Age and Developmental Stage Dependent Changes in Plasma Concentrations of Growth Hormone and Testosterone and Linear Growth Velocity in Boys between the Age of 1 and 20 Years
Age and Developmental Stage Dependent Changes in Plasma Concentrations of Growth Hormone and Testosterone and Linear Growth Velocity in Boys between the Age of 1 and 20 Years
Afzaal Ahmad Naseem1*, Maleeha Akram1, Sarwat Jahan2, Kiran Afshan2, Zubaria Iqbal1, Faheem Tahir3, Mazhar Qayyum1 and Syed Shakeel Raza Rizvi1
1Department of Zoology, Pir Mehr Ali Shah Arid Agriculture University, Shamsabad, Murree Road, Rawalpindi, Pakistan
2Department of Animal Sciences, Quaid-e-Azam University, Islamabad, Pakistan
3Reproductive Physiology, Public Health Laboratories Division, National Institute of Health, Islamabad, Pakistan
ABSTRACT
The accelerated longitudinal bone growth at puberty or pubertal growth spurt has been ascribed to reciprocated physiological influences of both somatotropic and gonadal axes, manifested as higher secretion of growth hormone (GH) and testosterone (T). GH and T affect linear growth velocity (LGV) directly through stimulation of chondrocytes and osteoblasts. Furthermore, T promotes LGV through augmentation of GH secretion. Nevertheless, age and developmental stage dependent changes in GH and T and LGV and their associations require further investigation. This study examined relationships between GH and LGV, T and LGV and GH and T in 540 normal healthy boys of 1 to 20 years (n=27 boys/age group). The concentrations of GH and T were measured using specific ELISA systems and LGV was calculated for each age/stage group. Pearson correlation r and ANOVA were employed. GH and LGV exhibited age related positive correlations from 1 to 20 years and developmental stage dependent positive correlations during infancy, pre-puberty, early, mid and late puberty/adolescence. T and LGV were positively correlated from 1st to 16th year and negatively correlated from 17th to 20th year. T and LGV exhibited positive correlations from infancy to mid-puberty and negative correlation at late puberty/adolescence. GH and T were positively correlated from 1 to 16 years and negatively correlated between 17 and 20 years. GH and T were positively correlated during infancy, pre-puberty and early and mid-puberty and negatively correlated at late puberty/adolescence. In conclusion, GH, T and LGV exhibit age and stage dependent changes from infancy to early adulthood.
Article Information
Received 15 July 2016
Revised 30 August 2016
Accepted 05 September 2016
Available online 26 December 2016
Authors’ Contributions
AAN conceived the study, performed experimental work and wrote the article. SSRR and MQ designed the experiments and supervised the work. MA and KA performed statistical analysis. FT, SJ and ZI helped in experimental work.
Key words
Growth hormone, Testosterone, Linear growth velocity, Pubertal growth spurt, Male puberty
* Corresponding author: [email protected]
0030-9923/2017/0001-0229 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.1.229.240
INTRODUCTION
Puberty is marked by the greatest growth and sexual development since the stages of fetal development and major transformations in body composition, linear growth and distribution of fat in different body regions (Rogol, 2002). Growth in height is driven by elongation of long bones due to chondrogenesis at the epiphyseal plates, also known as the growth plate. This process results from chondrocyte proliferation, hypertrophy, and extracellular matrix secretion. An individual grows in a dynamic pattern throughout infancy, childhood, puberty, and early adolescence through a complex process that is sustained through age- and gender-dependent interactions among key genetic, environmental, dietary, socioeconomic, developmental, behavioral, nutritional, metabolic, biochemical, and hormonal factors (Veldhuis et al., 2005; Banerjee and Clayton, 2007; Susan et al., 2008; Gajdos et al., 2010). Consequently, the growth rate varies during infancy, childhood, puberty, and early adolescence. A number of previous studies indicate that the height velocity in the new born adjusts toward the genetically predicted pathways (Veldhuis et al., 2005).
Growth velocity is increased in early or mid-puberty and is known as the pubertal growth spurt. The growth velocity decreases and may even be zero after epiphyseal fusion, i.e., after growth plate closure in late puberty (Murray and Clayton, 2013). During the pubertal growth spurt, proliferation and differentiation of chondrocytes, secretion of extracellular matrix, calcification of the hypertrophic zone, invasion and differentiation of osteoblast, and formation of blood vessel repeat continuously in the growth plate (Nilsson et al., 2005; Shim, 2015). The hormonal regulation of the growth spurt and the alterations in body composition depend on the release of the gonadotropins, leptin, the sex-steroids, and growth hormone (GH). Although, changes in hormonal regulation play a pivotal role all along the way from fetal growth to the aging process, the processes of growth and development under hormonal regulation are merely magnified during pubertal development. The somatic growth and maturation are influenced by several factors that act independently or in concert to modify an individual’s genetic growth potential (Rogol et al., 2000).
Essential to normal growth and development are adequate levels of several hormones, although deficiencies of these are a much less common cause of growth disturbances. The growth hormone (GH) secreted by anterior pituitary is closely correlated with the pattern of linear growth during infancy, pre-puberty, puberty and adolescence. GH stimulates growth of bone, cartilage and muscle. The 24 h pattern of spontaneous GH release changes with age in experimental animals and humans. In humans, spontaneous pulsatile release is present in all infant within the first 1-2 days of life, GH release is enhanced through the amplitude and frequency of pulses, then as the infant matures, there is a decrease in GH levels frequency, amplitude, and nadir. Studies on the 24 h pattern of spontaneous GH secretion in pre-pubertal children revealed GH peaks during waking hours as well as during sleep (Miller et al., 1982). Nevertheless, late pubertal boys secrete more GH per 24 h than boys in any other pubertal group and triple that of pre-pubertal boys (Martha et al., 1992). Shortly after cessation of linear growth in boys, the overall peripheral GH pulse pattern returns toward pre-pubertal levels so that the concentration profiles in young men are remarkably similar to those in pre-pubertal boys (Martha et al., 1990). Thus in young adults, total GH secretion is almost one-half that in late pubertal boys, but the 24 h burst frequency is the same (McGillivray et al., 1970).
GH stimulates linear growth through the secretion of insulin-like growth factor-I (IGF-I) in the liver and growth plate and hence GH and IGF-I are regarded as the main stimulators of longitudinal bone growth. They are also important for the acquisition of bone mass during the pre-pubertal period and maintenance of bone homeostasis throughout life. It has been demonstrated that longitudinal bone growth is mediated by GH, circulating IGF-I, and more importantly, local IGF-I in the growth plate. For differentiation, proliferation, and hypertrophy of chondrocytes; the production of extracellular matrix; and ossification in the growth plate, IGF-I produced from chondrocytes of the epiphyseal plate is important (Giustina et al., 2008). At puberty, there can be a 1.5- to 3-fold increase in the pulsatile secretion of GH and a more than 3-fold increase in the concentration of serum IGF-I (Albin et al., 2012; Shim, 2015).
