Anti-hyperlipidemic Effect of Berberis lycium Royle Root Bark Extract in Alloxanized Swiss Albino Mice
Anti-hyperlipidemic Effect of Berberis lycium Royle Root Bark Extract in Alloxanized Swiss Albino Mice
Tafail Akbar Mughal1, Shaukat Ali1*, Hafiz Muhammad Tahir1, Shumaila Mumtaz1, Samaira Mumtaz1, Ali Hassan1 and Syed Akif Raza Kazmi2
1Applied Entomology and Medical Toxicology Laboratory, Department of Zoology, Government College University, Lahore, Pakistan
2Department of Chemistry, Government College University, Lahore, Pakistan
Abstract | Hyperlipidemia is a condition in which blood is overloaded with lipids. This condition is challenging for living beings. There are many ways to solve this problem but herbal medicines take on paramount importance in this regard. The purpose of the current research was to inspect the effects of an aqueous extract of Berberis lycium Royle root bark on lipid profiles of Swiss albino mice in which diabetic phenotype was induced by alloxan. A single injection of Alloxan (150 mg/kg) was applied intravenously to induce diabetic phenotype. Alloxan induction raised the overall level of triglycerides, low density- lipids (LDLs), and cholesterol and reduced the level of high density lipids (HDLs). Oral administration of 200 mg/kg aqueous extract of B. lycium Royle root bark for 28 days rescued all these changes. This study shows that the root bark extract of B. lycium Royle has antihyperlipidemic properties.
Novelty Statement | This study reports that root bark extract of Berberis lycium Royle has very strong antihyperlipidemic properties.
Article History
Received: May 07, 2020
Revised: December 13, 2020
Accepted: December 23, 2020
Published: December 30, 2020
Authors’ Contributions
TAM designed. SM and TAM conducted the experiments. SA analyzed the data and wrote the first draft. HMT revised the draft. SN and AH designed the study and analyzed the data. SARK helped in literature review.
Keywords
Alloxan, Lipid profile, Berberis lycium Royle, Root bark
Corresponding author: Dr. Shaukat Ali
dr.shaukatali@gcu.edu.pk
To cite this article: Mughal, T.A., Ali, S., Tahir, H.M., Mumtaz, S., Mumtaz, S., Hassan, A. and Kazmi, S.A.R., 2020. Anti-hyperlipidemic effect of Berberis lycium royle root bark extract in Alloxanized Swiss Albino mice. Punjab Univ. J. Zool., 35(2): 261-268. https://dx.doi.org/10.17582/journal.pujz/2020.35.2.261.268
Introduction
Hyperlipidemia is a situation in which there is an overload of lipids, mostly cholesterol as well as triglycerides in the blood. There are many causes of hyperlipidemia, one of which is diabetes. Abnormal conditions of insulin production and insulin action are responsible for a metabolic disorder known as diabetes mellitus which results in the disturbance of lipid metabolism. Diabetes mellitus results in dysfunction, long term damage, and failure of numerous organs (Joshi et al., 2015). Medicinal plants are a potential source of antidiabetic drugs. Many of the synthetic drugs that have been created have been either directly or indirectly derived initially from plant sources (Swaroopa et al., 2017).
Natural products play a vital part in current drug development programs. Herbal preparations have different properties, including production, low costs, fewer side effects and ease of availability that make them more suitable components in existing therapies (Singab et al., 2014).
Berberis lycium Royle, which is native to the Himalayas, region is broadly used in folk medicines and as a food source. Plants also have been utilized to isolate a wide variety of nutritionally and medicinally important phytochemical constituents, including lipids, carbohydrates, proteins, alkaloids, tannins, vitamins, cardio-active glycosides, anthocyanins, cellulose, fiber content, phytic acid, β carotene, saponins, and phytate phosphorous. Plants contain numerous minerals, which are very important for the treatment of several disorders and contribute to a wide range of biological processes. These minerals include potassium, lead, sulphur, copper, sodium, phosphorus, zinc, calcium, manganese and iron. Traditionally, the Berberis lycium has been used to cure piles, diarrhea, gingivitis, diabetes, backache, intestinal colic, bone fractures, pustules, internal wounds, ophthalmia, fever, sun blindness, throat pain, menorrhagia, jaundice, rheumatism, scabies, and as diaphoretic expectorant and diuretic. B. lycium is reported to have anticoccidial, hepatoprotective, antifungal, antibacterial, antimutagenic, wound healing properties, pesticidal, and antidiabetic, supporting its traditional uses (Shabbir et al., 2012). In this research, this plant is used as antihyperlipidemic in alloxan-treated swiss albino mice.
