Acute Effect of Epidermal Growth Factor on Phosphate Diffusion across Intestinal Mucosa of Hens using the Ussing Chamber System
Acute Effect of Epidermal Growth Factor on Phosphate Diffusion across Intestinal Mucosa of Hens using the Ussing Chamber System
Xiaopeng Tang1,2, Rejun Fang3,4,*, Guochang Pan1,2 and Kangning Xiong1,2,*
1School of Karst Science, Guizhou Normal University, Guiyang, China
2State Key Laboratory Cultivation for Karst Mountain Ecology Environment of Guizhou Province, Guiyang, China
3College of Animal Science and Technology, Hunan Agricultural University, Changsha, China
4Hunan Co-Innovation Center of Animal Production Safety, Changsha, China
ABSTRACT
The aim of this study was to investigate the effect of epidermal growth factor (EGF) on phosphate absorption across intestinal mucosa of hens using the Ussing chamber system. First, four Loman laying hens with similar weight (1.25±0.13 kg) were sacrificed to detect the lactate dehydrogenase (LDH) activity of isolated small intestine at time on 0 min, 30 min, 60 min, 90 min and 120 min, to provide a appropriate operation time for Ussing chamber test. Then, a total of 20 Loman laying hens with similar weight (1.25±0.13 kg) were divided into 1 of 5 treatments: i) control group (0 ng/mL EGF), ii) 50 ng/mL EGF group, iii) 100 ng/mL EGF group, iv) 150 ng/mL EGF group, and v) 200 ng/mL EGF group for using chamber test. The results showed that (i) the LDH activity of isolated intestine increased (P < 0.05) sharply during 30 min, at 60 min, the LDH activity of isolated intestines reached a high level; (ii) EGF increased (P < 0.05) the transepithelial electrical resistance (TEER, Ω.cm2) significantly; (iii) EGF inhibited (P < 0.05) the phosphate diffusion across intestinal mucosa significantly. According to the results, the Ussing chamber system can use to evaluate phosphate diffusion across intestinal mucosa, and EGF may inhibit the absorption of phosphorus.
Article Information
Received 20 December 2018
Revised 12 January 2019
Accepted 06 February 2019
Available online 23 August 2019
Authors’ Contribution
XT and RF designed and performed the experiments. XT wrote the paper. GP and KX helped in writing the article.
Key words
Epidermal growth factor, Hens, Intestinal mucosa, Phosphate, Ussing chamber.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2209.2216
* Corresponding author: fangrj63@126.com;
xiongkn@163.com
0030-9923/2019/0006-2209 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Phosphate (Pi) is a key component of biological systems involved in a variety of physiologic processes including energy metabolism, cell signaling, nucleotide and phospholipids biosynthesis, and bone mineralization (Fang et al., 2012; Giral et al., 2012). Pi is absorbed by the epithelium of the small intestine via both a passive diffusion and an active sodium-dependent process (Penido and Alon, 2012; Fang et al., 2016). Previous studies have demonstrated that the active absorption of Pi across intestinal epithelium is mainly mediated by type IIb sodium-dependent transport (NaPi-IIb) protein (Xiang et al., 2012; Fang et al., 2012, 2016).
EGF is a small mitogenic polypeptide comprising 53 amino acid residues, which has established as a trophic factor for the intestinal maturation, epithelial cell homeostasis, and ions transport in the small intestine (Trapani et al., 2014; Tang et al., 2016, 2018a). Previous studies have demonstrated that EGF inhibited the expression of NaPi-IIb (Xu et al., 2001, 2003; Xing et al., 2017; Tang et al., 2018b), which implied that EGF inhibited the active absorption of Pi. However, there is no direct evidence that EGF can regulate phosphorus absorption in intestine. So, the present study would adopt an in vitro method (Ussing chamber system) to study the effect of EGF on phosphorus absorption.
The Ussing chamber system was first invented by Hans Ussing (Ussing and Zerahn, 1951), which has several components, including a circuit system, diffusion chambers, inserts, electrodes (Fig. 1), a data collection system, and a software support system (He et al., 2013). The Ussing chamber provides a physiologically relevant system for measuring the gastrointestinal epithelium permeability, gastrointestinal barrier function, and the transport of ions, nutrients and drugs across intestinal epithelial tissues (Maresca et al., 2002; Millar et al., 2002; Albin et al., 2007; Clarke, 2009; He et al., 2013). There are published studies involving the use of Ussing chambers to determine whether EGF affects glucose transport in the jejunum (Lennernäs et al., 1997; Millar et al., 2002), while, the study of EGF affects Pi transport using Ussing chamber is lacking. So, the aim of this study was to investigate the effect of EGF on phosphate absorption across intestinal mucosa of hens using the Ussing chamber system.