During puberty, girls and boys experience a growth that is faster than at any postnatal age since infancy. The peak growth spurt in boys occurs during mid-puberty (Tanner Stages 3–4), when testosterone (T) levels are rapidly rising (Blakemore et al., 2010). The increase in the production of sex steroids at the start of puberty is clearly linked to an increase of bone mineral acquisition during this period and contributes to the establishment of sex differences in bone growth (Callewaert et al., 2010; Ferlin et al., 2013). The pubertal growth spurt can be divided into three stages: first, the phase of minimal growth velocity just before the spurt (‘take-off’ velocity); then the stage of most rapid growth, or peak height velocity (PHV) followed by the stage of decreased velocity and cessation of growth at epiphyseal fusion. The precise pattern depends on the timing and tempo of pubertal development, since those with a significant delay often show a peri-pubertal decrement in growth velocity (pre-adolescent ‘dip’) before the accelerated phase of the pubertal growth spurt (Soliman et al., 2014).
It has been suggested that a close correlation exist between circulating levels of T and GH at infantile, prepubertal and pubertal stages of development (Rose and Nisula, 1989; Martha et al., 1992). A number of clinical observations have shown that both GH and sex steroid hormones must be present for normal pubertal growth to occur. During pubertal development, the interactions between GH and the sex steroid hormones are striking and pervasive and rising levels of T during puberty play a pivotal role in augmenting spontaneous GH secretion and production (Rose and Nisula, 1989; Martha et al., 1992). The ability of T to stimulate pituitary GH secretion, however, appears to be transient, expressed only peri-pubertally, because GH and IGF-1 levels decrease significantly during late puberty and into adulthood despite continued high concentrations of gonadal steroid hormones (Martha et al., 1989).
In addition to its growth promoting effect on bone through increases in GH secretion, T itself also contributes to bone formation and the pubertal growth spurt, perhaps through a direct interaction with growth plate chondrocytes (Nilsson et al., 2003; Shim, 2015). It has been demonstrated that dihydrotestosterone (DHT) can stimulate proliferation and proteoglycan synthesis in growth plate chondrocytes in vitro and testosterone stimulates chondrocyte proliferation in an organ culture model study with increased local IGF-I production (Nilsson et al., 2005; Shim, 2015).
The available data provides information on the secretions of GH and T at different stage of pubertal development. However, there was very little or no data available on the age and developmental stage related changes in the secretion of GH and T and their impact on LGV in our local population. Therefore, the present study was designed to determine age and development stage dependent relationships between plasma concentrations of GH and T and LGV in normal boys of 1 to 20 years of ages.
MATERIALS AND METHODS
Subjects
The healthy normal boys with minor illness like temperature, flu, and cough without any concurrent diseases between the ages of 1 to 20 years, visiting Pakistan Institute of Medical Sciences (PIMS), Islamabad and Benazir Bhutto Shaheed Hospital, Rawalpindi were included in this study. The boys were categorized into 20 different age groups of 1 to 20 years and into five developmental stages, i.e., infancy, pre-puberty, early puberty, mid-puberty and late puberty/adolescent based on criteria developed by Tanner and Whitehouse (1976). All boys belonged to middle class of about the same socioeconomic status and eating habits. The anthropometric parameters of boys with their body weight (BW) and height were recorded in the proforma, especially developed for the purpose.
The parents/guardians of children were briefed about the purpose of the study and their written consent was obtained. The study protocol was approved by the Research Ethical Committee of PMAS Arid Agriculture University, Rawalpindi. The boys with any chronic disease and/or endocrine disorder or family history of endocrinopathies were excluded from the study.
Calculation of LGV
The occurrence of pubertal growth spurt in boys was evaluated by LGV calculated with the help of the following relationship:
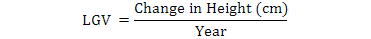
Where standing height was measured with portable Stadiometer/infant height scale to the nearest of 0.1 cm.
Blood specimens
Multiple blood samples (~1.5 ml) of each boy visiting PIMS, Islamabad and Benazir Bhutto Shaheed Hospital Rawalpindi were collected from antecubital vein in heparinized syringes. The blood samples were immediately centrifuged after collection, the plasma was separated and frozen at -20oC. All bleedings were carried out between 10:00 am and 12:00 pm to minimize the influence of the diurnal variation in the circulating concentrations of the hormones.
Hormonal analysis
Plasma GH concentrations were determined by using solid phase competitive ELISA (Amgenix International Inc. 3444 Pinotin Ct. San Jose, CA 95148, USA). The minimum detectable concentration of GH was 0.5 ng/ml. Plasma T concentrations were determined by using a fully automated Electrochemiluminescence Immunoassay testosterone kit on Roche Diagnostics Elecsys 2010 instrument. The detection range of the assay was from 0.087 to 52.0 nmol/l.
Statistical analysis
Pearson correlation r and analysis of variance (ANOVA) were done for the interpretation of results. Statistical significance was adjusted at p<0.05 (Steel and Torrie, 1980). Values were expressed as Mean ± SEM.
RESULTS
Growth hormone and linear growth velocity in normal boys
The age related changes in the mean plasma GH concentrations and LGV in healthy boys from 1 to 20 years are shown in Figure 1. The mean plasma GH concentrations progressively decreased (p>0.05) from 1st to 3rd year of age, remained more or less at about the same level (p>0.05) from 4th to 9th year of age, progressively increased (p<0.05) from 10th year to reach highest concentrations at 15th year, then gradually declined (p<0.05) from 16th to 19th year and increased non-significantly (p=0.23) at 20th year of age.
The mean LGV during the 1st year of life was the highest, which decreased abruptly and significantly (p=0.00) at 2nd year and further decreased (p>0.05) gradually from 3rd to 7th year of age. Thereafter, LGV values progressively but non-significantly (p>0.05) increased from 8th to 13th years of age. The mean LGV remained at about the same levels (p=0.37) at 14th year and significantly (p=0.003) increased to reach a peak value at 15th year of age. Subsequently, the LGV significantly decreased (p=0.00) at 16th year, while non-significantly (p>0.05) decreased at 17th to 19th year to reach the lowest observed values at 20th year of age.
