Black Garlic Phytochemical Potential and Antioxidant Capacity as a Feed Additive
Research Article
Black Garlic Phytochemical Potential and Antioxidant Capacity as a Feed Additive
Hamdi Mayulu1*, Endang Sawitri2
1Animal Sciences Departement of Agricultural Faculty, Mulawarman University, Kampus Gunung Kelua Jalan Pasir Belengkong Samarinda 75123, East Kalimantan, Indonesia; 2Laboratory of Physiology Science, Medical Faculty, Mulawarman University, Kampus Gunung Kelua Jalan Krayan Samarinda 75123, East Kalimantan, Indonesia.
Abstract | Black garlic is usually obtained from the thermal fermentation process which comprises incubation at controlled temperature and humidity for several weeks. During this process, browning and non-enzymatic reactions alter the taste, aroma, physicochemical properties, and organoleptic and bioactive compounds in garlic. Therefore, this study aims to investigate the phytochemical potential and antioxidant capacity of black garlic as a livestock feed additive. The Folin-Ciocalteau method was used to test for tannins and total phenolics, and the aluminum chloride (AlCl3) method was used to assess flavonoid content. In addition, antioxidant capacity was determined with Radical Scavenging Activity 2,2-diphenyl-1-picrylhydrazyl (DPPH) and expressed with IC50 value. The data obtained were analyzed using the single factor Analysis of Variance (ANOVA) with the IBM Statistical Package for Social Sciences (SPSS). The results showed that the concentrations of flavonoids, tannins, and total phenolics were 51.325±1.47 mgQE/g, 553.165±34.18 mg tannic acid/g, and 339.875±18.86 mg GAE/g respectively, while the DPPH radical scavenging activity was 58.5 µg/mL at 21 days of incubation (T3). This indicated that black garlic has broad potential to become a natural feed additive suitable for livestock production in line with the development and progress of science and modern technology.
Keywords | Black Garlic, Bioactive Compounds, Antioxidants, Feed Additives, Livestock
Received | March 21, 2023; Accepted | May 09, 2023; Published | May 29, 2023
*Correspondence | Hamdi Mayulu, Animal Sciences Departement of Agricultural Faculty, Mulawarman University, Kampus Gunung Kelua Jalan Pasir Belengkong Samarinda 75123, East Kalimantan, Indonesia; Email: hamdi_mayulu@faperta.unmul.ac.id
Citation | Mayulu H, Sawitri E (2023). Black garlic phytochemical potential and antioxidant capacity as a feed additive. Adv. Anim. Vet. Sci. 11(7):1047-1056.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.7.1047.1056
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Livestock require a complete diet to meet their nutritional needs, including macronutrients such as proteins, lipids, and carbohydrates, as well as micronutrients such as calcium, phosphorus, magnesium, and vitamins D, C, and K. These nutrients are obtained through the feed consumed by the animals (Mayulu et al., 2021). However, the quantity of nutrients required is sometimes lacking, and additional ingredients or certain combinations are needed as feed additives. This can increase livestock production, and feed efficiency, improve the rate of gain, as well as prevent and control disease or harmful environmental influences (Mayulu et al., 2020; Chen et al., 2021). Feed additives are divided into two, namely nutritional and non-nutritional. Meanwhile, a natural ingredient widely known to have various bioactive compounds that are beneficial to the health of both human and livestock production is garlic (Chen et al., 2021).
Garlic (Allium sativum L.) is a horticultural plant (Rios et al., 2021) that has long been used as a traditional medicine for various diseases (Shehri, 2021). This is due to its bioactive compounds (Yu et al., 2020) such as polysaccharides consisting of 85% fructose, 14% glucose, and 1% galactose, as well as saponins, polyphenols, flavonoids, phenolic acids, and various sulfur compounds (Shang et al., 2019; Najman et al., 2021). The main active components in garlic are organosulfur compounds such as diallyl thiosulfonate (allicin), S-allyl-cysteine (SAC), S-allyl-cysteine sulfoxide (alliin), Diallyl sulfide (DAS), Diallyl disulfide (DADS), Diallyl trisulfide (DATS), E-ajoene, and Z-ajoene, as indicated in Figure 1 (Shang et al., 2019; Shang et al., 2019; Gudalwar et al., 2021).
The medical use of garlic based on previous reports includes immunomodulatory, anti-inflammatory (Saryono et al., 2021), anti-bacterial, antibiotic (Najman et al., 2021), anti-cancer, anti-tumor (Leontiev et al., 2018; Chen et al., 2019), anti-Alzheimer’s, antidiabetic, renoprotective, anti-atherosclerotic (Batiha et al., 2020), and anti-fatigue. Garlic also regulates blood glucose and pressure helps digestion, as well as increases appetite (Lu et al., 2017). Furthermore, it has outstanding antioxidant capabilities as a powerful scavenger that inhibits free radical reactions, supports the action of endogenous antioxidant enzymes, and enhances the ability of the body to fight free radicals, making it effective in preventing various civilizational diseases (Najman et al., 2021). The efficacy of garlic can be increased through various thermal-based processing methods including heat treatment, aging, and fermentation (Yu et al., 2020). Fermented garlic without any additives will undergo changes in chemical compounds, organoleptic properties, and bioavailability culminating in black garlic products (Medina et al., 2019; Najman et al., 2021; Karnjanapratum et al., 2021; Lestari et al., 2022).
Black garlic has antioxidant activity with various nutrients and contains thiosulfate, polysaccharides, flavonoids, reducing sugars, proteins, phenolics, organic sulfur compounds, and melanoidin (Nakagawa et al., 2020; Yu et al., 2020; Liang et al., 2022, 2022), diallyl trisulfide, allyl methyl trisulfide, and small amounts of epicatechin (Moon et al., 2022). Based on previous studies, it has strong antibacterial properties (Bedrnícek et al., 2021; Moon et al., 2022) and compounds that can protect the genotoxin genome from damage in a dose-dependent manner. This ability is related to the antioxidant capacity and free-radical scavenging of organosulfur compounds (Medina et al., 2019). The phytochemical and antioxidant properties of black garlic have the potential to be further explored as non-nutrient feed additives for livestock. Therefore, this study aims to explore the phytochemical potential and antioxidant capacity of black garlic as a feed additive for livestock.