Materials and Methods
Ethical statement
All experimentations were carried out in agreement with the University guidelines and International laws for the maintenance of experimental animals as mentioned in (Ali et al., 2019, 2020a, b, c; Hussain et al., 2020; Ara et al., 2020; Khan et al., 2019; Mumtaz et al., 2019; Mughal et al., 2019; Dar et al., 2019). The Committee of the Faculty of Science, Government College University, Lahore, Pakistan has approved all procedures used in the experiments included in this study.
Collection of medicinal plants
Berberis lycium Royle plants were collected from Chinari, District Jhelum valley, Azad Kashmir, Pakistan. An Ethnobotanist from the Department of Botany, Faculty of Science, University of Azad Jammu and Kashmir Muzaffarabad, Pakistan identified the plants. The dust from the plants was removed by rinsing them with water. These plants were placed in the shade to dry, after that they were ground into a fine powder. Shade dried plant parts were crushed into a fine powder.
Preparation of plant extract
Ten- grams of shade dried plant material was boiled in 100 ml of distilled water. This solution was filtered through filter paper (Whatman No. 1) and the solution was concentrated with a rotary evaporator to obtain the extracts. The plant extract was dried in a vacuum oven (Vacucell, Einrichtungen GmbH) at 40°C for 24 hours.
Chemicals and drugs
Alloxan was purchased from Sigma Aldrich, (St Louis, MO, USA). Glibenclamide was purchased from Sigma-Aldrich (Chemie, Steinheim, Germany).
Doses
BLR-Extract: 200 mg/kg b.w; Glibenclamide: 10 mg/ kg b.w.
Animal selection and grouping
Twenty-four male Swiss albino mice, with average age eight weeks and 36.6±1.5 g body weight were obtained from the GC University, Lahore. Before starting the experimental procedure, all mice were housed together for seven days. Then the experimental animals were randomly assigned to one of four different groups. The first group (four animals) did not receive any treatment and this group was designated as the normal control group. A single intraperitoneal injection of alloxan (one injection contained 150 mg/kg) was given to 20 animals to induce diabetes. Animals that showed glucose levels that were higher than 150 mg/dL were further categorized into three additional groups:
Diabetic control group (6 animals); Diabetic+BLR extract group (6 animals) were diabetic mice that received a daily oral dose of BLR extract of 200 mg/kg for 28 consecutive days; Diabetic + glibenclamide group (6 animals) were diabetic phenotypic mice that received an oral dose of glibenclamide 10 mg/kg g for 28 consecutive days. The diagrammatic scheme of the experimental design is shown in Figure 1.
Animal management
Four polypropylene cages were used to house mice assigned to the different groups. Mice belonging to one group were housed in the same cage. Four or six mice were housed in each cage. Pure drinking water and Standard laboratory pelleted feed were given to animals ad libitum. The experimental room temperature was set at 22±3°C. A 12 h dark/ 12 h light cycle was maintained and 70% was the upper limit for humidity in the room. The oral dose of glibenclamide was administered using a gavage oral feeding tube.
Body weight measurement
The body weight of the mice in all groups was measured using a digital balance. The body weight of the mice was monitored from the beginning until the end of the study at seven-day intervals.
Induction of experimental diabetic phenotype
A single intraperitoneal dose of alloxan was administered to induce diabetic phenotype in the experimental animals, using a dose of 150 mg/kg in 0.9% saline. Freshly prepared alloxan was injected into twenty mice. Subsequently, the blood glucose levels of fasting mice were assessed and the diabetic group consisted of mice that exhibited a blood glucose level higher than 150 mg/dL.
Assessment of glucose levels
The tail vein of the mice was used to collect the blood samples. Fasting blood glucose was estimated with a One Touch Basic Glucometer after a 6- hour fasting period on the 0, 7th, 14th, 21st and 28th days.
Analysis of lipid profiles
Extraction of lipids
An approach proposed by Friedewald et al. (1972) was employed to perform serum lipids extraction.
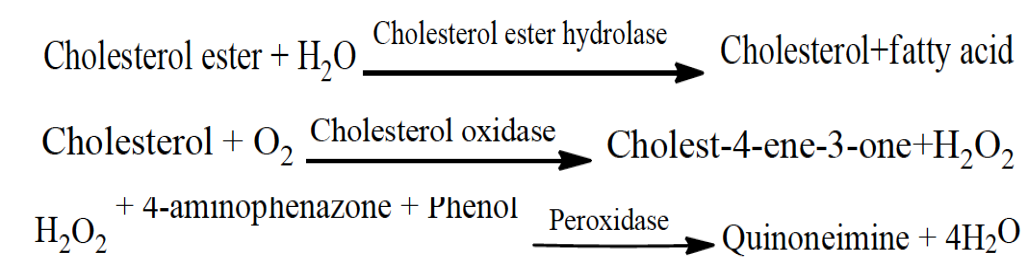
Estimation of total cholesterol
The enzymatic colorimetric method proposed by Allain et al. (1974) enzymatic colorimetric method was used to determine cholesterol level.