The mean plasma concentrations of GH and LGV were positively correlated at 1st (r = 0.76), 2nd (r=0.65) and 3rd year of age (r = 0.72). The mean plasma GH concentrations and LGV were negatively correlated at 4th year (r = -0.8) but positively correlated (r = 0.75) at 5th year and again negatively correlated (r = -0.71) at 6th year. The mean plasma GH concentrations and LGV were positively correlated (r = 0.63) at 7th year but negatively correlated at 8th (r = -0.62) and 9th (r = -0.64) years of age. The mean plasma GH concentrations and LGV were positively correlated at 10th (r = 0.74), 11th (r = 0.76), 12th (r = 0.76), 13th (r = 0.7), 14th (r = 0.78), 15th (r = 0.82), 16th (r = 0.78), 17th (r = 0.72), 18th (r = 0.7) and 19th (r = 0.65) year but negatively correlated (r = -0.66) at 20th year of age.
Figure 2 shows the relationship between plasma levels of GH and LGV at different developmental stages. The mean plasma levels of GH were higher at infancy, decreased significantly (p=0.001) during pre-puberty and then increased significantly (p=0.00) at early puberty reaching highest concentrations (p=0.00) during mid-puberty and dropped significantly (p=0.00) at late puberty to levels comparable to those observed during infantile stage of development in boys. Similarly, the mean LGV was highest at infancy, which significantly (p=0.00) decreased at pre-puberty. The mean values of LGV increased non-significantly at early (p=0.2) and mid-puberty (p=0.45) and then dropped significantly (p=0.00) to the lowest values at late puberty.
The levels of GH and LGV were positively correlated at infancy (r=0.7), pre-puberty (r=0.8), early puberty (r=0.6), mid-puberty (r=0.6) and late puberty/adolescence (r=0.8).
Mean Plasma testosterone concentrations and LGV in normal boys
The age related changes in the mean plasma T concentrations and LGV in boys between the age of 1 and 20 years are also shown in Figure 3. The mean plasma concentrations of T were higher at 1st year of age, which declined significantly (p=0.007) at 2nd year and further decreased (p=0.93) to the lowest concentrations at 3rd year of age. The levels of T increased (p=0.83) at 4th year of age and fluctuated in a non-significant (p>0.05) manner from 5th to 8th years of age. The concentrations of T increased 3 fold at 9th year (p=0.03) and 10th year of age (p=0.01). The levels of T increased non-significantly (p=0.32) from 11th year to 12th year and significantly (p=0.04) increased at 13th year. The T concentrations abruptly increased (p=0.00) at 14th year reaching first peak at 15th year but significantly (p=0.01) declined at 16th year. The levels of T abruptly increased (p=0.001) to reach a second and highest peak at 18th year of age. The concentrations of T decreased significantly (p=0.00) at 19th year and further decreased (p=0.009) at 20 years of age.
The mean LGV of boys at 1st year of age had highest value, which decreased abruptly and significantly (p=0.00) at 2nd year and further decreased (p>0.05) gradually from 3rd to 7th year of age. Thereafter, LGV values progressively and non-significantly (p>0.05) increased from 8th to 13th years of age. The mean LGV values slightly increased (p=0.37) at 14th year and further increased significantly (p=0.03) to reach a peak value at 15th year of age. From the peak value, the LGV significantly decreased (p=0.00) at 16th year, while non-significantly (p>0.05) decreased at 17th, 18th and 19th year to reach the lowest observed values at 20th year of age.
The mean plasma T concentrations and LGV were positively correlated with each other at 1st (r = 0.7), 2nd (r = 0.64) and 3rd (r = 0.62) years but negatively correlated (r = -0.61) at 4th year and again positively correlated at 5th (r = 0.74) and 6th (r = 0.72) years of age. There was a negative correlation (r = -0.63) between mean plasma T concentrations and LGV at 7th year but a positive correlation at 8th (r = 0.71), 9th (r = 0.74), 10th (r = 0.82), 11th (r = 0.78), 12th (r = 0.71), 13th (r = 0.72), 14th (r = 0.74), 15th (r = 0.85) and 16th (r = 0.74) years of age. The mean plasma T concentrations and LGV were negatively correlated at 17th (r = -0.71) and 18th (r = -0.8) but positively correlated at 19th (r = 0.64) and 20th (r = 0.66) years of age.
Figure 4 shows the changes in the mean plasma concentrations of T and LGV at infancy, pre-puberty, early, mid and late puberty in boys. The levels of T were quite higher at infancy, which were considerably lowered (p=0.07) at pre-puberty and again increased significantly (p=0.00) during early puberty. The levels of T abruptly increased (p=0.00) at mid puberty, which further increased (p=0.00) to highest concentrations during late puberty/adolescence. The mean LGV was highest at infancy, which drastically and significantly (p=0.00) decreased at pre-puberty. The mean values of LGV increased non-significantly at early (p=0.2) and mid-puberty (p=0.45) and then dropped significantly (p=0.00) to the lowest values at late puberty.
The mean plasma T values and LGV were positively correlated at infancy (r = 0.7), pre-puberty (r = 0.67), early puberty (r = 0.72) and mid-puberty (r = 0.71) but negatively correlated at late puberty/adolescence (r = -0.6).
Mean Plasma GH and T concentrations in normal boys
The age related changes in the mean plasma GH and T concentrations in boys between the age of 1 and 20 years are shown in Figure 5. The mean plasma GH concentrations progressively decreased (p>0.05) from 1st to 3rd year of age, remained more or less at about the same level (p>0.05) from 4th to 9th year of age, progressively increased (p<0.05) from 10th year to reach highest concentrations at 15th year, then gradually declined (p<0.05) from 16th to 19th year and increased non-significantly (p=0.23) at 20th year of age.
The mean plasma concentrations of T were higher at 1st year of age, which declined significantly (p=0.007) at 2nd year and further decreased (p=0.93) to the lowest concentrations at 3rd year of age. The levels of T increased (p=0.83) at 4th year of age and fluctuated in a non-significant (p>0.05) manner from 5th to 8th years of age. The concentrations of T increased 3 fold at 9th year (p=0.03) and 10th year of age (p=0.01). The levels of T increased non-significantly (p=0.32) from 11th year to 12th year but significantly (p=0.04) increased at 13th year. The T concentrations abruptly increased (p=0.00) at 14th year reaching first peak at 15th year but significantly (p=0.01) declined at 16th year. The levels of T abruptly increased (p=0.001) to reach a second and highest peak at 18th year of age. The concentrations of T decreased significantly (p=0.00) at 19th year and further decreased (p=0.009) at 20 years of age.