MATERIALS AND METHODS
Material
The main material in this study was single-bulb garlic and the chemicals used in the analysis included Folin-Ciocalteu reagent, sodium carbonate, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, aluminum chloride. (AlCl3), 95% ethanol, distilled water, 5% NaNO2, diethyl ether, Na2CO3, and ascorbic acid.
Study procedure
This study was conducted in three stages, namely (1) single garlic preparation, (2) thermal fermentation process, and (3) phytochemical test and antioxidant capacity. The tested phytochemicals included flavonoids, tannins, and total phenolics, while the antioxidant capacity was assessed through the DPPH radical scavenging activity. The following is a brief schematic showing the stages of this study (Figure 2).
Black garlic sample preparation
Single garlic (single bulb) as raw material for making black garlic was obtained from the traditional market. The first step was to clean the garlic and set them into a thermal fermenter according to the curing time. Thermal processing was carried out at high temperatures ranging from 60-90oC, and controlled humidity of 70-90% for a period of 0 days (T0), 7 days (T1), 14 days (T2), and 21 days (T3). The process was performed without the participation of microorganisms such as bacteria, fungi, and even preservatives, but only under the influence of enzymes that occur naturally in raw materials. This is due to natural reactions and changes in the constituent biochemical compounds of garlic (Medina et al., 2019; Najman et al., 2021).
Flavonoid test
The concentration of flavonoids in garlic was determined using the aluminum chloride (AlCl3) method with some adjustments and quercetin as a standard (Hue et al., 2022; Kumar et al., 2022). The extract (1 mg) was dissolved with 95% ethanol to 10 ml and added with 0.7 ml of distilled water and 0.1 ml of 5% NaNO2. The mixture was allowed to stand for 5 minutes, then 0.1 ml of 10% AlCl3 was added and incubated for 10 minutes. Absorbance was measured at a wavelength of 510 nm using a spectrophotometer with a blank in the form of 1 ml of sample replaced with 1 ml of 95% ethanol solvent. The final results were expressed as milligrams of quercetin equivalent per gram dry weight (mg QE/g dry weight) (Zou et al., 2004; Hapsari et al., 2022; Hue et al., 2022).
Tannin test
The tannin concentration was determined using the standard Folin-Ciocalteau method with slight modifications (Manoshree and Devangi, 2019). The test procedure began with weighing a sample of 0.5 g and extracting 10 ml of diethyl ether for 20 hours, followed by filtering. The residue obtained was boiled with 100 ml of distilled water for 2 hours, then cooled and filtered to produce the extract. The extract obtained was added to distilled water until the volume reached 100 ml. About 0.1 ml of the extract together with 0.1 ml of 50% Folin-Ciocalteau reagent was vortexed. Furthermore, the mixture of extracts and Folin-Ciocalteau was added to 2 ml of Na2CO3 and vortexed. The absorbance was read at a wavelength of 760 nm after incubation for 30 minutes at room temperature and the results obtained were plotted against the standard curve of tannic acid. Tannin concentrations are expressed in mg of tannic acid/kg extract (Malangngi et al., 2012).
Total phenolic test
Identification of total phenolics was carried out by the Folin-Ciocalteau method with slight modifications, and UV-Vis spectrophotometry using gallic acid as the standard (Kim et al., 2013; Nayeem et al., 2022). The first step was to extract the sample by peeling the skin of the garlic and mashing it to make a paste. Garlic and black garlic paste of about 0.2 g were extracted with 5 ml of 80% ethanol for 24 hours at room temperature. The supernatant obtained was then vortexed and centrifuged at 700x g for 10 minutes and filtered through a PVDF syringe filter (0.45µM) (Nakagawa et al., 2020). About 0.4 ml of the sample was reacted with 2 ml of tenfold diluted Folin-Ciocalteau reagent and incubated for 8 minutes. Afterward, the reaction mixture was added with 1.6 ml of 7.5% (w/v) Na2CO3 solution and incubated in the dark at room temperature for one hour. The absorbance was measured by UV-Vis spectrophotometry and the readings were taken at the maximum absorption wavelength of 765 nm after incubation for 3 repetitions. The result in the form of the total phenolic concentration was expressed as milligrams of gallic acid equivalent (mg GAE)/g lyophilized sample (Medina et al., 2019; Capra et al., 2022).
DPPH radical scavenging activity
The antioxidant capacity of single and black garlic was tested in vitro using the 2,2-Diphenyl-1-Picrylhdrazyl (DPPH) method (Rammal et al., 2012; Paat et al., 2022). The testing procedure began with taking single and black garlic samples. The extract obtained was diluted, then 2 ml was added with 2 ml of DPPH (0.15 mM in ethanol) and mixed until homogeneous. The mixture was incubated in a dark place at room temperature for 30 minutes and also served as a control prepared to consist of 2 ml DPPH with 2 ml of ethanol. The absorbance was measured at a wavelength of 517 nm, while ascorbic acid was used as a positive control and ethanol as a blank (Rammal et al., 2012; Bedrnícek et al., 2021). The DPPH ability of the extract was calculated using the equation (Rammal et al., 2012; Ro et al., 2022).
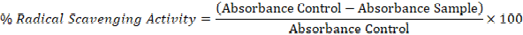
Description: Absorbance control: Absorbance DPPH + Etanol; Absorbance sample: Absorbance + sample
The final result of the DPPH scavenging activity calculation was expressed by the IC50 value through the linear regression line equation (y=a+bx) (Conte et al., 2022). This value represents the concentration of the extract/fraction/test compound/sample that can inhibit and/or capture free radicals by 50% (Ramírez et al., 2022; Ro et al., 2022). Therefore, the smaller the IC50 value, the more active or stronger the antioxidant potential of a compound. Antioxidant capacity based on the IC50 value can be categorized as follows.