The reaction sequence was as follows:
Procedures
Samples of mice serum, standards and the reagent blank were preincubated for 5 minutes at 37 °C. Samples (10 μL) or standard (10 μL) and reagent blank (1,000 μL) were pipetted into cuvettes and mixed carefully by inversion. The cuvettes were put into the holder and a stopwatch was started to assess the time. The absorbance of the sample, standard and the reagent blank was measured at 400 nm wavelength within 60 minutes. As the final point, the absorbance of the sample (ΔA sample) and the standard (ΔA standard) against the reagent blank were calculated.
TC concentration (mg⁄dl)= ΔAsample / ΔA standard × Cstandard
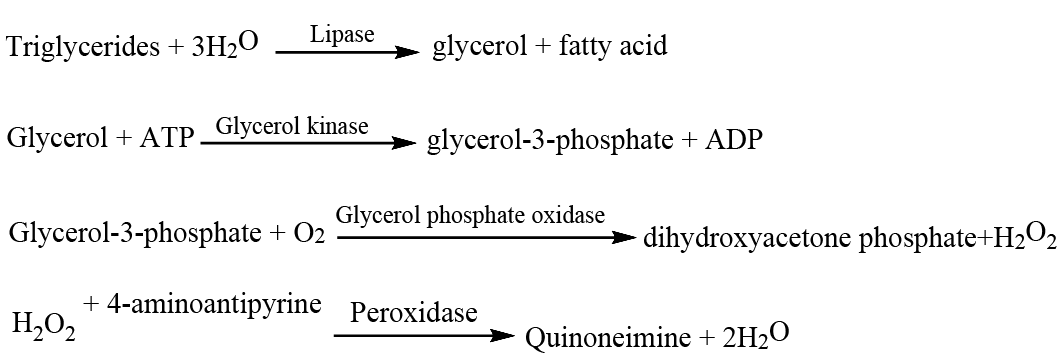
Estimation of triglycerides
The enzymatic colorimetric method published by Hearne and Fraser (1981) enzymatic colorimetric method was used to determine triglyceride (TG) levels.
The reaction sequence was as follows:
To examine serum of the mice, the samples and the standard reagents are prepared for use on an automated analyzer. The enzymatic test was carried out at a wavelength (540 nm), a 1 cm optical path, at 37oC and the measurements were compared against a reagent blank.
Procedure
The samples, standards and blank were preincubated for 5 minutes at 37 °C. The reagent blank (1,000 μL) and samples (10 μL) or standards (10 μL) were placed into cuvettes and mixed gently by inversion. The cuvettes were inserted into the cell holder and a stopwatch was used to assess the time. The absorbance of the samples, standards and blank was measured at 540 nm. Finally, the absorbance of the sample (ΔA sample) and the standards.
(ΔA standard) against the reagent blank were calculated.
TG level concentration (mg/dl) = ΔA sample/ ΔA standard×Cstandard
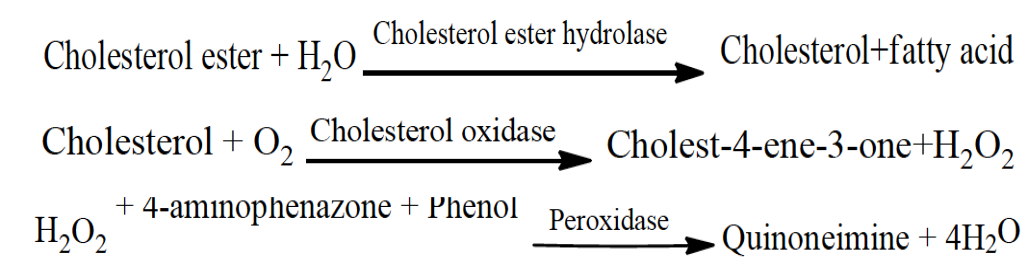
Estimation of high density lipoprotein (HDL) cholesterol
The VLDL and LDL from serum were precipitated by phosphotungstate in the presence of magnesium ions. After elimination by centrifugation, the clear supernatant comprising the high density lipoproteins (HDL)-fraction and the cholesterol content was confirmed enzymatically according to methods mentioned inSeigler and Wu (1981).