The mean plasma GH and T concentrations were positively correlated at 1st (r = 0.72), 2nd (r = 0.76), 3rd (r = 0.61), 4th (r = 0.6) and 5th (r = 0.7) years while negatively correlated at 6th (r = -0.63), 7th (r = -0.62), 8th (r = -0.68) and 9th (r = -0.68) years of age. There was a positive correlation between mean plasma concentrations of GH and T at 10th (r = 0.73), 11th (r = 0.7), 12th (r = 0.72), 13th (r = 0.77), 14th (r = 0.81), 15th (r = 0.85) and 16th (r = 0.71) year but a negative correlation at 17th (r = -0.71) and 18th (r = -0.76) years. The mean plasma GH and T concentrations were positively correlated (r = 0.63) at 19th year while negatively correlated (r = -0.65) at 20th year of age.
Figure 6 shows plasma GH and T levels at different developmental stages. The mean plasma levels of GH were higher at infancy, decreased significantly (p=0.001) during pre-puberty and then increased significantly (p=0.00) at early puberty reaching highest concentrations (p=0.00) during mid-puberty and dropped significantly (p=0.00) at late puberty to levels comparable to those observed during infantile stage of development in boys. The levels of T were quite higher at infancy, which were considerably lowered (p=0.07) at pre-puberty and again increased significantly (p=0.00) during early puberty. The levels of T abruptly increased (p=0.00) at mid puberty, which further increased (p=0.00) to highest concentrations during late puberty/adolescence.
The levels of GH and T were positively correlated at infancy (r = 0.8), pre-puberty (r = 0.7), early puberty (r = 0.81) and mid puberty (r = 0.83) but negatively correlated at late puberty/adolescence (r = -0.74).
DISCUSSION
In this study, the mean LGV of boys at the end of 1st year was 26.84 cm. The boys grew 9.47 cm during the 2nd year, 7.79 cm during the 3rd year and 7.03 cm during the 4th year. The LGV of boys averaged 5.33 cm between 5 and 9 years and 6.7 cm/year between the age of 10 and 12 years. The mean LGV increased to 9.44 cm/year between the age of 13 and 15 years. Thereafter, the mean LGV decreased to 3.67 cm/year from 16 to 18 years and to 1.84 cm/year during 19 and 20 years of age. In a previous investigation, it has been demonstrated that during 1st year of life, babies by growing on average of 10 inches (25 cm) in length triple their birth weights. The baby’s growth in length considerably slows down after 1st year of age and from 2nd year the longitudinal growth usually continues at a steady rate of 2.45 inches (~6.17 cm) per year till 9th year of age. The height velocity decreases to 1.33 inches (~3.5 cm) per year during early puberty and a major growth spurt occurs at the time of mid puberty at a height velocity of 2.59 inches (~6.64cm) per year. The increase in height was reduced to 0.5 inches (~1.25cm) per year following puberty and adolescence. Similar results were reported in boys and girls showing that height and weight steadily increase from age of 5th year until 15th year with significant differences (Garcia-Mayor et al., 1997). Usually, the growth spurt occurs between the age of 8th and 13th year in girls and 10th and 15th year in boys. It has earlier been reported that before puberty both boys and girls have an average of about two inches of skeletal growth per year (Gordon and Laufer, 2005). Earlier studies also demonstrate that body length increased by approximately 1.5 times birth length between 8 and 12 months of age and between 12 and 24 months baby grows about 80-95cm (31-35 inches) in length and growth rate is about 7-13 cm per year and the mean body length by 2nd year was 85-95 cm. Similar to these observations, the average body length of boys was 74.8 cm at 1st year and 83.1 cm during the 2nd year in our study. The linear growth within the first 2 years of life generally decelerates but then remains relatively constant throughout childhood until the onset of the pubertal growth spurt (Rogol et al., 2000). During puberty, there is a growth spurt that lasts for about one or two years for each individual wherein a skeletal growth of approximately four inches per year is observed, but this peak velocity lasts only a few months (Gowers, 2005; Gordon and Laufer, 2005). At the age of 1 year, the baby grows to a height of 50% over birth length (Wright et al., 2010).
In the present study, LGV of boys was 18.2 cm/year during infancy, which dropped to 6.06 cm/year during pre-puberty. The boys grew at an average growth velocity of 6.65 cm/year at early puberty, 7.4 cm/year during mid-puberty, 2.9 cm/year at late puberty and 1 cm/year during adolescence. Our findings are in line with earlier observations in American boys wherein LGV averaged approximately 18.75 cm/year during infancy. The annual height velocity decreased considerably during pre-puberty and a plateau-like phase emerged in mid childhood, wherein height velocity approaches 5.5 cm/year before puberty (Veldhius et al., 2005). The boys continued to gain height at a pre-pubertal rate until 11 years of age, when testes volume began to increase beyond 7-10 ml. The pubertal males then achieved a peak height velocity of 9.5 cm/year at about 13.5 year of age, which coincided with pubertal genital stages 3 and 4 (Veldhius et al., 2005). The pubertal growth rate declined rapidly to 1 cm/year during adolescence and continued to be maintained by about 21 years of age (Veldhuis et al., 2005).
The growth rate is dependent upon the amount of GH secreted by the pituitary somatotropes with the higher concentrations of GH inducing higher rate of growth in humans (Rogol, 2010). In this investigation, the mean plasma GH concentrations, which were higher at 1st year decreased at 2nd and 3rd year and showed slight fluctuations between 3rd and 9th year. The mean plasma GH concentrations progressively increased from 10th year, reaching peak levels at 15th year of life. The mean plasma GH steeply declined from 15th to 19th year, but markedly increased at 20th year of age. Some previous observations in the rat (Ojeda and Jameson, 1997; Sonntage, 1979), rhesus monkey (Wilson et al., 1986; Wheeler and Styne, 1988) and man (Martha et al., 1989, 1992) have also shown age-related changing in the secretion of GH and support our findings.
The GH levels in this study were higher at infancy, decreased at pre-puberty, increased at early and mid-puberty and again decreased at late puberty. In another study, GH levels increase throughout the first stages of puberty in boys to reach a maximum at the time of peak height velocity (Zhang et al., 2000). Earlier findings have also reported that plasma levels of GH, which are low at the pre-pubertal stage, rise with the onset of puberty in humans, peak during mid to late puberty and decline progressively during post pubertal period to reach very low concentrations at adulthood (Martha et al., 1989, 1992; Rose et al., 1991).
In the present study, the mean plasma concentrations of GH and LGV exhibited age related positive correlation in boys between the age of 1 and 20 years. Similarly, there was a positive correlation between mean plasma concentrations of GH and LGV during different development stages from infancy to pre-puberty, early and mid-puberty to late puberty/adolescence. It has already been established that growth spurt at puberty is resultant of higher concentrations of GH in growing boys (Rogol, 2010).