Table 1: Antioxidant capacity based on IC50 value.
|
IC50 value |
Antioxidant capacity |
|
< 50µg/mL |
Very strong |
|
50-100 µg/mL |
Strong |
|
100-150 µg/mL |
Currently |
|
150-200 µg/mL |
Weak |
Source: Paat et al., 2022
Statistic analysis
The results of all data were expressed as mean ± standard deviation values and analyzed using a single factor Analysis of Variance (ANOVA) with the IBM Statistical Package for the Social Sciences (SPSS).
RESULTS AND DISCUSSION
Characteristics of black garlic
Fresh single garlic was used the basic ingredient for producing black garlic through a thermal incubation process which consisted of heating, fermentation, and aging. During these processes, it underwent Maillard or amino-carbonyl reaction which led to several changes in physicochemical characteristics, and nutritional content (Jing, 2020; Karnjanapratum et al., 2021; Barido et al., 2022; Mayulu and Sawitri, 2023), as well as organoleptic, and sensory properties (Najman et al., 2020). It also culminated in the formation of new bioactive or neo-formed compounds such as melanoidin which are nitrogen-containing heterogeneous compounds (Nakagawa et al., 2020) and Amadoris (ACS) (Yu et al., 2020; Ahmed and Wang, 2021). The quantified Amadori compounds in black garlic included N-(1-deoxy-D-fructose-1-yI)-L-proline (Fru-Pro), N-(1-deoxy-D-fructose-1-yI)- L-valine (Fru-Val), N-(1-deoxy-D-fructose-1-yl)-L-leucine (Fru-Leu), N-(1-deoxy-D-fructose-1-yl)- L-methionine (Fru-Met), and N-(1-deoxy-D-fructose-1-yl)-L-arginine (Fru-Arg) (Rios et al., 2019; Yu et al., 2020).
Amadori compounds are important intermediates in the Maillard reaction which in turn can produce various heat treatment products such as the slightly sweet taste and pleasant aroma of black garlic. Furthermore, the distinctive sweet taste was from the reaction of glucose or fructose with amino acids, culminating in a change in sugar and a significant increase in the fructose content (Najman et al., 2020; Setyoningrum et al., 2021). The Maillard reaction also enhanced the synthesis of organic acids such as formic, succinic, 3-hydroxy propionic, or pyroglutamic acids due to the oxidation of the aldehyde groups in aldoses, or hexose degradation which increased the acetic acid content (Najman et al., 2022).
Other chemical changes that occurred include the degradation of fructans into monomers, an increase in the content of S-allyl-L-cysteine 6 times compared to fresh garlic, a rise in the concentration of polyphenols, flavonoids (Rios et al., 2021), S-allymercaptocys-teine (SAMC), pyruvate (Shehri, 2021), total phenolic acid, 5-hydroxy-methyl furfural, melanoidin, and thiosulfinate (Barido et al., 2022). Moreover, the water activity of garlic during the thermal process to black garlic also decreased from 0.97 to 0.93 but the total dissolved solids (Brix) increased from 40.47 to 45.67 (Medina et al., 2019). Carbohydrates also increased 1-2 times, sucrose 1.3-1.6 times, fructose 6-108 times, glucose 2-13 times, and amino acids 2.5 times compared to fresh garlic (Jing, 2020).
The Maillard and/or browning reaction caused a decrease in pH from 5.94 to 3.49-3.69 (Medina et al., 2019), a change in the color from white to caramel, brown to black, a sweet taste, slightly tart with a dash of dried plums and apricots, vanilla or licorice. The decrease in pH was caused by an increase in the product content such as a rise in the synthesis of organic acids due to the oxidation of the aldehyde groups in aldoses and acetic acid during the fermentation process (Najman et al., 2020). Furthermore, the texture of garlic became more rubbery or jelly-like (Cinar et al., 2022) while the consistency appeared softer, and smoother, resembling cream cheese (Najman et al., 2020). The browning intensity of garlic during the thermal process decreased from 47.16 (fresh garlic) to 17.58 (black garlic). The intensity can occur earlier at higher temperatures, hence, an increase in temperature and the browning product formation have a positive relationship. The color change of garlic from white to black due to a non-enzymatic reaction (Maillard reaction) indicates the formation of various compounds (Medina et al., 2019).
Compounds that were unstable in garlic during heat treatment were converted into stable soluble components with high antioxidant capacity (Medina et al., 2019). Thermal treatment can accelerate the degradation of polysaccharides with high molecular weight into oligosaccharides and monosaccharides with low molecular weight which are consequently converted into water-soluble bioactive compounds such as S-allyl cysteine, tetrahydro-β-carbolines, polyphenols alkaloids, and flavonoids (Karnjanapratum et al., 2021). These compounds have a strong relationship with biological and pharmacological properties such as antioxidant, anticancer, antitumor, anti-allergic, and hypolipidemic activity (Karnjanapratum et al., 2021). Besides, physicochemical changes due to processing indicate an increase in black garlic bioactivity (Lestari et al., 2022).
Based on the results, black garlic had different characteristics as demonstrated in Figure 3. The appearance with increasing incubation time became darker, rubbery consistency with a sweet taste, and a pleasant aroma was produced. Karnjanapratum et al. (2021) reported that black garlic has a soft and mushy texture, chewy consistency with a sweet taste and pleasant aroma. This is related to the formation of aldehydes which are the dominant compound in garlic (Rios et al., 2021). The texture of black garlic in the 7-day fermentation (T1) was soft and a bit hard, in the 14-day (T2) the texture was mushy, and in the 21-day (T3), it was soft with chewy and elastic consistency (Figure 3). This was caused by the water content, which decreased from 72.60% to 50-70% culminating in the soft and elastic texture with a water content of about 40-50% (Sunanta et al., 2021). According to Hue et al. (2022), garlic had a better appearance in terms of elasticity and consistency when incubated at 70oC.
Black garlic phytochemicals
The phytochemicals of black garlic are very diverse, including S-allyl cysteine, tetrahydro-β-carboline, polyphenolic alkaloids, flavonoid-like compounds (Karnjanapratum et al., 2021), thiosulfinates (Sunanta et al., 2021), amino acids (Afzaal et al., 2021), tannins and volatile compounds including acetic acid (35.34%), Trisulfide, di-2-propenyl (17.72%), Trisulfide, methyl 2-propenyl (10.62%), and 2-Furancarboxaldehyde (7.93%) (Cinar et al., 2022). Bioactive compounds such as allicin produced by chemical extraction and synthesis from garlic have been widely used in livestock production due to their low cost, high-purity ingredients, and significant medicinal properties (Chen et al., 2021).