Materials and methods
Animals
All animal studies were approved by the Animal Care Committee of Guizhou Normal University (Guiyang, China). The experimental procedures were conducted in accordance with the Chinese guidelines for animal welfare.
Loman laying hens with similar weight (1.25±0.13 kg) were purchased from Hunan Institute of Animal Science and Veterinary Medicine (Changsha, China).
LDH activity determination
The release of LDH into the buffer can reflect the activity of isolated small intestine (Todd et al., 2016; Fadda et al., 2017; Tang et al., 2018a). To detect the LDH activity of isolated small intestine, four hens were sacrificed after 12 h of starvation. A 5 cm of duodenum (from distal of the gizzard to 1 cm distal of the bile duct), jejunum (1 cm distal from the bile duct to the Meckel’s diverticulum), and ileum (Meckel’s diverticulum to 5 cm proximal to the ileocecal junction) (Fang et al., 2012) were opened longitudinally, and immersed in a dish containing 20 mL Hepes-Tris buffer (6 g Hepes, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 8.18 g NaC1, adjust the pH to 7.4 using 1 M Tris), respectively. At 0 min, 30 min, 60 min, 120 min and 180 min, 0.5 mL buffer was collected to analysis LDH activity. The determination of LDH activity was used a Lacate Dehydrogenase Assay kit (No. A020-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the manufacturer. Experiments were performed in four times.
Ussing chamber assay
A total of 20 Loman laying hens with similar weight (1.25±0.13 kg) were divided into 1 of 5 treatments: (i) Control group (0 ng/mL EGF), (ii) 50 ng/mL EGF group, (iii) 100 ng/mL EGF group, (iv) 150 ng/mL EGF group, and (v) 200 ng/mL EGF group. Each treatment had 4 replicates with one hen. All hens were fasted for 12 h before sacrificed. The duodenum, jejunum, and ileum were obtained immediately after slaughter for Ussing chamber studies. The Ussing chamber system (VCC MC6) was manufactured by Physiologic Instruments, Inc. (San Diego, CA, USA).
After the removal of muscle layers and serosal layer, the remaining mucosa samples were mounted between the two halves of Ussing chambers inserts (P2313) with an exposed serosal area of 0.71 cm2. Mucosal compartments were filled with 4 mL Hepes-Tris buffer (pH 7.4) containing 0 ng/mL EGF (Peprotech, Rocky Hill, NJ, USA), 50 ng/mL EGF, 100 ng/mL EGF, 150 ng/mL EGF, 200 ng/mL EGF, respectively, and serosal compartments filled with 4 mL Hepes-Tris buffer (pH 7.4) simultaneously, circulated with carbogen gas (95% O2, 5% CO2) at a temperature of 37°C. After an equilibration period of 10 min, 1 mL of 0.35% Pi solutions was added into mucosal compartment, and 1 mL of 0.35% mannitol solution was added into serosal compartment. After 45 min of incubation, 0.5 mL of samples was collected from both compartments for Pi concentration analysis to calculate the absorption rate of Pi across intestinal mucosa. The determination of Pi concentration was use a Phosphate Assay Kit (No. C006, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the manufacturer. During the experiment, TEER was monitored during the whole experimental period using a computer controlled voltage clamp device (Physiologic Instruments Inc., San Diego, CA, USA). Experiments were performed in four times. The absorption rate of Pi was calculated by following equations:
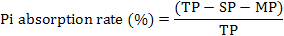
Where TP is total Pi content, SP is the Pi content in serosal compartment, and MP is the Pi content in mucosal compartment.
Statistical analysis
All data were expressed as mean±standard deviation (SD). Data for single factor experiments were performed by one-way ANOVA procedure of SPSS 21.0 software (SPSS, Inc., Chicago, IL, USA). Differences among treatment mean were determined using Duncan’s multiple comparison test. Linear correlation analyses between EGF concentrations and TEER, and Pi absorption rate were performed by linear regression procedure of GraphPad Prism 7.0 software (Graph-pad Software Inc., San Diego, USA). P < 0.05 was considered significant.