Earlier studies demonstrated that GH increases longitudinal growth directly through its effect on chondrocytes and osteocytes (Locatelli and Bianchi, 2014). The growth promoting effect of GH on bone and cartilage is exerted through local or systemic production of insulin like growth factor-I (IGF-I) (Locatelli and Bianchi, 2014). Interestingly, GH and IGF-I may have effects that are not dependent on each other, since prepubertal growth inhibition is more severe in IGF-I knockout mice than in GH-deficient mice, showing that IGF-I has effects on bone that are independent of GH. It has, therefore, been suggested that during puberty, both GH-dependent and GH-independent mechanisms come into play (Mohan et al., 2003). Mice deficient in liver IGF-I display high GH, unchanged IGF-I, and increased cortical bone growth after ovariectomy, suggesting a direct role of GH in bone growth (Fritton et al., 2010). On the other hand, GH supplementation increases bone formation in IGF-I knockout mice (Bikle et al., 2001; Olsen et al., 2011), again demonstrating separate roles for these hormones.
The present investigation shows that the mean plasma concentrations of T were higher at 1st year of age and declined to lower concentrations at 2nd year of age. The mean plasma concentrations of T remained very low between 3 and 9 years of age and started to rise at 10th year of age. The mean plasma concentrations of T abruptly increased at 14th year and reached first peak at 15th year of age and a second highest peak at 18th year of age and slightly declined at 19th and 20th year of age. These findings are consistent with previous studies, which showed that the plasma levels of T increase after 7 to 9 years in both boys and girls, though T concentrations increase more dramatically in boys (Hoekstra et al., 2006). Likewise, Chada et al. (2003) have reported higher concentrations of T at birth, which gradually decreased to low concentrations by the end of 2nd year of life. The concentrations of T remained low during the next three years and then began to rise at the onset of puberty. Thus, the concentrations of T continued to increase through various stages of puberty and reached maximum values at adolescence (Chada et al., 2003).
In this investigation, the levels of T were quite higher at infancy, which decreased considerably at pre-puberty and again increased during early puberty. The levels of T abruptly increased at mid-puberty, which further augmented to highest concentrations during late puberty/adolescence. A number of previous studies showed that the circulating concentrations of T are high during infancy, which decline to low levels at pre-pubertal developmental stage, again rise at puberty reaching the highest values during mid to late puberty and decline to normal high concentrations at adulthood (Martha et al., 1989; Chada et al., 2003). It has been demonstrated that serum T is secreted with a diurnal variation in pre-puberty as well as during puberty. The most pronounced diurnal rhythm is found in early and mid-puberty (Ankarberg-Lindgren and Norjavaara, 2004). At early puberty, the increase in the amplitude of T secretion first appear in the night and later in the early morning hours (Ankarberg-Lindgren and Norjavaara, 2004).
In the current study, the mean plasma concentrations of GH and T were positively correlated with each other from 1 to 16 years of age and negatively correlated with each other between 17 and 20 years of age. In a similar manner, the mean plasma concentrations of GH and T were positively correlated with each other during infancy, pre-puberty and early and mid-puberty. The mean plasma concentrations of GH and T were negatively correlated with each other at late puberty/adolescence. These findings are consistent with some earlier studies wherein changes in growth rate during different developmental stages seem to coincide with alterations in the secretion of GH and T (Rogol, 2010). It has been suggested that the combined physiological effects of the somatotropic and gonadal axes results in accelerated linear growth during normal puberty that transform a child to an adult (Giustina et al., 1994; Clark and Rogol, 1996). It has been argued that in influencing growth rate, T may directly exert its effect on osteocytes and chondrocytes and/or may indirectly affect growth rate through its effect on GH secretion. It has long been debated that the fundamental force behind pubertal growth spurt is the relationship between rising concentrations of T, GH, and IGF-I (Maurus et al., 1996). Nevertheless, reduction in axial and appendicular bone size in both GH-intact and GH-deficient rats following orchidectomy at 6 weeks of age indicates that the effect of T on bone growth is additive and independent of GH (Kim et al., 2003). Furthermore, reduction of periosteal bone formation by orchidectomy in mice at 3 weeks of age without a decrease in serum IGF-I shows that the effect of T on bone growth is unrelated to IGF-I and hence GH secretion (Venken et al., 2006). It has also been demonstrated that androgens can increase periosteal bone growth even in the absence of the GH receptor and with no increase in IGF-I. In this regard, it has been reported that even in wild-type orchidectomized mice, androgen supplementation restores bone growth without an IGF-I increase (Venken et al., 2007; Olsen et al., 2011). In line with these observations, we have observed that the mean plasma concentrations of T and LGV were positively correlated with each other from 1st year of life to 16th year of age. Similarly, the mean plasma concentrations of T and LGV exhibited positive correlation from infancy to mid-puberty and negative correlation at late puberty/adolescence.
As far as the influence of T on bone growth through its effect on GH secretion is concerned, it has been reported that androgens specifically and significantly augment GH secretory rate by amplifying the magnitude of GH secretory episodes (Link et al., 1986; Ulloa-Aguirre et al., 1990; Keenan et al., 1993; Eakman et al., 1996). Some previous studies have also shown that T induces expression of GH/IGF-1 axis, leading to rapid growth. Bhasin et al. (2001) determined the effects of graded doses of T on body composition, muscle size, and IGF-I levels and found that IGF-I was positively correlated with T. Furthermore, it has been demonstrated that resistance exercise and training resulted in lower T levels, which were associated with lower GH levels (Vingren et al., 2010). A more recent study showed that T injections stimulate the secretion of GH by the pituitary somatotropes and that cultured pituitary cells showed increased GH expression after T stimulation (Li and Li, 2014). It was also determined that osteocalcin-mediated GH receptor (GHR) and IGF-1 expression in the liver is stimulated by T. These inducing effects were also detected in cultured cells, uninfluenced by GH (Li and Li, 2014). It has also been reported that the GH releasing effects of T are not exerted at the pituitary level but hypothalamic GHRH neurons are stimulated by T for affecting GH secretion (Rizvi et al., 2000). Furthermore, it has been demonstrated that T does not affect the sensitivity of the pituitary somatotropes to GHRH, but augments the secretion of GH via stimulation of hypothalamic GHRH neurons (Rizvi et al., 2000).