Table 2: Phytochemical content of garlic based on the fermentation time.
|
Treatment |
Bioactive compounds |
||
|
Flavonoid (mg QE/g) |
Tannins (mg Tannic acid/kg) |
Total phenolic (mg GAE/g) |
|
|
T0 |
40.02±1.27 |
463.165±29.46 |
38.97±1.99 |
|
T1 |
28.89±3.25 |
108.165±41.25 |
47.435±3.81 |
|
T2 |
43.53±2.04 |
534.83±10.61 |
230.77±1.99 |
|
T3 |
51.325±1.47 |
553.165±34.18 |
339.875±18.86 |
Description: T0: Fresh garlic single bulb; T1: Garlic single bulb fermented for 7 days; T2: Single bulb garlic fermented for 14 days; and T3: garlic single bulb fermented for 21 days.
Flavonoid
Phytochemicals in black garlic include various bioactive compounds, such as flavonoids. They are a very diverse group of phenolic compounds, not only in terms of their chemical structure with different numbers and distribution of hydroxyl groups but also in terms of thermal stability (Najman et al., 2020). The main subgroup of flavonoids present in black garlic are flavonols, followed by flavanones, and flavones. Flavanones in fresh garlic act as main metabolites that have biological functions such as acting as defensive compounds and antioxidants against stress (Sunanta et al., 2021). The concentration of flavonoids in black garlic varied greatly, depending on processing conditions such as processing time, temperature, and sensitivity (Sunanta et al., 2021). The concentration increased significantly during thermal fermentation specifically the aging period (Lu et al., 2017; Setyoningrum et al., 2021) hence, the fermentation time plays an important role in increasing flavonoids (Setyoningrum et al., 2021). This increase has several underlying reasons, including (1) the thermal fermentation process breaks down or releases bound forms such as glycosylation and esterification into phenolic acids and complex polyphenols, causing an increase in free forms (Sunanta et al., 2021); and (2) a decrease in enzymatic oxidation involving antioxidant compounds (Setyoningrum et al., 2021) (Figure 4).
Based on the results, the highest average concentration of flavonoids reached 51.325 ± 1.47 mg QE/g, namely at T3. This concentration is not significantly different from the results obtained by Najman et al. (2020), namely an average of 58.42 ± 5.38 mg/100 g d.m. Flavonoid concentrations tend to increase 2-5 times under the influence of thermal treatment, depending on the time and conditions of fermentation (Najman et al., 2020), but in this study, it only increased 0.3 times when compared to T0. Additionally, flavonoids reportedly have several health-promoting properties in both humans and livestock, such as anti-bacterial and anti-inflammatory properties (Buiatte et al., 2022).
The use of flavonoids for livestock has been widely studied, especially in relation to their efficacy in (1) improving the health of livestock; (2) acting as an antibacterial compound to disrupt bacterial cell membranes as well as inhibit bacterial toxin production and enzymes that support DNA replication; and (3) modulating the intracellular signaling pathways of the innate and adaptive immune systems through several mechanisms of action such as inhibiting COX-2 activity with the NF-ҡB and MAPK pathways (Buiatte et al., 2022). Therefore, the flavonoids contained in black garlic have the potential to be used in livestock production.
Tannins
Black garlic also contains secondary metabolites known as tannins (Kumar et al., 2022). They function as a plant chemical defense system against pathogen invasion and insect attack (Huang et al., 2018; Das et al., 2020). Tannins are secondary polyphenols found in plants (Menci et al., 2021) with antimicrobial, antiparasitic, antiviral, antioxidant, anti-inflammatory, and immunomodulating properties. Moreover, they naturally bind and precipitate proteins (Sawitri, 2016; Huang et al., 2018; Das et al., 2020). Tannins have traditionally been considered an anti-nutritional factor but when applied in the right way and concentration, they can improve the gut microbial ecosystem and performance, thereby increasing productivity (Huang et al., 2018).
Based on the results in Figure 5, black garlic had a relatively high tannin content, namely 553.165 mg Tannic Acid/g in T3 followed by T2, which reached 534.83 mg Tannic Acid/g. Therefore, it has the potential to be explored and applied more broadly to livestock production. Bioactive compounds in tannins have been widely used in modern livestock production. For example, forage inclusions containing in-vivo condensed tannins can increase antioxidant activity in the serum of cattle and sheep (Huang et al., 2018). Furthermore, condensed tannins in-vitro and in-vivo were reported to have anti-parasitic properties, particularly against gastrointestinal nematode parasites of ruminants (Woolsey et al., 2022). The addition of quebracho tannin in sheep rations also raised the antioxidant status of liver muscle and plasma, as well as the stability of meat color by delaying myoglobin oxidation during refrigerated storage (Luciano et al., 2009; Huang et al., 2018). The application in ruminants can increase protein utilization and production efficiency by reducing degradation in the rumen without affecting feed intake and nutrient digestion (Huang et al., 2018). They have been shown to also improve the ratio of protein by-pass (Menci et al., 2021) and reduce CH4 emissions above 20g/kg, which are beneficial to the environment (Gutierrez et al., 2021).
Based on previous reports, using condensed tannins in ruminant production such as meat-producing livestock products at low to moderate concentrations can increase production efficiency by reducing protein degradation in the rumen (Huang et al., 2018). However, at high concentrations, they inhibit feed intake (Rira et al., 2022) due to their astringent properties (Nascimento et al., 2021) and protect proteins that reduce rumen microbial activity (Huang et al., 2018). This implies that the optimum concentration standard for their use in rations must be considered. The most popular application of condensed tannins (CT) for ruminants is to reduce bloating, and the recommended concentration is 1.0 mg CT/g dry materials (Huang et al., 2018). The abilities possessed by tannins vary depending on the source, composition, and chemical structure, as well as the method/standard of analysis used in determining the concentration for use (Huang et al., 2018). Meanwhile, protein deposition capacity, anti-microbial, anti-parasitic, and antioxidant activities are the most relevant properties to consider for their use in livestock production, especially ruminants (Huang et al., 2018).