The reactions were as follows:
For this research, mouse serum was sampled and standard reagents were used on an automated analyzer. The enzymatic assay was carried out at 593 nm wavelength, 1 cm optical path, 37 °C temperature and measurements done against the reagent blank.
Procedure for precipitation
100 μL of reagent and 10μL of the samples were pipetted into a centrifuge tube, mixed well, allowed to stand for 5 minutes at 37°C and centrifuged at 3000 rpm for 20 minutes. The supernatant (sample) was collected for HDL test.
Procedure for determination of HDL
The reagent blank, samples and calibrator were preincubated for 5 minutes at 37°C. The reagent blank (10 μL distilled water and 750 μL enzymes) and samples (10 μL samples and 750 μL enzymes) or calibrator (10 μL calibrator and 750 μL enzymes) were pipetted into cuvettes and mixed gently by inversion. The cuvettes were inserted into the cell holder and a stopwatch was used to assess the time. The absorbance of the samples, standards and the reagent blank was measured at 593 nm after 5 minutes. Finally, the absorbance of the samples (ΔA sample) and the calibrator (ΔA standard) against the reagent blank were calculated.
HDL concentration (mg⁄dl)= ΔAsample / ΔAcalibratorx Ccalibrator
Estimation of low density lipoprotein (LDL) cholesterol
Indirect method TC, TG and HDL are measured and LDL cholesterol is calculated from the primary measurements by use of the empirical Freidewald Formula equation:
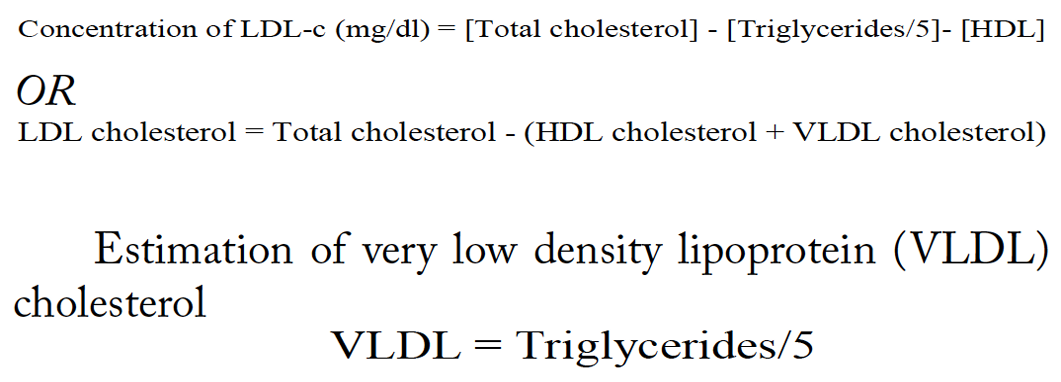
Statistical data analysis
Data were presented as mean±standard error of the mean. Shapiro-Wilk test was used to check the distribution of the data. Parametric tests were used on normally distributed data and then examined statistically via one-way ANOVA, followed by “Dunnett’s Multiple Comparison Test”, to find any substantial variances among the group means. GraphPad Prism version 5.0 for Windows (Graph Pad Software, San Diego, CA, USA) was used for analyses. Values of P ≤ 0.05 were considered as significant.
Results and Discussion
Effect on body weight
Alloxan caused a gradual decrease in body weight. When the alloxan-treated mice were administered the extract and glibenclamide, the alloxan-induced changes in body weight were rescued (Table 1).
Effect on glucose level
Alloxan caused a gradual increase in blood glucose levels. When the alloxan-treated mice were administered the extract and glibenclamide, the alloxan-induced phenotypes in blood glucose were rescued (Table 2).
Effect on lipid profile
Total cholesterol
In this study, intraperitoneal administration of a single dose of alloxan (150 mg /kg body weight) caused a highly significant increase in the overall cholesterol level of the alloxan-treated group (185.6±5) as compared to control (91.76±3). When extract and glibenclamide were given to alloxan-treated mice they caused a significant decrease in total cholesterol level (alloxan-treated+extract: 116.14±2; alloxan-treated+ glibenclamide: 99.43±4) as compared to the alloxan-treated group (185.6±5) (Table 3).
Triglycerides
Intraperitoneal administration of a single dose of alloxan (150mg /kg body weight) caused a highly significant increase in the triglycerides level of the alloxan-treated groups (174.62±6) as compared to control (90.16±3). When extract and glibenclamide were given to the alloxan-treated mice they caused a significant decrease in the triglycerides level (alloxan-treated +extract: 130.98±3; alloxan-treated+ glibenclamide: 111.68±8) as compared to the alloxan-treated group (174.62±6) (Table 3).