It has been reported that T has an osteoanabolic effect on the skeleton during puberty and this effect is mediated by periosteal bone formation, which is partly dependent on androgen receptor (AR) signaling, and partly on estradiol, which may then interact with the dominant regulator of bone formation, that is GH-IGF-I (Sinnesael et al., 2013). Bone also produces osteocalcin, which may further stimulate the testes to produce T. The role of T in trabecular bone maintenance has been clearly established in mice. Nevertheless, estrogen is clearly important, not only for bone gain but also as a risk factor for fractures in men with low estrogen concentrations (Sinnesael et al., 2013). In a number of previous studies, the elevation in pubertal GH secretion has been attributed to concurrent elevations in sex steroid concentrations (Giustina et al., 1997; Rogol, 2010), particularly estrogen, which in the male is primarily derived from T via aromatization (Metzger and Kerrigan, 1994; Veldhuis et al., 1997). It has been suggested that the stimulation of GH secretion by T requires prior aromatization to oestrogen. Evidence comes from studies reporting that administration of an oestrogen receptor antagonist abrogates the GH-stimulatory effect of T in hypogonadal men and reduces GH secretion in normal healthy men (Weissberger and Ho, 1993). Furthermore, in boys with delayed puberty, dihydrotestosterone, an androgen that cannot be aromatized to an oestrogen, does not stimulate GH secretion (Veldhius et al., 1997). Thus, the central ‘push’ mechanism of T involves oestrogen receptor mediation (Meinhardt and Ho, 2006).
Conclusion
In conclusion, this study demonstrates that both somatotropic and reproductive axes are involved in causing accelerated linear growth at puberty and that both of them can have interdependent as well as independent effects on linear bone growth.
Conflict of interest statement
We declare that we have no conflict of interest.
ACKNOWLEDGEMENTS
The authors acknowledge the financial assistance provided by the Pir Mehr Ali Shah Arid Agriculture University Rawalpindi and Mr. Chaudhry Muhammad Naeem, Chief Executive Officer, MedLab Services Rawalpindi for providing the kits for hormonal analysis to carry out this research.
REFERENCES
Albin, A.K., Niklasson, A., Westgren, U. and Norjavaara, E., 2012. Estradiol and pubertal growth in girls. Horm. Res. Paediatr., 78: 218-225. http://dx.doi.org/10.1159/000343076
Ankarberg-Lindgren, C. and Norjavaara, E., 2004. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 ml is a transition stage to puberty. Eur. J. Endocrinol., 151: 747-757. http://dx.doi.org/10.1530/eje.0.1510747
Banerjee, I. and Clayton, P., 2007. The genetic basis for the timing of human puberty. J. Neuroendocrinol., 19: 831-838. http://dx.doi.org/10.1111/j.1365-2826.2007.01598.x
Bhasin, S., Woodhouse, L., Casaburi, R., Singh, A.B., Bhasin, D., Berman, N., Chen, X., Yarasheski, K.E., Magliano, L., Dzekov, C., Dzekov, J., Bross, R., Phillips, J., Sinha-Hikim, I., Shen, R. and Storer, T.W., 2001. Testosterone dose-response relationships in healthy young men. Am. J. Physiol. Endocrinol. Metab., 281: 1172-1181.
Bikle, D., Majumdar, S., Laib, A., Powell-Braxton, L., Rosen, C., Beamer, W., Nauman, E., Leary, C. and Halloran, B., 2001. The skeletal structure of insulin-like growth factor I–deficient mice. J. Bone Miner. Res., 16: 2320-2329. http://dx.doi.org/10.1359/jbmr.2001.16.12.2320
Blakemore, S.J., Burnett, S. and Dahl, R.E., 2010. The role of puberty in developing adolescent brain. Hum. Brain Map, 31: 926-933. http://dx.doi.org/10.1002/hbm.21052
Callewaert, F., Sinnesael, M., Gielen, E., Boonen, S. and Vanderschueren, D., 2010. Skeletal sexual dimorphism: relative contribution of sex steroids. J. Endocrinol., 207: 127-134. http://dx.doi.org/10.1677/JOE-10-0209
Chada, M., Prusa, R., Bronsky, J., Kotaska, K., Sidlova, K., Pechova, M. and Lisa, L., 2003. Inhibin, follicle stimulating hormone, luteinizing hormone and testosterone during childhood and puberty in males: changes in serum concentrations in relation to age and stage of puberty. Physiol. Res., 52: 45-51.
Clark, P.A. and Rogol, A.D., 1996. Growth hormones and sex steroid interactions at puberty. Endocrinol. Metab. Clin. N. Am., 25: 665-681. http://dx.doi.org/10.1016/S0889-8529(05)70346-7
Eakman, G.D., Dallas, J.S., Ponder, S.W. and Keenan, B.S., 1996. The effects of testosterone and dihydrotestosterone on hypothalamic regulation of growth hormone secretion. J. clin. Endocrinol. Metab., 81: 1217-1223. http://dx.doi.org/10.1210/jcem.81.3.8772602
Ferlin, A., Selice, R., Carraro, U. and Foresta, C., 2013. Testicular function and bone metabolism – beyond testosterone. Nat. Rev. Endocrinol., 9: 548-554. http://dx.doi.org/10.1038/nrendo.2013.135
Fritton, J.C., Emerton, K.B., Sun, H., Kawashima, Y., Mejia, W., Wu, Y., Rosen, C.J., Panus, D., Bouxsein, M., Majeska, R.J., Schaffler, M.B. and Yakar, S., 2010. Growth hormone protects against ovariectomy-induced bone loss in states of low circulating insulin-like growth factor (IGF-1). J. Bone Miner. Res., 25: 235-246. http://dx.doi.org/10.1359/jbmr.090723
Gajdos, Z.K.F., Henderson, K.D., Hirschhorn, J.N. and Palmert, M.R., 2010. Genetic determinants of pubertal timing in general population. Mol. cell. Endocrinol., 324: 21-29. http://dx.doi.org/10.1016/j.mce.2010.01.038
Garcia-Mayor, R.V., Andrade, M.A., Rios, M., Lage, M., Dieguez, C. and Casanueva, F.F., 1997. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J. clin. Endocrinol. Metab., 82: 2849-2855. http://dx.doi.org/10.1210/jcem.82.9.4235
Giustina, A., Bresciani, E., Bossoni, S., Chiesa, L., Misitano, V., Wehrenberg, W.B. and Veldhuis, J.D., 1994. Reciprocal relationship between the level of circulating cortisol and growth hormone secretion in response to growth hormone releasing hormone in man: studies in patients with adrenal insufficiency. J. clin. Endocrinol. Metab., 79: 1266-1272. http://dx.doi.org/10.1210/jcem.79.5.7962318
Giustina, A., Scalvini, T., Tassi, C., Desenzani, P., Poiesi, C., Wehrenberg, W.B., Rogol, A.D. and Veldhuis, J.D., 1997. Maturation of the regulation of growth hormone secretion in young males with hypogonadotropic hypogonadism pharmacologically exposed to progressive increments in serum testosterone. J. clin. Endocrinol. Metab., 82: 1210-1219. http://dx.doi.org/10.1210/jcem.82.4.3871
Giustina, A., Mazziotti, G. and Canalis, E., 2008. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev., 29: 535-559. http://dx.doi.org/10.1210/er.2007-0036
Gordon, M.C. and Laufer, M.R., 2005. Physiology of puberty. In: Pediatric and adolescent gynecology (eds. S.J.H. Emans, D.P. Goldstein and M.R. Laufer), Williams and Wilkins, Lippincott, Philadelphia, pp. 120-155.