Total polyphenols
Phenolic compounds are included in the bioactive components found in plants and they have good health-promoting activities (Lu et al., 2017; Cinar et al., 2022). They play an important role in reproduction, growth, and act as a defense mechanism against pathogens, parasites, and predators which contributes to the color of plants (Idehen et al., 2017). Fresh garlic contains more than 20 phenolic compounds including β-resorcylic acid, gallic acid, rutin, protocatechuic acid, pyrogallol (Shang et al., 2019), astragilin, quercitrin, isoquercitrin, nirurin and quercetin (Sawitri, 2016). However, the numbers are three times higher in single black garlic (garlic bulbs) and six times higher in black garlic (garlic cloves) (Medina et al., 2019).
The results showed that the total concentration of black garlic polyphenols increased with rising incubation time, and the highest amount was obtained at T3, namely 339.875 ± 18.86 mg GAE/g with incubation for 21 days. In other words, there was an increase of about 8.7 times the average of fresh garlic at T0=38.97±2.36 mg GAE/g). This is consistent with Kim et al. (2013), who obtained the highest content of polyphenolic compounds in black garlic, reaching 982.14 mg GAE/kg or a significant escalation of 4-10 times (Figure 6). Najman et al. (2020) also obtained a value of 27.50 ± 0.57 mg/100 g d.m or a two-fold increase. Although both experienced an upsurge, the concentration of polyphenolic compounds obtained in this study was different. This is due to differences in the heat treatment conditions applied including time, temperature, and humidity (Najman et al., 2020).
The increase in polyphenols is related to the Maillard reaction during the incubation process. It might occur due to the liberation of new low molecular weight derivatives, fragmentation of high molecular weight polyphenols (Karnjanapratum et al., 2021), and as a conversion result of the bound phenolic to free forms (Capra et al., 2022). Bedrnícek et al. (2021) revealed that the concentration of total polyphenols can be affected by a certain temperature gradient. Meanwhile, polyphenols are one of the main sources of antioxidants. Organic derivatives from plants are the most important and safe antioxidants to prevent various diseases, reactive nitrogen species in the human body, and overproduction of reactive oxygen species, which have the potential to suppress the effects of oxidative stress in livestock (Zakeya et al., 2022).
Black garlic antioxidant capacity
Antioxidants are compounds that can inhibit oxidation reactions by binding to free radicals and molecules with significant reactive properties (Azhar and Yuliawati, 2021). Typical antioxidants found in black garlic are phenolic acids, flavonoids, and sulfur compounds such as S-allyl-L-cysteine (SAC), diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DAT) (Ding et al., 2021). The antioxidant capacity cannot be separated from the contribution of bioactive compounds such as Amadori, which are produced in the Maillard reaction during the thermal fermentation process (Yu et al., 2020). Therefore, the capacity and activity are affected by the processing method and conditions (Lu et al., 2017). The antioxidant capacity of black garlic increased during processing under controlled temperature and humidity up to 5.7-7.8 times (Medina et al., 2019; Yu et al., 2020; Shehri, 2021) with stronger properties than fresh garlic (Lu et al., 2017; Barido et al., 2022) both in vitro and in vivo (Medina et al., 2019). The antioxidant properties of black garlic are closely related to polyphenols (Lu et al., 2017).
The DPPH radical scavenging activity was observed by increasing the thermal fermentation time, where T3 with incubation for 21 days had an IC50 value of 58.5 µg/mL. This indicates that black garlic which has been incubated for 21 days, can inhibit the oxidation process and capture DPPH free radicals by 50% with 58.5 µg/mL. In other words, T3 has stronger antioxidant properties compared to T0, T1, and T2 (Figur 7). According to Capar et al. (2022), a low IC50 value is considered to indicate greater antioxidant capacity. The decrease in the IC50 value during thermal fermentation is related to the increase in the flavonoid and phenolic compounds, both of which have antioxidant properties (Setyoningrum et al., 2021), as well as melanoidins, 5-Hydroxymethylfurfural (HMF), vitamin C, selenium (Cinar et al., 2022), and S-allyl-L-cysteine (SAC) (Medina et al., 2019). Furthermore, a previous study showed that SAC has excellent antioxidant activity, mainly manifested through its ability to remove reactive oxygen and nitrogen species in the body, reduce oxidative stress, and enhance the main function of the antioxidant defense (Ro et al., 2022).
Given the strong antioxidant capacity of black garlic, it has the potential to be developed into a natural antioxidant product. This is supported by several scientific reviews related to the application of antioxidants in livestock production. For example, antioxidants are permitted in commercial feed to prevent lipid peroxidation and oxidative rancidity during the production, processing, and storage of feeds (Salami et al., 2016). Moreover, natural antioxidants added to livestock products (meat) help maintain a protective effect against lipid oxidation and extend shelf life (Barido et al. al., 2022).
CONCLUSIONS AND Recommendations
Garlic and its derivatives have been widely documented in various literature for their multiple biological impacts on health promotion and treatment of various diseases, especially in humans. The results obtained in this study showed that black garlic contains various bioactive compounds, particularly flavonoids, tannins, and phenolics. Besides, its antioxidant capacity reflected in the DPPH radical scavenging activity is better than that of fresh garlic. The bioactive components, especially tannins, can act as alternative antibiotics in rations. They have great potential to become natural feed additives that can contribute to livestock production, especially pollution-free meat, animal welfare improvement, and sustainable animal husbandry development with the progress of modern science and technology. The utilization of plant extracts also contributes to reducing the use of synthetic feed additives. Furthermore, the use of black garlic and its extracts, along with the increasing public demand for livestock products especially meat, has a unique development and utilization value as a green additive while considering the implications for human health. Further investigations on black garlic, as well as its bioactive compounds and antioxidant activity in livestock commodities, can be performed by exploiting modern technological advances through biotechnology, nanotechnology, and pharmacology approaches. This will contribute to promoting and propagating the nutritional and medicinal values, to enhance livestock production and health.
