production of protein hydrolysates with functional properties produced to be used as functional materials. Therefore, limited enzymatic hydrolysis is performed to obtain a hydrolysate with an appropriate molecular size. However, an insufficient period of hydrolysis causes limited quantification of enzymatic hydrolysis, making it nearly impossible to recover all proteins present in fish. Accordingly, different methods are usedto obtain a hydrolysate or peptide fraction having both a desired molecular size and a functional property (Jeon et al., 1999). Visceral organs of fish have rich enzyme sources. Most of these enzymes exhibit high catalytic activity at relatively low concentrations. Considering the specific properties, fish processing by-products are now widely used for enzyme extraction. Among these proteolytic enzymes, the commercial proteolytic enzymes pepsin, trypsin, chymotrypsin and collagenases extracted from marine fish organs are especially notable (Kim and Mendis, 2006). The amino acid composition of the protein hydrolysate is highly hygroscopic (Bhaskar and Mahendrakar, 2008; Ayana and Turhan, 2010). Fisheries products have an important place in human nutrition with quality protein and fatty acid content. Cholesterol is a heat resistant molecule (melting point 148°C). In addition, there is no enzyme system that breaks down cholesterol. The removal of cholesterol from the body can take place through the elimination of bile through the gastrointestinal tract (Hara and Radin, 1978). Therefore, cholesterol levels in biological systems are considered to be constant except extreme conditions such as extreme stress. Cholesterol ester is also a very strong lipid class such as cholesterol. However, digestion can only be achieved by the activity of the enzyme cholesterol esterase released from the pancreas (Harper, 1959). As observed in HPTLC, when both cholesterol and cholesterol ester ratios of total fat contents of muscles and visceral organs were examined, it was seen that the ratio of both of them in total lipid classes increased up to two folds during the first 30 min of heat period applied to hydrolysates. Again, the ratio of TAG in total fat decreased dramatically within the first 30 min, while the proportions of other lipid classes remained constant or slightly increased over this time. Considering the increase in Col and ColE as well as the decrease in TAG, an excessive decrease in TAG caused a relative increase in other lipid classes. In fact, a slight decrease was observed in these lipid classes over the course of heat treatment. During the increasing temperature cycles, the change remained relatively constant in the visceral organs. However, after 90 min of hydrolysis in muscle, both FFA and MAG-DAG began to disrupt, the TAG ratio in total fat content increased relatively but the proportions of other lipid classes remained stable.
Conclusion
In conclusion, this study concludes that the duration of hydrolysis is effective on the protein profile of both hydrolysates and that the increase in protein is continued for both hydrolysates until the longest heating time period, and only the amount of protein in the hydrolysates obtained from the muscles decreases within the first 30 min. It has been observed that heating time period had an effect only on the phospholipid content. It was observed that both FFA and MAG-DAG started to break down after 90 min of hydrolysis in the muscular tissue, while the ratio of TAG in the total fat content relatively increased. It was also thought that the analytical techniques used in the profile determination of fish protein hydrolysates were effective.
Acknowledgements
We’d like to thank Assoc. Dr. Özgür Kaynar for their help and support in the laboratory studies, and Research Assistant Fatih Korkmaz for his moral support. This work was supported by the Atatürk University Scientific Research Projects Unit with the code BAP 2016/187.
Compliance with ethical standards
This study was funded by Atatürk University (BAP 2016/187).
Ethical approval
In this study only byproduct were involved. This article does not contain any studies with animals performed by any of the authors.
Statement of conflict of interest
We have not any conflict of interest to declare.
References
Alimentar, E., 2013. Production of fish protein hydrolysates by a marine proteolytic strain. Master thesis, Instituto Português do Mar e da Atmosfera, Divisão de Aquacultura e Valorização.
Aslam, M.S., Gull, I., Abbas, Z. and Athar, M.A. 2018. Soluble expression of IFNα2-Tα1 fusion protein in Escherichia coli by N-terminal SUMO fusion and its anti-proliferative activity. Pakistan J. Zool., 50:1413-1419.
Ayana, B. and Turhan, K.N., 2010. Edible films/coatings containing antimicrobial agent and their applications in food packaging. Gıda, 35: 151-158.
Benhabiles, M.S., Abdi, N., Drouiche, N., Lounici, H., Pauss, A., Goosen, M.F.A. and Mameri, N., 2012. Fish protein hydrolysate production from sardine solid waste by crude pepsin enzymatic hydrolysis in a bioreactor coupled to an ultrafiltration unit. Mat. Sci. Engin., 32: 922-928. https://doi.org/10.1016/j.msec.2012.02.013
Bhaskar, N. and Mahendrakar, N.S., 2008. Protein hydrolysate from visceral waste proteins of Catla (Catla catla): Optimization of hydrolysis conditions for a commercial neutral protease. Bioresour. Technol., 99: 4105-4111. https://doi.org/10.1016/j.biortech.2006.12.015
https://doi.org/10.1016/j.biortech.2007.09.006
Ekici, K. and Akyüz, N., 2003. A study with SDS-PAGE technique for the species identification of raw meat. YYÜ Vet. Fak. Derg., 14: 78-82.