Gowers, S.G., 2005. Development in adolescence. Psychiatry, 4: 6-9. http://dx.doi.org/10.1383/psyt.4.6.6.66353
Hoekstra, R.A., Bartels, M. and Boomsma, D.I., 2006. Heritability of testosterone levels in 12-year old twins and its relation to pubertal development. Twin Res. Hum. Genet., 9: 558-565. http://dx.doi.org/10.1375/twin.9.4.558
Keenan, B.S., Richards, G.E., Ponder, S.W., Dallas, J.S., Nagamani, M. and Smith, E.R., 1993. Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J. clin. Endocrinol. Metab., 76: 996-1002. http://dx.doi.org/10.1210/jcem.76.4.8473416
Kim, B.T., Mosekilde, L., Duan, Y., Zhang, X.Z., Tornvig, L., Thomsen, J.S. and Seeman, E., 2003. The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J. Bone Miner. Res., 18: 150-155. http://dx.doi.org/10.1359/jbmr.2003.18.1.150
Li, Y. and Li, K., 2014. Osteocalcin induces growth hormone/insulin like growth factor-1 system by promoting testosterone synthesis in male mice. Horm. Metab. Res., 46: 768-773.
Link, K., Blizzard, R.M., Evans, W.S., Kaiser, D.L., Parker, M.W. and Rogol, A.D., 1986. The effect of androgens on the pulsatile release and the twenty-four hour mean concentration of growth hormone in prepubertal males. J. clin. Endocrinol. Metab., 62: 159-164. http://dx.doi.org/10.1210/jcem-62-1-159
Locatelli, V. and Bianchi, V.E., 2014. Effect of GH/IGF-I on bone metabolism and osteoporosis. Int. J. Endocrinol., 1-26.
Martha, J.P.M., Rogol, A.D., Veldhuis, J.D., Kerrigan, J.R., Goodman, D.W. and Blizzard, R.M., 1989. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J. clin. Endocrinol. Metab., 60: 563-570. http://dx.doi.org/10.1210/jcem-69-3-563
Martha, J.P.M., Bilizzard, R.M., Thorner, M.O. and Rogol, A.D., 1990. Atenolol enhances nocturnal growth hormone (GH) release in GH-deficient children during long-term GH-releasing hormone therapy. J. clin. Endocrinol. Metab., 70: 56-61. http://dx.doi.org/10.1210/jcem-70-1-56
Martha, J.P.M., Gorman, K.M., Blizzard, R.M., Rogal, A.D. and Veldhuis, J.D., 1992. Endogenous growth hormone secretion and clearance rates in normal boys, as determined by deconvolution analysis, relationship to age, pubertal status and body mass. J. clin. Endocrinol. Metab., 74: 336-344. http://dx.doi.org/10.1210/jcem.74.2.1730812
Mauras, N., Rogol, A.D., Haymond, M.W. and Veldhuis, J.D., 1996. Sex steroids, growth hormone, insulin-like growth factor-1: neuroendocrine and metabolic regulation in puberty. Horm. Res., 45: 74-80. http://dx.doi.org/10.1159/000184763
McGillivray, M.H., Frohman, L.A. and Doe, J., 1970. Metabolic clearance and production rates of human growth hormone in subjects with normal and abnormal growth. J. clin. Endocrinol. Metab., 30: 632-638. http://dx.doi.org/10.1210/jcem-30-5-632
Meinhardt, U.J. and Ho, K.K.Y., 2006. Modulation of growth hormone action by sex steroids. Clin. Endocrinol., 65: 413-422. http://dx.doi.org/10.1111/j.1365-2265.2006.02676.x
Metzger, D.L. and Kerrigan, J.D., 1994. Estrogen receptor blockade with tamoxifen diminishes growth hormone secretion in boys: evidence for a stimulatory role of endogenous estrogens during male adolescence. J. clin. Endocrinol. Metab., 79: 513-518. http://dx.doi.org/10.1210/jcem.79.2.8045971
Miller, J.D., Tannunbaum, G.S., Colle, E. And Guyda, H.J., 1982. Daytime pulsatile growth hormone secretion during childhood and adolescence. J. clin. Endocrinol. Metab., 55: 989-994. http://dx.doi.org/10.1210/jcem-55-5-989
Mohan, S., Richman, C., Guo, R., Amaar, Y., Donahue, L.R., Wergedal, J. and Baylink, D.J., 2003. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology, 144: 929-936. http://dx.doi.org/10.1210/en.2002-220948
Murray, P.G. and Clayton, P.E., 2013. Endocrine control of growth. Am. J. med. Genet. C. Semin. med. Genet., 163: 76-85. http://dx.doi.org/10.1002/ajmg.c.31357
Nilsson, O., Chrysis, D., Pajulo, O., Boman, A., Holst, M., Rubinstein, J., Martin, E.R. and Savendahl, L., 2003. Localization of estrogen receptors-alpha and -beta and androgen receptor in the human growth plate at different pubertal stages. J. Endocrinol., 177: 319-326. http://dx.doi.org/10.1677/joe.0.1770319
Nilsson, O., Marino, R., De Luca, F., Phillip, M. and Baron, J., 2005. Endocrine regulation of the growth plate. Horm. Res., 64: 157-165. http://dx.doi.org/10.1159/000088791
Ojeda, S.R. and Jameson, H.E., 1997. Developmental patterns of plasma and pituitary growth hormone (GH) in the female rat. Endocrinology, 100: 881-887. http://dx.doi.org/10.1210/endo-100-3-881
Olsen, L.E., Ohlsson, C. and Mohan, S., 2011. The role of GH/IGF-1 mediated mechanisms in sex differences in cortical bone size in mice. Calcif. Tissue Int., 88: 1-8. http://dx.doi.org/10.1007/s00223-010-9436-2
Rizvi, S.S.R., Weinbauer, G.F., Arslan, M., Partsch, C.J. and Nieschlag, E., 2000. Testosterone modulates growth hormone secretion at the hypothalamic but not at the hypophyseal level in the adult male rhesus monkey. J. Endocrinol., 65: 337-344. http://dx.doi.org/10.1677/joe.0.1650337
Rogol, A.D., Clark, P.A. and Roemmich, J.N., 2000. Growth and pubertal development in children and adolescents: effect of diet and physical activity. Am. J. Clin. Nutr., 72: 521-528.