ACKNOWLEDGMENT
The authors are grateful to the Head and Staff of Post-Harvest Laboratory and Agricultural Product Packaging, Faculty of Agriculture, Mulawarman University who provided facilities to support this study.
Novelty Statement
The utilization of fermented black garlic as a feed additive for livestock has not been widely studied to improve livestock performance.
AUTHOR’S CONTRIBUTION
Hamdi Mayulu: Conceptualization, data analysis, and interpretation, funding acquisition, original draft and review writing, as well as editing. Endang Sawitri: Analysis in the laboratory and verification of laboratory data.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Afzaal M, Saeed F, Rasheed R, Hussain M, Aamir M, Hussain S, Mohamed AA, Alamri MS, Anjum FM (2021). Nutritional, biological, and therapeutic properties of black garlic: A critical review. Int. J. Food Prop., 24(1): 1387-1402. https://doi.org/10.1080/10942912.2021.1967386
Ahmed T, Wang CK (2021). Black garlic and its bioactive compounds on human health diseases: A review. Molecules, 26(5028): 1-38. https://doi.org/10.3390/molecules26165028
Azhar SF, Yuliawati KM (2021). Pengaruh waktu aging dan metode ekstraksi terhadap aktivitas antioksidan black garlic yang dibandingkan dengan bawang putih (Allium sativum L.). J. Riset Farmasi, 1(1): 16-23. https://doi.org/10.29313/jrf.v1i1.43
Barido FH, JangA, Pak JI, Kim YJ, Lee SK (2022). Combined effects of processing method and black garlic extract on quality characteristics, antioxidative, and fatty acid profile of chicken breast. Poult. Sci., 101: 1-11. https://doi.org/10.1016/j.psj.2022.101723
Batiha GES, Beshbishy AM, Wasef LG, Elewa YHA, Sagan AAA, Hack MEAE, Taha AE, Elhakim YMA, Devkota HP (2020). Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients, 12: 1-21. https://doi.org/10.3390/nu12030872
Bedrnícek J, Laknerová I, Lorenc F, Moraes PP, Jarošová M, Samková E, Tríska J, Vrchotová N, Kadlec J, Smetana P (2021). The use of a thermal process to produce black garlic: differences in the physicochemical and sensory characteristics using seven varieties of fresh garlic. Foods, 10(2703): 1-17. https://doi.org/10.3390/foods10112703
Buiatte V, Dominguez D, Lesko T, Jenkins M, Chopra S, Lorenzoni AG (2022). Inclusion of high-flavonoid corn in the diet of broiler chickens as a potential approach for the control of necrotic enteritis. Poult. Sci., 101: 1-6. https://doi.org/10.1016/j.psj.2022.101796
Capar TD, Inanir C, Cimen F, Ekicil L, Yalcin H (2022). Black garlic fermentation with green tea extract reduced HMF and improved bioactive properties: Optimization study with response surface methodology. J. Food Measur. Character., 16: 1340–1353. https://doi.org/10.1007/s11694-021-01247-4
Chen C, Cai J, Liu S, Qiu G, Wu X, Zhang W, Chen C, Qi W, Wu Y, Liu Z (2019). Comparative study on the composition of four different varieties of garlic. PeerJ., 7: 1-17. https://doi.org/10.7717/peerj.6442
Chen J, Wang F, Yin Y, Ma X (2021). The nutritional applications of garlic (Allium sativum) as natural feed additives in animals. PeerJ, 9: 1-15. https://doi.org/10.7717/peerj.11934
Cinar A, Altuntas S, Demircan H, Dundar AN, Taner G, Oral RA (2022). Encapsulated black garlic: Comparison with black garlic extract in terms of physicochemical properties, biological activities, production efficiency, and storage stability. Food Biosci., 50: 1-12. https://doi.org/10.1016/j.fbio.2022.101979
Conte FL, Pereira AC, Brites G, Ferreira I, Silva AC, Sebastiao AI, Matos P, Pereira C, Batista MT, Sforcin JM, Cruz MT (2022). Exploring the antioxidant, anti-inflammatory, and antiallergic potential of Brazilian propolis in monocytes. Phytomedicine Plus, 2: 1-9. https://doi.org/10.1016/j.phyplu.2022.100231
Das AK, Islam MN, Faruk MO, Ashaduzzaman M, Dungani R (2020). Review on tannins: Extraction, processes, application, and possibilities. South Afr. J. Bot., 135: 58-70. https://doi.org/10.1016/j.sajb.2020.08.008
Ding Y, Zhou X, Zhong Y, Wang D, Dai B, Deng Y (2021). Metabolite, volatile and antioxidant profiles of black garlic stored in different packaging materials. Food Contr., 127: 1-10. https://doi.org/10.1016/j.foodcont.2021.108131
Gudalwar B, Minakshee G, Nimbalwar, Panchale AW, Wadekar AB, Manwar JV, Bakal RL (2021). Allium sativum, a potential phytopharmacological source of natural medicine for better health. GSC Adv. Res. Rev., 6(3): 220–232. https://doi.org/10.30574/gscarr.2021.6.3.0049
Gutierrez EC, Aguirre EA, Jimenez LER, Ortega OAC, Canul AJC, Foggi G, Hernandez JCA, Perez EVB, Ronquillo MG (2021). Effect of tannins from tropical plants on methane production from ruminants: A systematic review. Vet. Anim. Sci., 14: 1-12. https://doi.org/10.1016/j.vas.2021.100214
Hapsari S, Yohed I, Kristianita RA, Jadid N, Aparamarta HW, Gunawan S (2022). Phenolic and flavonoid compounds extraction from Calophyllum inophyllum leaves. Arab. J. Chem., 15: 1-10. https://doi.org/10.1016/j.arabjc.2021.103666
Huang Q, Liu X, Zhao G, Hu T, Wang Y (2018). Potential and challenges of tannins as an alternative to in-feed probiotics for farm animal production. Anim. Nutr., 4: 137-150. https://doi.org/10.1016/j.aninu.2017.09.004