HDL-C
Intraperitoneal administration of a single dose of alloxan (150mg /kg body weight) caused a highly significant decrease in the HDL-C levels in the alloxan-treated group (23.38±3) as compared to controls (45.96±3). When the extract and glibenclamide were given to alloxan-treated mice they caused no significant change in HDL-C level (alloxan-treated + extract: 42.54±0.8; alloxan-treated+ glibenclamide: 41.16±1.8) as compared to the alloxan-treated group (45.96±3) (Table 3).
LDL-C
Intraperitoneal administration of a single dose of alloxan (150mg /kg body weight) caused a highly significant increase in the LDL-C levels in alloxan-treated (127.296±6) mice as compared to controls (27.708±4). When the extract and glibenclamide were given to alloxan-treated mice they caused a significant decrease in the LDL-C level (alloxan-treated + extract: 62.964±5; alloxan-treated + glibenclamide: 51.014±4) as compared to the alloxan-treated group (127.296±6) (Table 3).
VLDL-C
Intraperitoneal administration of a single dose of alloxan (150 mg /kg body weight) caused a highly significant increase in the VLDL-C level of the alloxan-treated group (34.924±1.1) as compared to control (18.032±0.5). When the extract and glibenclamide were given to the alloxan-treated mice they caused a significant decrease in VLDL-C level (alloxan-treated +extract: 26.196±0.6; alloxan-treated + glibenclamide: 22.336±1.6) as compared to the alloxan-treated group (34.924±1.1) (Table 3).
The present study showed that an aqueous extract of B. lyceum Royle root bark rescued the changes made by alloxan in Swiss albino mice. The present study revealed that B. lycium Royle root bark extract has an antihyperlipidemic effect in alloxan-treated Swiss albino mice.
The current results revealed a highly significant increase in plasma glucose, cholesterol, LDL and triglycerides in the alloxan-treated mice while a reduction in body weight and HDL level was seen. The same results were seen in Wistar rats, in which diabetes was induced by alloxan-induction (Luka and Tijjani, 2013; Bako et al., 2014). The level of TG, cholesterol and LDL in the plasma was decreased after treatment with the plant extract, while the level of HDL was increased. Similar results were reported in alloxan-induced diabetic rabbits (Wojtowicz et al., 2007). These results are in agreement with the current research. B. lycium Royel root bark powder significantly lessened the total LDL, triglycerides and cholesterol of treated mice as compared to alloxan-treated ones.
Table 1: The measurement of body weight.
|
Group |
Treatment |
Body weight (Mean ± SEM g) |
|||||
|
Before induction of diabetes |
Day 0 after treatment |
Day 7 after treatment |
Day 14 after treatment |
Day 21 after treatment |
Day 28 after treatment |
||
|
I |
Control |
38.6±0.43 |
38.6± 0.43 |
39.2± 0.62 |
39.3± 0.22 |
39.6± 0.37 |
39.8± 0.73 |
|
II |
Diabetic |
38.2±0.31 |
36± 0.32a |
34± 0.47aa |
32± 0.7aaa |
28.7± 0.17 aaa |
25.7±0.37aaa |
|
III |
Diabetic+ Extract |
38.4±0.34 |
36.4± 0.34 |
34.9± 0.39 |
35.7± 0.17bbb |
36.8± 0.34bbb |
37± 0.13bbb |
|
IV |
Diabetic+ glibenclamide |
38.8±0.17 |
37.8± 0.17 |
35± 0.27 |
36.4± 0.43ccc |
38.2± 0.6ccc |
38.6± 0.43ccc |
Keys: (a) specifies the significant difference among control and diabetic group; (b) indicates the significance difference between diabetic group and Diabetic + extract treated group and (c) presents the significance difference between diabetic group and Diabetic + glibenclamide treated group. Each value in table signifies the mean value of six replicates and SEM. Numerical icon: aaa, bbb, ccc= p ≤ 0.001.
Table 2: Effect on blood glucose level.
|
Group |
Treatment |
Blood glucose level (Mean ± SEM mg/dl) |
||||
|
Day 0 |
Day 7 |
Day 14 |
Day 21 |
Day 28 |
||
|
I |
Control |
117 ±4.04 |
116.25±3.52 |
121.75±4 |
123±3.09 |
120.5± 2.19 |
|
II |
Diabetic |
322.5± 26.44aaa |
327.5± 25.52aaa |
355±39.42 aaa |
342.75± 37.19 aaa |
333.75± 33.5 aaa |
|
III |
Diabetic+Extract |
297.25±3.9 |
256.5±9.6 |
186.75± 2.4bb |
154.5± 3.96 bbb |
128±2.7 bbb |
|
IV |
Diabetic+glibenclamide |
292±15.32 |
252.25±16 |
174.75± 3.5cc |
129.25± 3.66 ccc |
115.25± 6.16 ccc |
Keys: (a) specifies the significant difference among control and diabetic group; (b) indicates the significance difference between diabetic group and Diabetic + extract treated group and (c) presents the significance difference between diabetic group and Diabetic + glibenclamide treated group. Each value in table signifies the mean value of six replicates and SEM. Numerical icons: bb, cc = p ≤ 0.01, aaa, bbb, ccc= p ≤ 0.001.
