Advances in Animal and Veterinary Sciences
Research Article
Influence of Probiotic Supplementation on Oxidative Stress and Lipid Disorders in Quail
Enas A. Nosser1, Marwa I. Khalifa2*
1Department of Biochemistry, Faculty of Veterinary Medicine, Aswan University, 81528 Sahary City, Egypt; 2Department of Food Hygiene, Faculty of Veterinary Medicine, Aswan University, 81528 Sahary City, Egypt.
Abstract | Oxidative stress and ROS generation which induced via free radicals which up-raised by consumption of oxidized oils rich in polyunsaturated fatty acids, triggers inflammatory changes, DNA damage and oxidative induced tissue damage mainly in liver and kidney. Several feed additives were used to counteract such type of cellular stressors especially those which have antioxidant properties. In our current study, we checked the benefits of using lactobacillus acidophilus which are considered as feed additives and a novel inhibitor of food-induced oxidative stress. Our results confirmed the nobility of the lactobacillus acidophilus in counteracting and prevention of all cellular damage especially in liver and kidney which induced by oxidized oils consumed by quails. Furthermore its ability to reduce cholesterol levels in the blood and creating a balance between the pro-oxidant and the antioxidant levels. Our results strongly suggested the lactobacillus acidophilus can act as a potent prophylactic inhibitor of the cellular oxidative stress induced by the oxidized oils in food.
Keywords | Antioxidants, Oxidative stress, Biochemical parameters, Lipid profile, Probiotic, Quail.
Received | October 30, 2019; Accepted | January 17, 2020; Published | April 15, 2020
*Correspondence | Marwa I. Khalifa, Department of Food Hygiene, Faculty of Veterinary Medicine, Aswan University, 81528 Sahary City, Egypt; Email: [email protected]
Citation | Nosser EA, Khalifa MI (2020). Influence of probiotic supplementation on oxidative stress and lipid disorders in quail. Adv. Anim. Vet. Sci. 8(5): 478-483.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.478.483
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Nosser and Khalifa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oxidative stress (OS) induced due to the imbalance occurs between pro-oxidants and antioxidants. The free radicals, reactive oxygen species (ROS) and aldehydic products are a natural products of normal cell metabolism and vital for living organism homostasis (Ray et al., 2012). Nonetheless, excessive secretion of those chemical reactive substances and toxins produced from OS are harmful to body cells via destruction of cell membrane components, DNA damage, protein denaturation, and lipid per-oxidation. Moreover the inability of the body to detoxify and getting rid of these products which ends with harmful and genotoxic effects of the body cells (Katerji et al., 2019).
Cellular oxidative stress strongly initiated by consuming oxidized oils. Oxidized oils contain high levels of polyunsaturated fatty acids such as linoleic acid or arachidonic acid which induces free radicals production, ROS generation, aldehydic compounds, and chemical toxins, in which those products collectively attack important macromolecules causing destructions to cellular membrane constituents especially phospholipids, which undergoes per-oxidation, this lipid per-oxidation promoted via up-regulated levels of malanialdehyde (MDA) (Liang et al., 2015) Oxidative stress leading to the development of many hazardous conditions including cardiac disease, neoplasms, diabetes, Parkinson’s disease, neurological disorders, and aging. Additionally, there are many other biomarkers of metabolic oxidative status such as total antioxidant capacity (TOC) which is decreased with increased lipid peroxides (Tan et al., 2018).
The severity of liver injury from free radicals resulted from consumption of oxidized oil by quail which could be evaluated by De Ritis ratio (AST/ALT) as perfect index of hepatic fibrosis than absolute evaluation of transaminase enzymes in serum of living organisms (Senanayake et al., 2015).
To control OS several additives were used in food and feed, especially additives which have antioxidant properties such as Vit A, Vit C, tocopherols, selenium, polyphenols, palmitic acid and sesamol (Parkash & Kumar 2010).
Probiotics nowadays announced to hamper OS. As probiotics are pure cultures of normally inhabitant microorganisms isolated from milk of different ruminant species and colonized aseptically to be served as food or feed additives for its beneficial health values (Khalifa and Noseer, 2019).
Huss et al 2008 have been used the quails instead of mice as an experimental model in research plans due to ease housing and management in collecting the blood samples besides it is economic.
This experiment was designed to evaluate the influence of probiotic against OS generated by oxidized oils in quail ration.
Materials and Methods
Experimental Design
All experimental procedures were reviewed and approved by the Animal Care and Use Committee of Aswan University, Aswan, Egypt.
A total number of 45 quails aged 15 days old were introduced and housed together for 5 days and feeding on the same basal diet. Then the 45 birds were divided into three equal groups (n= 15), the first group fed on the basal diet and act as control, the 2nd group (OXO) received basal diet mixed with oxidized oil (2%) and the 3rd group (OXP) fed on oxidized oil 2% of basal diet plus probiotic in water in a dose of 2 g/L.
All groups were housed in clean cages at temperature arranged between 25:27 ºC.
The oxidized oil was added to diet as a mean of developing and generating OS.
Oxidized Oil
The oxidized oil was prepared thermally by heating at 180 Cº for 48 hours in a 1000 ml beaker in the oven and preserved at -20 °C. The chemical and physical analytical tests were performed to compare between fresh oil and thermally oxidized oil as Iodine Value. Through titration of fresh and oxidized oil against iodine. (Shafaeizadeh et al., 2011).
Probiotics Source
The probiotics used in this experiment (consists of mixture of lactobacillus acidophilus 6 billion/cfu, Saccharomyces cervices 2 billion/cfu in a water soluble dextrose carrier) was purchased commercially and added to the drinking water as recommended dose.