Hue CT, Tan LQ, Hung HV, Le QTN, Nguyen TH, Huong NTL, Ha NM, Trinh DK (2022). Assessment of the physicochemical properties and biological activity of Vietnamese single-bulb black garlic. Food Biosci., 49: 1-10. https://doi.org/10.1016/j.fbio.2022.101866
Idehen E, Tang Y, Sang S (2017). Bioactive phytochemicals in barley. J. Food Drug Anal., 25: 148-161. https://doi.org/10.1016/j.jfda.2016.08.002
Jing H (2020). Black garlic: Processing, composition change and bioactivity. EFood, 1(3): 242–246. https://doi.org/10.2991/efood.k.200617.001
Karnjanapratum S, Supapvanich S, Kaewthong P, Takeungwongtrakul S (2021). Impact of steaming pretreatment process on characteristics and antioxidant activities of black garlic (Allium sativum L.). J. Food Sci. Technol., 58(5): 1869-1876. https://doi.org/10.1007/s13197-020-04698-7
Kim JS, Kang OJ, Gweon OC (2013). Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods, 5: 80-86. https://doi.org/10.1016/j.jff.2012.08.006
Kumar A, Ramamoorthy D, Verma DK, Kumar A, Kumar N, Kanak KR, Marwein BM, Mohan K (2022). Antioxidant and phytonutrient activities of spirulina platensis. Energy Nexus, 6: 1-9. https://doi.org/10.1016/j.nexus.2022.100070
Leontiev R, Hohaus N, Jacob C, Gruhlke MCH, Slusarenko AJ (2018). A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of Allicin. Sci. Rep., 8: 1-19. https://doi.org/10.1038/s41598-018-25154-9
Lestari AR, Batubara I, Wahyudi ST, Ilmiati A, Achmadi SS (2022). Bioactive compounds in Garlic (Allium sativum) and black garlic as antigout agents, using computer simulation. Life, 12(1131): 1-15. https://doi.org/10.3390/life12081131
Liang J, Zhao Y, Yang F, Zheng L, Ma Y, Liu Q, Cai L, Gong W, Wang B (2022). Preparation and structure-activity relationship of highly active black garlic polysaccharides. Int. J. Biol. Macromol., 220: 601-612. https://doi.org/10.1016/j.ijbiomac.2022.08.115
Lu X, Li X, Qiao Z, Qiu, Liu P (2017). Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal., 25: 340-349. https://doi.org/10.1016/j.jfda.2016.05.011
Luciano, G., F. J. Monahan, V. Vasta, L. Biondi, M. Lanza, M. Bella, P. Pennisi, and A. Priolo. (2009). Lamb meat colour stability as affected by dietary tannins. Italian Journal of Animal Science, 8 (2): 507-509. https://doi.org/10.4081/ijas.2009.s2.507
Malangngi LP, Sangi MS, Paedong JJE (2012). Penentuan kandungan tanin dan uji aktivitas antioksidan ekstrak biji buah alpukat (Persea americana Mill.). J. Mipa Unsrat Online, 1(1): 5-10. https://doi.org/10.35799/jm.1.1.2012.423
Manoshree M, Devangi C (2019). Sensitivity of AOAC and folin’s method in estimation of tannins from different plants. World J. Pharma. Res., 8(8): 764-770.
Mayulu H, Sawitri E (2023). The potential of black garlic as livestock feed supplement. Technium BioChemMed., 5: 40-53. https://doi.org/10.47577/biochemmed.v5i.8540
Mayulu H, Topan EA, Haris MI, Daru TP (2021). Evaluation of dry matter intake and average daily gain of beef cattle in Samarinda city. J. Southw. Jiaotong Univ., 56(1): 164-175. https://doi.org/10.35741/issn.0258-2724.56.1.15
Mayulu H, Rahayu F, Christiyanto M, Haris MI, Daru TP, Rahmatullah SN (2020). Evaluation of digestibility value and rumen fermentation kinetic of goat’s local feedbased ration. Eur. J. Mol. Clin. Med., 7(8): 3703-3711. https://ejmcm.com/article_6631_cc0d10c17b9dd4f4d8ef9281272e6859.pdf
Medina MAT, Amo TM, Bedmar ZF, Font R, Celestino MR, Aparicio JP, Ortega AM, Moraga AA, Rojas RM (2019). Physicochemical characterization and biological activities of black and white garlic: in vivo and in vitro assays. Foods, 8(220): 1-18. https://doi.org/10.3390/foods8060220
Menci R, Coppa M, Torrent A, Natalello A, Valenti B, Luciano G, Priolo A, Niderkorn V (2021). Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed Sci. Technol., 278: 1-13. https://doi.org/10.1016/j.anifeedsci.2021.114977
Moon SB, Choi NR, Kim JN, Kwon MJ, Kim B, Ha KT, Lim EY, Kim YT, Kim BJ (2022). Effects of black garlic on the pacemaker potentials of interstitial cells of Cajal in murine small intestine in vitro and on gastrointestinal motility in vivo. Anim. Cells Syst., 26(1): 37-44. https://doi.org/10.1080/19768354.2022.2049640
Najman K, Sadowska A, Hallmann E (2020). Influence of thermal processing on the bioactive, antioxidant, and physicochemical properties of conventional and organic agriculture black garlic (Allium sativum L.). Appl. Sci., 10: 1-17. https://doi.org/10.3390/app10238638
Najman K, Sadowska A, Hallmann E (2021). Evaluation of bioactive and physicochemical properties of white and black garlic (Allium sativum L.) from conventional and organic cultivation. Appl. Sci., 11: 1-24. https://doi.org/10.3390/app11020874
Najman K, Krol K, Sadowska A (2022). The physicochemical properties, volatile compounds and taste profile of black garlic (Allium sativum L.) cloves, paste and powder. Appl. Sci., 12: 1-19. https://doi.org/10.3390/app12094215
Nakagawa K, Maeda H, Yamaya Y, Tonosaki Y (2020). Maillard reaction intermediates and related phytochemicals in black garlic determined by EPR and HPLC analyses. Molecules, 25(4578): 1-10. https://doi.org/10.3390/molecules25194578