Results and discussion
The LDH activity of isolated small intestine
LDH is a stable glycolytic enzyme, when cells or organs suffered from stress, the cell membrane permeability would change, and LDH can be quickly released (Todd et al., 2016). So the release of LDH into the Hepes-Tris buffer can reflect the activity of isolated small intestine. As shown in Figure 2, the LDH activity of isolated duodenum (Fig. 2A), jejunum (Fig. 2B), and ileum (Fig. 2C) increased sharply during 30 min (P < 0.05). After 60 min, the LDH activity of isolated intestines reached a high levels, it indicated that the activity of isolated intestines is weaken. So the Ussing chamber experiment should finished in 60 min. In the present study we incubated the isolated small intestine in chambers for 45 min.
Effects of EGF on TEER
The intestinal mucosal plays an essential role in the separation of the inside of the body from the outside environment (Oshima and Miwa, 2016). The integrity of intestinal is the foundation of nutrition absorption for animals, and can be evaluated through the measurement of the TEER (Garcia-Hernandez et al., 2015). TEER cans reflect the barrier function of intestine (Chen et al., 2015). Hence, the measurement of TEER has been mainly applied for assessing the permeability of tight junctions or the membrane perturbation by toxicants on intestinal epithelium (Chen et al., 2015). The Ussing chamber model is one of in vitro methods to measure TEER (Li et al., 2004; He et al., 2013). In the present study, the TEER of duodenum (Fig. 3A), jejunum (Fig. 3B) and ileum (Fig. 3C) treated with EGF (50, 100, 150, 200 ng/mL) both higher (P < 0.05) than that control group (none EGF); in duodenum (Fig. 3A) and jejunum (Fig. 3B), the 100, 150 and 200 ng/mL EGF group had a higher (P < 0.05) TEER than that 50 ng/mL EGF group; in ileum (Fig. 3C)
100 ng/mL EGF group had a higher (P<0.05) TEER than that 50, 150 and 200 ng/mL EGF group. Both in duodenum (Fig. 3A), jejunum (Fig. 3B) and ileum (Fig. 3C) the EGF concentration had no liner relationship between EGF concentration and TEER (P>0.05). The results of present study indicated that EGF had a protective effect on the maintenance of intestinal integrity. Previous studies have demonstrated that EGF had the ability to increase TEER, and decrease epithelial paracellular permeability (Basuroy et al., 2006; Flores-Benitez et al., 2009; Garcia-Hernandez et al., 2015). The present study also confirmed that EGF can increase the TEER of isolated small intestines, which means that EGF plays an important role in maintaining intestinal integrity.
Effects of EGF on phosphate diffusion
Phosphorus is one of most important mineral element in life process (Fang et al., 2012; Giral et al., 2012). It is well know that Pi is mainly absorbed by simple diffusion and active absorption, and the active absorption of Pi is mainly mediated by NaPi-IIb (Forster et al., 2012; Xiang et al., 2012; Fang et al., 2012, 2016). Previous studies have demonstrated that EGF inhibited the expression of NaPi-IIb (Xu et al., 2001, 2003; Xing et al., 2017; Tang et al., 2018b). It means that EGF inhibited the active absorption of Pi in intestines. But whether EGF would promote simple diffusion of Pi is not know. The Ussing chamber technique is a simple, but powerful technique to investigate ion transport (Li et al., 2004). While, the study of EGF affects Pi transport using Ussing chamber is lacking. Hence, this study used Ussing chamber technique to study the effects of EGF on Pi absorption, to clarify the influence of EGF on phosphorus absorption. The results of Pi absorption rate was presented in Figure 4. It showed that the addition of EGF (50, 100, 150, 200 ng/mL) in Hepes-Tris buffer significantly (P<0.05) inhibited the diffusion of Pi from mucosal compartment to serosal compartment both in duodenum (Fig. 4A), jejunum (Fig. 4B) and ileum (Fig. 4C). However, both in duodenum (Fig. 4A), jejunum (Fig. 4B) and ileum (Fig. 4C) the EGF concentration had no liner relationship between EGF concentration and Pi absorption rate (P > 0.05). The reasons of EGF inhibited the diffusion of Pi may be interpreted from the following two aspects: firstly, EGF has trophic effects on intestine (Bedford et al., 2015; Xu et al., 2015). It can protect intestinal mucosa from exogenous injury to some extent in short times, which confirmed by the increase of TEER in the present study. So, the permeability of intestine was decreased, which resulted in a decreased Pi diffusion. Secondly, as previous studies showed, EGF inhibited the expression of NaPi-IIb (Xu et al., 2001, 2003; Xing et al., 2017; Tang et al., 2018b). It means that EGF inhibited the active absorption of Pi in intestines, which also resulted in a decreased Pi absorption rate.