Rogol, A.D., 2002. Androgens and puberty. Mol. cell. Endocrinol., 198: 25-29. http://dx.doi.org/10.1016/S0303-7207(02)00365-9
Rogol, A.D., 2010. Sex steroids, growth hormone, leptin and the pubertal growth spurt. Endocrinol. Dev., 17: 77-85.
Rose, S.R. and Nisula, B.C., 1989. Circadian variation of thyrotropin in childhood. J. clin. Endocrinol. Metab., 68: 1086-1090. http://dx.doi.org/10.1210/jcem-68-6-1086
Rose, S.R., Municchi, G., Barnes, K.M., Kamp, G.A., Uriarte, M.M., Rose, J.L., Cassorla, F. and Cutler, Jr. G.B., 1991. Spontaneous growth hormone secretion increases during puberty in normal girls and boys. J. clin. Endocrinol. Metab., 73: 428-435. http://dx.doi.org/10.1210/jcem-73-2-428
Shim, K.H., 2015. Pubertal growth ad epiphyseal fusion. Annls. Pediatr. Endocrinol. Metab., 20: 8-12. http://dx.doi.org/10.6065/apem.2015.20.1.8
Sinnesael, M., Claessens, F., Boonen, S. and Vanderschueren, D., 2013. Novel insights in the regulation and mechanism of androgen action on bone. Curr. Opin. Endocrinol. Diabet. Obes., 20: 240-244. http://dx.doi.org/10.1097/MED.0b013e32835f7d04
Soliman, A., De Sanctis, V. and Elalaily, R., 2014. Nutrition and pubertal development. Indian J. Endocrinol. Metab., 18: 39-47. http://dx.doi.org/10.4103/2230-8210.145073
Sonntage, W.E., 1979. Luteinizing hormone releasing hormone and somatostatin in the anterior and posterior hypothalamus during development of the male and female rat. Doctoral Dissertation, Tulane University, New Orleans.
Steel, R.G.D. and Torrie, J.H. 1980. Principles and procedures of statistic - A biometriccal approach. McGraw Hill, New York, 2nd Edition.
Susan, Y.E., Selevan, S.G., Pescovitz, O.H. and Skakkebaek, N.E., 2008. Role of environmental factors in the timing of puberty. Pediatrics, 121: 167-171. http://dx.doi.org/10.1542/peds.2007-1813C
Tanner, J.M. and Whitehouse, R.H., 1976. Clinical longitudinal standards for height, weight, height velocity, weight velocity and stages of puberty. Arch. Dis. Child., 51: 170-179. http://dx.doi.org/10.1136/adc.51.3.170
Ulloa-Aguirre, A., Blizzard, R.M., Garcia-Rubi, E., Rogol, A.D., Link, K., Christie, C.M., Johnson, M.L. and Veldhuis, J.D., 1990. Testosterone and oxandrolone, a non-aromatizable androgen, specifically amplify the mass and rate of growth hormone (GH) secreted per burst without altering GH secretary burst duration or frequency or GH half-life. J. clin. Endocrinol. Metab., 71: 846-854. http://dx.doi.org/10.1210/jcem-71-4-846
Veldhuis, J.D., Metzger, D.L., Martha, P.M. Jr., Mauras, N., Kerrigan, J.R., Keenan, B., Rogol, A.D. and Pincus, S.M., 1997. Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)–insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J. clin. Endocrinol. Metab., 82: 3414-3420. http://dx.doi.org/10.1210/jc.82.10.3414
Veldhuis, J.D., Keenan, D.M., Mielke, K., Miles, J.M. and Bowers, C.Y., 2005. Testosterone supplementation in healthy older men drives GH and IGF-I secretion without potentiating peptidyl secretagogue efficacy. Eur. J. Endocrinol., 153: 577-586. http://dx.doi.org/10.1530/eje.1.02001
Venken, K., De Gendt, K., Boonen, S., Ophoff, J., Bouillon, R., Swinnen, J.V., Verhoeven, G. and Vanderschueren, D., 2006. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J. Bone Miner. Res., 21: 576-585. http://dx.doi.org/10.1359/jbmr.060103
Venken, K., Moverare-Skrtic, S., Kopchick, J.J., Coschigano, K.T., Ohlsson, C., Boonen, S., Bouillon, R. and Vanderschueren, D., 2007. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J. Bone Miner. Res., 22: 72-82. http://dx.doi.org/10.1359/jbmr.060911
Vingren, J.L., Kraemer, W.J., Ratamess, N.A., Anderson, J.M., Volek, J.S. and Maresh, C.M., 2010. Testosterone physiology in resistance exercise and training. Sports Med., 40: 1037-1053. http://dx.doi.org/10.2165/11536910-000000000-00000
Weissberger, A.J. and Ho, K.K., 1993. Activation of the somatotropic axis by testosterone in adult males: evidence for the role of aromatization. J. clin. Endocrinol. Metab., 76: 1407-1412. http://dx.doi.org/10.1210/jc.76.6.1407
Wilson, M.E., Gordon, T.P. and Collins, D.C., 1986. Ontogeny of luteinizing hormone secretion and first ovulation in seasonal breeding rhesus monkey. Brain Res., 248: 177-179. http://dx.doi.org/10.1016/0006-8993(82)91160-X
Wheeler, M.D. and Styne, D.N., 1988. The nonhuman primate as a model of growth hormone physiology in the human being. Endocr. Rev., 9: 213-245. http://dx.doi.org/10.1210/edrv-9-2-213
Wright, C.M., Williams, A.F., Elliman, D., Bedford, H., Birks, E., Butler, G., Sachs, M., Roy, R. and Cole, T.J., 2010. Using the new UK-WHO growth charts. Br. Med. J., 340: 647-650. http://dx.doi.org/10.1136/bmj.c1140
Zhang, J., Peddada, S.D., Malina, R.M. and Rogol, A.D., 2000. Longitudinal assessment of hormonal and physical alterations during normal puberty in boys VI. Modeling of growth velocity, mean growth hormone (GH mean) and serum testosterone (T) concentrations. Am. J. Hum. Biol., 12: 814-824. http://dx.doi.org/10.1002/1520-6300(200011/12)12:6<814::AID-AJHB9>3.0.CO;2-U
To share on other social networks, click on any share button. What are these?