Table 3: Effect on lipid profile.
|
Group |
Treatment |
Lipids levels (Mean ± SEM mg/dl) |
||||
|
Total cholesterol |
Triglycerides |
HDL cholesterol |
LDL cholesterol |
VLDL cholesterol |
||
|
I |
Control |
91.76±3 |
90.16±3 |
45.96±3 |
27.708±4 |
18.032±0.5 |
|
II |
Diabetic |
185.6±5aaa |
174.62±6aaa |
23.38±3aaa |
127.296±6 aaa |
34.924±1.1aaa |
|
III |
Diabetic + Extract |
116.14±2bbb |
130.98±3bbb |
26.98±5bbb |
62.964±5bbb |
26.196±0.6bbb |
|
V |
Diabetic + glibenclamide |
99.43±4ccc |
111.68±8ccc |
26.08±3ccc |
51.014±4ccc |
22.336±1.6ccc |
Keys: (a) specifies the significant difference among control and diabetic group; (b) indicates the significance difference between diabetic group and Diabetic + extract treated group and (c) presents the significance difference between diabetic group and Diabetic + glibenclamide treated group. Each value in table signifies the mean value of six replicates and SEM. Numerical icon: aaa, bbb, ccc= p ≤ 0.001.
Oral administration of 200 mg/kg and 600mg/kg extract fruit of Berberis lycium for six weeks resulted in a significant reduction in total cholesterol, triglyceride, and LDLs levels. Berberis lycium treatment increased the levels of HDLs. Oral administration of 200 mg/kg and 600mg/kg extract fruit of Berberis lycium for six weeks resulted in a significant reduction in total cholesterol, triglyceride, and LDLs levels. Berberis lycium treatment increased the levels of HDLs.
Oral administration of Berberis lycium fruit extract (200 mg/kg and 600mg/kg) for six weeks caused a significant elevation in the levels of HDLs while, a significant decline in LDLs, triglyceride and total cholesterol were found in the serum of diabetic phenotypic mice (Furman et al., 2020). Belwal et al. (2020) also have reported that the ethanolic as well as the aqueous extract of Berberis aristata showed better antidiabetic activity through eliciting decreased total cholesterol and elevated HDL-C levels, in diabetic and hyperglycemic patients leaf extracts of B. lycium lessened the lipid profile (Hussain et al., 2017). Many plant extracts lower the serum lipids especially total triglycerides, cholesterol, and level of low density lipoprotein, which are the therapeutic properties to combat atherosclerosis which is one of the main problems of diabetes (Luka and Tijjani, 2013).
Different atheromatous problems e.g., ischemic heart diseases are persistently present in diabetic patients (Assmann et al., 1997). In diabetic patients, the atheromatous disease is due to the decrease in the level of high-density protein (Rang and Urban, 1995). It was discovered that treatment with B. lycium Royle root extract caused a decrease in the levels of LDL and an increase in level of HDL that can save diabetic patients from athermanous disease.
Berberis lycium Royle possesses berberine, berbamine, chinabine, karakoramine, palmatine balauchistanamine, gilgitine, jhelumine, punjabine, sindamine, chinabine acetic acid, maleic acid, ascorbic acid (Khare, 2004). Its rook bark contains active components such as isoquinoline alkaloid that is responsible for its antihyperlipidemic effects in alloxan induced diabetic phenotypic mice (Saeed, 1976; Birdsall and Kelly, 1997; Chand et al., 2007). Critical valuation exposed that pharmacological studies are deficient in the documentation of active ingredients responsible for pharmacological actions. Therefore, a further investigation aimed towards the identification and isolation of active constituents in future studies is suggested.
Conclusions and Recommendations
Current research proved that B. lycium Royle root bark extract contained antihyperlipidemic potential in alloxan-treated mice. It significantly increased the level of HDL while decreased the raised level of total LDL, triglycerides, cholesterol, in alloxan-induced diabetic mice.
Acknowledgment
The authors thank the Department of Zoology, Government College University Lahore, for providing all facilities for the completion of this study.
Conflict of interests
The authors have declared no conflict of interest.