Conclusion
In summary, the results of this research suggested that: (i) it’s better finished in 60 min when used the Ussing chamber system to evaluate the absorption of nutrients in small intestine; (ii) EGF enhanced the TEER of intestinal mucosa and inhibited the diffusion of Pi.
Acknowledgements
This research was supported by grants from the National Top Discipline Construction Project of Guizhou Province: Geography in Guizhou Normal University (85 2017 Qianjiao Keyan Fa) and the Project of Innovation Program for Postgraduate Education of Guizhou Province: Xiong Kangning`s studio of postgraduate supervisors for the karst environment of Guizhou Province (04 2016 Qianjiao Yanhe GZS Zi).
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Albin, D.M., Wubben, J.E., Rowlett, J.M., Tappenden, K.A. and Nowak, R.A., 2007. Changes in small intestinal nutrient transport and barrier function after lipopolysaccha ride exposure in two pig breeds. J. Anim. Sci., 85: 2517-2523. https://doi.org/10.2527/jas.2006-237
Basuroy, S., Seth, A., Elias, B., Naren, A.P. and Rao, R., 2006. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J., 393: 69-77. https://doi.org/10.1042/BJ20050959
Bedford, A., Chen, T., Huynh, E., Zhu, C., Medeiros, S., Wey, D., de Lange, C. and Li, J., 2015. Epidermal growth factor containing culture supernatant enhances intestine development of early-weaned pigs in vivo: Potential mechanisms. J. Biotechnol., 196-197: 9-19. https://doi.org/10.1016/j.jbiotec.2015.01.007
Chen, S., Einspanier, R. and Schoen, J., 2015. Transepithelial electrical resistance (TEER): A functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol., 144: 509-515. https://doi.org/10.1007/s00418-015-1351-1
Clarke, L.L., 2009. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol., 296: 1151-1166. https://doi.org/10.1152/ajpgi.90649.2008
Fadda, L.M., Al-Rasheed, N.M., Hasan, I.H., Ali, H.M. and Khalaf, R., 2016. Bax and cd68 expression in response to liver injury induced by acetaminophen: The hepatoprotective role of thymoquinone and curcumin. Pakistan J. Zool., 49: 85-93. https://doi.org/10.17582/journal.pjz/2017.49.1.85.93
Fang, R., Xiang, Z., Cao, M. and He, J., 2012. Different phosphate transport in the duodenum and jejunum of chicken response to dietary phosphate adaptation. Asian-Australas. J. Anim. Sci., 25: 1457-1465. https://doi.org/10.5713/ajas.2012.12187
Fang, R., Xiang, Z., Hu, L., Su, W., Tang, X. and Wang, X., 2016. Effects of mechanistic target of rapamycin signaling pathway on the estrogen-mediated NaPi-IIb protein expression in pig small intestinal epithelial cells. J. Anim. Sci., 94: 303-306. https://doi.org/10.2527/jas.2015-9866
Flores-Benitez, D., Rincon-Heredia, R., Razgado, L.F., Larre, L., Cereijido, M. and Contreras, R.G., 2009. Control of tight junctional sealing: Roles of epidermal growth factor and prostaglandin E2. Am. J. Physiol. Cell. Physiol., 297: C611-C620. https://doi.org/10.1152/ajpcell.00622.2008
Forster, I.C., Hernando, N., Biber, J. and Murer, H., 2012. Phosphate transport kinetics and structure-function relationships of SLC34 and SLC20 Proteins. Curr. Top. Membr., 70: 313-356. https://doi.org/10.1016/B978-0-12-394316-3.00010-7
Garcia-Hernandez, V., Flores-Maldonado, C., Rincon-Heredia, R., Verdejo-Torres, O., Bonilla-Delgado, J., Meneses-Morales, I., Gariglio, P. and Contreras, R.G., 2015. EGF regulates claudin-2 and-4 expression through SRC and STAT3 in MDCK cells. J. cell. Physiol., 230: 105-115. https://doi.org/10.1002/jcp.24687
Giral, H., Cranston, D., Lanzano, L., Caldas, Y., Sutherland, E., Rachelson, J., Dobrinskikh, E., Weinman, E.J., Doctor, R.B., Gratton, E. and Levi, M., 2012. NHE3 regulatory factor 1 (NHERF1) modulates intestinal sodium-dependent phosphate transporter (NaPi-2b) expression in apical microvilli. J. biol. Chem., 287: 35047-35056. https://doi.org/10.1074/jbc.M112.392415