Nascimento TVC, Oliveira RL, Menezes DR, de Lucena ARF, Queiroz MAA, Lima AGVO, Ribeiro RDX, Bezerra LR (2021). Effects of condensed tannin-amended cassava silage blend diets of feeding behavior, digestibility, nitrogen balance, milk yield and milk composition in dairy goats. Animal, 15: 1-7. https://doi.org/10.1016/j.animal.2020.100015
Nayeem N, Imran M, Asdaq SMB, Rabbani SI, Alanazi FA, Alamri AS, Alsanie WF, Alhomrani M (2022). Total phenolic, flavonoid contents, and biological activities of stem extract of Astragalus spinosus (Forssk.) Muschl. Grown in Northen Border Province, Saudi Arabia. Saudi J. Biol. Sci., 29: 1277-1282. https://doi.org/10.1016/j.sjbs.2021.12.029
Paat SFA, Fatimawali, Antasionasti I (2022). Antioxidant activity test of ethanol extract of lemon peel (Citrus lemon L.) by DPPH method (1.1-Diphenil-2-Picrylhdarzyl). Pharmacon, 11(1): 1315-1320. https://ejournal.unsrat.ac.id/index.php/pharmacon/article/download/39143/35580
Ramírez PJG, Mathey LIP, Rodriguez RVG, Jimenez M, Beristain CI, Medina AS, Pineda LAP (2022). Effect of relative humidity on the metabolite profiles, antioxidant activity and sensory acceptance of black garlic processing. Food Biosci., 48: 1-9. https://doi.org/10.1016/j.fbio.2022.101827
Rammal FH, Hijazi A, Hamad H, Daher A, Reda M, Badran B (2012). In vitro antioxidant activity of ethanolic and aqueous extracts from crude Malva parviflora L. grown in Lebanon. Asian J. Pharma. Clin. Res., 5(3): 234-238. https://innovareacademics.in/journal/ajpcr/Vol5Suppl3/1250.pdf
Ríos KLR, Montilla A, Olano A, Villamiel M (2019). Physicochemical changes and sensorial properties during black garlic elaboration: A review. Trends Food Sci. Technol., 88: 459-467. https://doi.org/10.1016/j.tifs.2019.04.016
Rios KLR, Martinez MG, Pastrana DMR, Sanchez EM, Villamiel M, Montilla A, Silva EMM, Barrios MEV (2021). Ohmic heating pretreatment accelerates black garlic processing. LWT Food Sci. Technol., 151: 1-10. https://doi.org/10.1016/j.lwt.2021.112218
Rira M, Morgavi DP, Popova M, Maxin G, Doreau M (2022). Microbial colonization of tannin-rich tropical plants: Interplay between degradability, methane production and tannin disappearance in the rumen. Animal, 16: 1-12. https://doi.org/10.1016/j.animal.2022.100589
Ro KS, Chen Y, Du L, Wang L, Zhao L, XieJ, Wei D (2022). Improvement of S-allyl-L-cysteine content, probiotic properties and constipation prevention effect of black garlic by the lactic acid bacteria fermentation. Process Biochem., 115: 110-117. https://doi.org/10.1016/j.procbio.2022.02.009
Salami SA, Guinguina A, Agboola JO, Omede AA, Agbonlahor EM, Tayyab U (2016). Review: In vivo and postmortem effects of feed antioxidants in livestock: A review of the implications on authorization of antioxidant feed additives. Animal, 10(8): 1375-1390. https://doi.org/10.1017/S1751731115002967
Saryono, Nani D, Proverawati A, Sarmoko (2021). Immunomodulatory effects of black garlic (Allium sativum L.) on streptozotocin-induced diabetes in Wistar rats. Heliyon, 7: 1-5. https://doi.org/10.1016/j.heliyon.2021.e08493
Sawitri E (2016). Apoptosis of colorectal cancer cell on sprague-dawley rats induced with 1,2 dimethylhidrazine and Phyllanthus niruri linn extrac. Int. J. Sci. Eng., 10(1): 45-50.
Setyoningrum F, Priadi G, Afiati F, Herlina N, Solikhin A (2021). Composition of spontaneous black garlic fermentation in a water bath. Food Sci. Technol., 41(2): 557-562. https://doi.org/10.1590/fst.28720
Shang A, Cao SY, Yu X, Gan RY, Tang GY, Corke H, Mavumengwana V, Li HB (2019). Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods, 8(246): 1-31. https://doi.org/10.3390/foods8070246
Shehri SAA (2021). Efficacy of black garlic extract on anti-tumor and antioxidant activity enhancement in rats. Clin. Nutr. Open Sci., 36: 126-139. https://doi.org/10.1016/j.nutos.2021.03.005
Sunanta P, Pankasemsuk T, Jantanasakulwong K, Chaiyaso T, Leksawasdi N, Phimolsiripol Y, Rachtanapun P, Seesuriyachan P, Sommano SR (2021). Does curing moisture content affect black garlic physiochemical quality? Horticulturae, 7(535): 1-16. https://doi.org/10.3390/horticulturae7120535
Woolsey ID, Zeller WE, Blomstrand BM, Øines Ø, Enemark HL (2022). Effects of selected condensed tannins on Cryptosporidium parvum growth and proliferation in HCT-8 cell cultures. Exp. Parasitol., 241: 1-6. https://doi.org/10.1016/j.exppara.2022.108353
Yu J, Shan Y, Li S, Zhang L (2020). Potential contribution of Amadori compounds to antioxidant and angiotensin I converting enzyme inhibitory activities of raw and black garlic. LWT Food Sci. Technol., 129: 1-7. https://doi.org/10.1016/j.lwt.2020.109553
Zakeya N, Ibrahim M, Mamiro B, Ndossi H, Kilonzo M, Mkangara M, Chacha M, Chilongola J, Kideghesho J (2022). Potential of natural phenolic antioxidant compounds from Bersama abyssinica (Meliathacea) for treatment of chronic diseases. Saudi J. Biol. Sci., 29: 1-7. https://doi.org/10.1016/j.sjbs.2022.03.023
Zou Y, Lu Y, Wei D (2004). Antioxidant activity of flavonoid rich extract of Hypericum pertoratum L. in vitro. J. Agric. Food Chem., 52(16): 5032-5039. https://doi.org/10.1021/jf049571r
To share on other social networks, click on any share button. What are these?