References
Ali, S., Awan, Z., Mumtaz, S., Shakir, H.A., Ahmad, F., Tahir, H.M. and Ulhaq, M., 2020a. Cardiac toxicity of heavy metals (cadmium and mercury) and pharmacological intervention by vitamin C in rabbits. Environ. Sci. Pollut. Res. Int., 27: 29266-29279. https://doi.org/10.1007/s11356-020-09011-9
Ali, S., Bashir, S., Mumtaz, S., Shakir, H.A., Ara, C., Ahmad, F., Tahir, H.M., Faheem, M., Irfan, M., Masih, M., Ulhaq, M. and Andleeb, A., 2020b. Evaluation of cadmium chloride-induced toxicity in chicks via hematological, biochemical parameters and cadmium level in tissues. Environ. Sci. Pollut. Res. Int., https://doi.org/10.1007/s12011-020-02453-9
Ali, S., Ejaz, M., Dar, K.K., Nasreen, N., Ashraf, N., Gillani, S.F., Shafi, N., Safeer, S., Khan, M.A., Andleeb, S. and Mughal, T.A., 2020c. Evaluation of chemopreventive and chemotherapeutic effect of Artemisia vulgaris against diethylnitrosamine induced hepatocellular carcinogenesis in Balb C mice. Braz. J. Biol., 80: 489-496. https://doi.org/10.1590/1519-6984.185979
Ali, S., Hussain, S., Khan, R., Mumtaz, S., Ashraf, N., Andleeb, S., Shakir, H.A., Tahir, H.M., Khan, M.K.A. and Ulhaq, M., 2019. Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ. Sci. Pollut. Res. Int., 26: 3909–3920. https://doi.org/10.1007/s11356-018-3819-8
Allain, C.C., Poon, L.S., Chan, C.S., Richmond, W. and Fu, P.C., 1974. CHOD-PAP method for determination of total cholesterol. Clin. Chem., 20: 470-475. https://doi.org/10.1093/clinchem/20.4.470
Ara, C., Asmatullah, Butt, N., Ali, S., Batool, F., Shakir, H.A. and Arshad, A., 2020. Abnormal steroidogenesis, oxidative stress and reprotoxicity following prepubertal exposure to butyl paraben in mice and protective effect of Curcuma longa. Environ. Sci. Pollut. Res. Int., https://doi.org/10.1007/s11356-020-10819-8
Assmann, G., de Backer, G., Bagnara, S., Betteridge, J., Crepaldi, G., Fernandez-Cruz, A. and Ricci, G., 1997. Olive oil and the Mediterranean diet: implications for health in Europe. Br. J. Commun. Nurs., 6: 675-677. https://doi.org/10.12968/bjon.1997.6.12.675
Bako, H.Y., Mohammad, J.S., Waziri, P.M., Bulus, T., Gwarzo, M.Y. and Zubairu, M.M., 2014. Lipid profile of alloxan-induced diabetic wistar rats treated with methanolic extract of adansonia digitate fruit pulp. Sci. World. J., 9: 2.
Belwal, T., Bisht, A., Devkota, H.P., Ullah, H., Khan, H., Bhatt, I.D. and Echeverria, J., 2020. Phytopharmacology and clinical updates of Berberis species against diabetes and other metabolic diseases. Front. Pharmacol., 11: 41-54. https://doi.org/10.3389/fphar.2020.00041
Birdsall, T.C. and Kelly, G.S., 1997. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 2: 94-103.
Burstein, M., Scholnick, H.R. and Morfin, R., 1970. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid. Res., 11: 583-595.
Chand, N., Durrani, F.R., Qureshi, M.S. and Durrani Z., 2007. Role of Berberis lycium in reducing serum cholesterol in broilers. Asian-Australasian. J. Anim. Sci., 20: 563-568. https://doi.org/10.5713/ajas.2007.563
Dar, K.K., Ali, S., Ejaz, M., Nasreen, S., Ashraf, N., Gillani, S.F., Shafi, N., Safeer, S., Khan, M.A., Andleeb, S. and Mughal, T.A., 2019. In vivo induction of hepatocellular carcinoma by diethylnitrosoamine and pharmacological intervention in balb c mice using Bergeniaciliata extracts. Braz. J. Biol., 79: 629-638. https://doi.org/10.1590/1519-6984.186565
Friedewald, W.T., Levy, R.I. and Fredrickson, D.S., 1972. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin. Chem., 18: 499-502. https://doi.org/10.1093/clinchem/18.6.499
Furman, B.L., Candasamy, M., Bhattamisra, S.K. and Veettil, S.K., 2020. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J. Ethnopharmacol., 247: 112-264. https://doi.org/10.1016/j.jep.2019.112264
Hussain, Q., Mushtaq, W., Ishtiaq, M., Anjum, M., Faisal, M. and Mazhar, M., 2017. Comparative in vivo antidiabetic evaluation of leaves and bark of Berberis lyceum Royle in alloxan induced diabetic rabbits. Int. J. Biosci., 11: 91–98. https://doi.org/10.12692/ijb/11.2.91-98
Hussain, S., Ali, S., Mumtaz, S., Shakir, H.A., Ahmad, F., Tahir, H.M. and Ulhaq, M., 2020. Dose and duration-dependent toxicological evaluation of lead acetate in chicks. Environ. Sci. Pollut. Res. Int., 27: 15149-15164. https://doi.org/10.1007/s11356-020-08016-8
Joshi, R.K., Setzer, W.N. and da Veiga Junior, V.F., 2015. Aromatic and medicinal plants with anti-diabetic potential from India: A review. Am. J. Essent. Oil., 2: 22-28.