He, L., Yin, Y., Li, T., Huang, R., Xie, M., Wu, Z. and Wu, G., 2013. Use of the Ussing chamber technique to study nutrient transport by epithelial tissues. Front. Biosci., 18: 1266-1274. https://doi.org/10.2741/4178
Lennernäs, H., Nylander, S. and Ungell, A.L., 1997. Jejunal permeability: A comparison between the Ussing chamber technique and the single-pass perfusion in humans. Pharmaceut. Res., 14: 667-671. https://doi.org/10.1023/A:1012121632357
Li, H., Sheppard, D.N. and Hug, M.J., 2004. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros., 3: 123-126. https://doi.org/10.1016/j.jcf.2004.05.026
Maresca, M., Mahfoud, R., Garmy, N. and Fantini, J., 2002. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr., 132: 27-31. https://doi.org/10.1093/jn/132.9.2723
Millar, G.A., Hardin, J.A., Johnson, L.R. and Gall, D.G., 2002. The role of PI3-kinase in EGF-stimulated jejunal glucose transport. Can. J. Physiol. Pharm., 80: 77-84. https://doi.org/10.1139/y02-012
Oshima, T. and Miwa, H., 2016. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol., 51: 768-778. https://doi.org/10.1007/s00535-016-1207-z
Penido, M.G. and Alon, U.S., 2012. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol., 27: 2039-2048. https://doi.org/10.1007/s00467-012-2175-z
Tang, X., Liu, H., Yang, S., Li, Z., Zhong, J. and Fang, R., 2016. Epidermal growth factor and intestinal barrier function. Mediat. Inflamm., 2016: 1927348. https://doi.org/10.1155/2016/1927348
Tang, X., Liu B., Wang, X., Yu, Q. and Fang, R., 2018a. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int. J. mol. Sci., 19: 848. https://doi.org/10.3390/ijms19030848
Tang, X., Xu, R., Li, C., Peng, P., Yu, Q. and Fang, R., 2018b. Effects of epidermal growth factor on intestinal type IIb sodium-phosphate cotransporter expression of weaned piglets challenged by lipopolysaccharide. Chinese J. Anim. Nutri., 30: 4020-4027.
Todd, K., Ghiso, J. and Rostagno, A., 2016. Oxidative stress and mitochondria-mediated cell death mechanisms triggered by the familial Danish dementia ADan amyloid. Neurobiol. Dis., 85: 130-143. https://doi.org/10.1016/j.nbd.2015.10.003
Trapani, V., Arduini, D., Luongo, F. and Wolf, F.I., 2014. EGF stimulates Mg2+ influx in mammary epithelial cells. Biochem. biophys. Res. Commun., 454: 572-575. https://doi.org/10.1016/j.bbrc.2014.10.125
Ussing, H.H. and Zerahn, K., 1951. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand., 23: 110-127. https://doi.org/10.1111/j.1748-1716.1951.tb00800.x
Xiang, Z., Fang, R., Hu, L. and Su, W., 2012. Molecular cloning and functional characterization of swine sodium dependent phosphate cotransporter type IIb (NaPi-IIb) gene. Mol. biol. Rep., 39: 10557-10564. https://doi.org/10.1007/s11033-012-1941-0
Xing, T., Tan, X., Yu, Q., Yang, T. and Fang, R., 2017. Identifying the location of epidermal growth factor-responsive element involved in the regulation of type IIb sodium-phosphate cotransporter expression in porcine intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr., 101: 1249-1258. https://doi.org/10.1111/jpn.12645
Xu, H., Collins, J.F., Bai, L., Kiela, P.R. and Ghishan, F.K., 2001. Regulation of the human sodium-phosphate cotransporter NaPi-IIb gene promoter by epidermal growth factor. Am. J. Physiol. Cell Physiol., 280: C628-C636. https://doi.org/10.1152/ajpcell.2001.280.3.C628
Xu, H., Inouye, M., Hines, E.R., Collins, J.F. and Ghishan, F.K., 2003. Transcriptional regulation of the human NaPi-IIb cotransporter by EGF in Caco-2 cells involves c-myb. Am. J. Physiol. Cell Physiol., 28: C1262-C1271. https://doi.org/10.1152/ajpcell.00456.2002
Xu, S., Wang, D., Zhang, P., Lin, Y., Fang, Z., Che, L. and Wu, D., 2015. Oral administration of Lactococcus lactis-expressed recombinant porcine epidermal growth factor (rpEGF) stimulates the development and promotes the health of small intestines in early-weaned piglets. J. appl. Microbiol., 119: 225-235. https://doi.org/10.1111/jam.12833
To share on other social networks, click on any share button. What are these?