Hara, A. and Radin, N.S., 1978. Lipid extraction of tissues with a low-toxicity solvent. Analyt. Biochem., 90: 420-426. https://doi.org/10.1016/0003-2697(78)90046-5
Harper, A.E., 1959. Amino acid balance and imbalance. 1. Dietary level of protein and amino acid imbalance. J. Nutr., 68: 405-418. https://doi.org/10.1093/jn/68.3.405
Ibarra, J.P., Teixeira, A., Simpson, R., Valencia, P., Pinto, M. and Almonacid, S., 2013. Addition of fish protein hydrolysate for enhanced water retention in Sous Vide processing of salmon. J. Fd. Process. Technol., 4: 1-7. https://doi.org/10.4172/2157-7110.1000241
Jeon, Y.J., Byun, H.G. and Kim, S.K., 1999. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochem., 35: 471-478. https://doi.org/10.1016/S0032-9592(99)00098-9
Kaynar, Ö., İleritürk, M. and Hayırlı, A., 2013. Evaluation of computational modifications in HTLC with gel analysis software and flatbed scanner for lipid separation. J. Planar Chromatogr. Modern TLC, 26: 202-208. https://doi.org/10.1556/JPC.26.2013.3.1
Khantaphant, S. and Benjakul, S., 2008. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. Part B: Biochem. mol. Biol., 151: 410-419.
Kim, J.M., Whang, J.H. and Suh, H.J., 2004. Enhancement of angiotensin I converting enzyme inhibitory activity and improvement of the emulsifying and foaming properties of corn gluten hydrolysate using ultrafiltration membranes. Eur. Fd. Res. Technol., 218: 133-138. https://doi.org/10.1007/s00217-003-0825-x
Kim, S.K. and Mendis, E., 2006. Bioactive compounds from marine processing byproducts–a review. Fd. Res. Int., 39: 383-393. https://doi.org/10.1016/j.foodres.2005.10.010
Kim, S. and Venkatesan, J., 2014. Seafood processing by-products: Trends and applications. Springer New York. pp. 1–10.
Kristinsson, H.G. and Rasco, B.A., 2000. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Fd. Sci. Nutr., 40: 43-81. https://doi.org/10.1080/10408690091189266
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685. https://doi.org/10.1038/227680a0
Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J., 1951. Protein measurement with the folin phenol reagent. J. biol. Chem., 193: 265-275.
Muzaifa, M., Safriani, N. and Zakaria, F., 2012. Production of protein hydrolysates from fish byproduct prepared by enzymatic hydrolysis. AACL Bioflux, 5: 36-39.
Pacheco-Aguilar, R., Mazorra-Manzano, M.A. and Ramírez-Suárez, J.C., 2008. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Fd. Chem., 109: 782-789. https://doi.org/10.1016/j.foodchem.2008.01.047
Silva, J.F.X., Ribeiro, K., Silva, J.F., Cahú, T.B. and Bezerra, R.S., 2014. Utilization of tilapia processing waste for the production of fish protein hydrolysate. Anim. Feed Sci. Technol., 196: 96-106. https://doi.org/10.1016/j.anifeedsci.2014.06.010
Souissi, N., Bougatef, A., Triki-Ellouz, Y. and Nasri, M., 2007. Biochemical and functional properties of Sardinelle (Sardinella aurita) by-product hydrolysates. Fd. Technol. Biotechnol., 45: 187-194.
Türkmen, Z., Mercan, S. and Cengiz, S., 2008. Simultaneous high performance thin layer chromatographic determination of heroine, morphine, cocaine and MDMA. J. Foren. Med., 22: 13-24.
Ünlüsayın, M., Gümüş, B., Erdilal, R. and Gülyavuz, H., 2011. The ınfluence of different salting processes on protein loss of Cuttlefish (Sepia officinalis). Ege J. Fish. aquat. Sci., 28: 71-74.
Wu, R., Chen, L., Liu, D., Huang, J., Zhang, J., Xiao, X., Lei, M., Chen, Y. and He, H., 2017. Preparation of antioxidant peptides from salmon byproducts with bacterial extracellular proteases. Mar. Drugs, 15: 1-19. https://doi.org/10.3390/md15010004
To share on other social networks, click on any share button. What are these?