Khan, R., Ali, S., Mumtaz, M., Andleeb, S., Ulhaq, M., Tahir, H.M., Khan, M.K.A., Khan, M.A., Shakir, H.A., 2019. Toxicological effects of heavy metals (cadmium and mercury) on blood and thyroid gland and pharmacological intervention by vitamin c in rabbits. Environ. Sci. Pollut. Res. Int., 26: 16727-16741. https://doi.org/10.1007/s11356-019-04886-9
Khare, C.P., 2004. Indian Herbal Remedies, Springer, New York, pp. 98-100. https://doi.org/10.1007/978-3-642-18659-2
Lopes-Virella, M.F., Stone, P., Ellis, S. and Colwell, J.A., 1977. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem., 23: 882-884. https://doi.org/10.1093/clinchem/23.5.882
Luka, C.D. and Tijjani, H., 2013. Comparative studies of the aqueous extracts of Ocimum gratissimum, Aloe vera, Brassica oleracea and Ipomoea batatason some biochemical parameters in diabetic rats. IOSR J. Pharm. Biol. Sci., 6: 23-29. https://doi.org/10.9790/3008-0632329
Mughal, T.A., Saleem, M.Z., Ali, S., Anwar, K.K., Bashir, M.M., Babar, M. and khan, M.A., 2019. Evaluation of hepatotoxicity of carbon tetrachloride and pharmacological intervention by vitamin E in Balb C mice. Pak. J. Zool., 51: 755-761. https://doi.org/10.17582/journal.pjz/2019.51.2.755.761
Mumtaz, S., Ali, S., Khan, R., Andleeb, S., Haq, M., Khan, M.A. and Shakir, H.M.A., 2019. The protective role of ascorbic acid in the hepatotoxicity of cadmium and mercury in rabbits. Environ. Sci. Pollut. Res. Int., 26: 14087–14096. https://doi.org/10.1007/s11356-019-04620-5
Rang, H.P. and Urban, L., 1995. New molecules in analgesia. Br. J. Anaesth., 75: 145-156. https://doi.org/10.1093/bja/75.2.145
Saeed, M., 1976. Dehati moalig. 2nd ed. Hamd. Found. Pak., pp. 246-247.
Seigler, L. and Wu, W.T., 1981. Separation of serum high-density lipoprotein for cholesterol determination: ultracentrifugation vs precipitation with sodium phosphotungstate and magnesium chloride. Clin. Chem., 27: 838-841. https://doi.org/10.1093/clinchem/27.6.838
Shabbir, A., Shahzad, M., Arfat, Y., Ali, L., Aziz, R.S., Murtaza, G., Waqar, S.A. and Alamgeer, 2012. Berberis lycium Royle: A review of its traditional uses, phytochemistry and pharmacology. Afr. J. Pharm. Pharmacol., 6: 2346-2353. https://doi.org/10.5897/AJPP12.927
Singab, A.N., Youssef, F.S. and Ashour, M.L., 2014. Medicinal plants with potential antidiabetic activity and their assessment. Med. Aromat. Plants, 3: 2167-0412.
Swaroopa, P., Jaya, V., Reddy, S., Koshma, M., Sudharani, S., Basha, S.J., Werner, M., Gabrielson, D. and Eastman, G., 1981. Ultramicro determination of serum triglycerides by bioluminescent assay. Clin. Chem., 21: 268. https://doi.org/10.1093/clinchem/27.2.268
Wojtowicz, W.M., Wu, W., Andre, I., Qian, B., Baker, D. and Zipursky, S.L., 2007. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell, 130: 1134-1145. https://doi.org/10.1016/j.cell.2007.08.026
To share on other social networks, click on any share button. What are these?