Ration Formation
The basal ration used in this experiment is in referring to that used by (Khalifa and Noseer, 2019).
Sample Collection
The blood samples were collected from heart puncture of a live quail at age of 25, 35 & 45days with 10 days intervals. The blood samples were leaved to coagulate and centrifuged at 3000 rpm, and then the serum obtained and admitted for biochemical determination by micro lab 300 devices (ID 6788483548 France).
Biochemical Parameters
The next tests were applied on serum for evaluation of the probiotic effect against OS. Cholesterol, alanine transaminase (ALT) and aspartic acid transaminase (AST) as indicators to liver function, Kidney function expressed as creatinine & uric acid ,Oxidative stress biomarkers malonialdehyde (MDA) and total antioxidant capacity (TOC).
Cholesterol
Cholesterol measured by using 10ul of serum + 1ml of reagent at 546 nm wave length. Spectrum kits (Young et al., 1975).
Liver Functions
Liver enzymes (ALT& AST) measured by using 100ul of serum + 1ml of working reagent for both ALT & AST at wave length 340 spectrum kits (Zilva & Pannall, 1979).
Kidney Function
Kidney test uric acid investigated by 1ml of serum + 1ml of reagent at wave length 546 and creatinine measured by 100 ul of serum + 1ml of working reagent at wave length 492 (Tietz, 1986) spectrum kits.
Oxidative Stress
MDA was measured in serum by mixing 0.2 ml +1ml of chromagen boil in water bath for 30 min and read against 534 wave length (Ohkawa et al., 1979). According to explained in Biodignostic commercial kits.
TOC was measured in serum by incubating 0.02 ml+0.5 R1 for 5 min at 37ºC and add 0.5 of working reagent at 505 wave length (Koracevic et al., 2001).
Statistical Analysis
Data, bars and markers in the figures were represent the mean ± s.d. We used the two-tailed Student’s t-test, we compared each of a number of treatments with a single control. Differences were considered statistically significant when the P value and the significance were measured (** p < 0.01, * p < 0.05 vs. OXO).
Results and Discussion
Addition of the probiotics is a novel prophylactic way for avoiding the excess generation of ROS, hydrogen peroxides, other toxins and cellular stressors via promotion of beneficial microorganism in gut so it demonstrated as natural antioxidants (Mishra et al., 2015). Probiotics are member of live micro-organisms safe species exerts general beneficial health effects according to (FAO/WHO 2002). Lactobacillus species are considered as a beneficial example which exceed more than 50 species, and can be taken from fermented food like yogurts and dietry supplements .moreover lactobacillus species are mostly efficient for poultry (Bernardeau & Vernoux 2013). Probiotics bacteria have multiple therapeutic effects especially for regulating gut function. Probiotics are able to lower the cholesterol level due to inhibition of hydroxymethyl-glutaryl-coenzyme A (HMG COA), which is a key regulatory enzyme for cholesterol biosynthesis and converts cholesterol in the intestine to coprostanol which easily excreted (Khalifa and Noseer, 2019).
In this study the total cholesterol was increased in the quails feed on oxidized oil (OXO), while the group fed diet with probiotics (OXP) showed lower levels almost nearby to control group Figure 1.
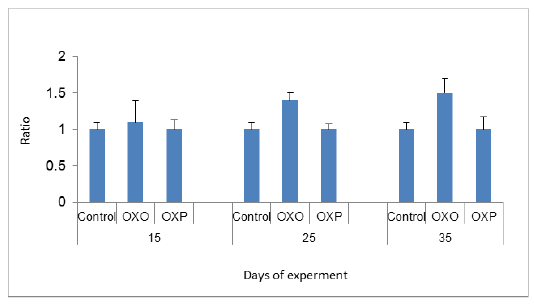
Figure 1: Cholesterol levels in different groups, P value and the significance was measured (* p < 0.05 vs. OXO).
Which confirmed by Shafaeizadeh et al., (2011); where they reported that blood cholesterol levels markedly increased after a diet rich in oxidized oils. The kidney function was estimated in all quail groups revealed that decreased creatinine level in probiotics treated group (OXP) in compared to (OXO) group indicated the ability of probiotics to get rid of toxins including creatinine ,thus protecting renal cells. Consequently the Uric acid level decreased in probiotics groups (OXP). Haque et al 2017 reported similar findings. Figure 2 & 3.

Figure 2: Uric acid ratio in different groups across days of experiment. P value and the significance was measured (* p < 0.05 vs. OXO).
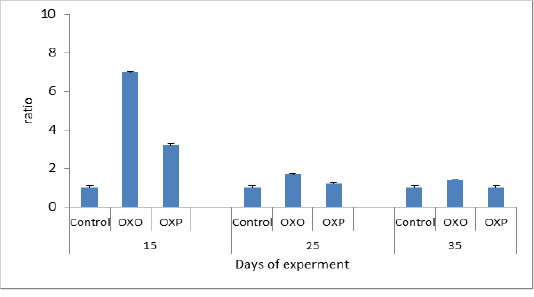
Figure 3: Creatinine ratio in different groups across experiment. P value and the significance was measured (** p < 0.01 vs. OXO).
In this study the oxidized oil found to be exerted harmful effects causing several alterations of lipid metabolism and also decreases activities of antioxidant enzymes and the major organ involved in lipid metabolism is the liver. Figure 4 and 5 measuring indicator enzymes of liver function tests such as alanine transaminase (ALT) and aspartic acid transaminase (AST) levels in serum of treated group with oxidized oil without antioxidants (OXO) was increased indicating negative liver function and consequently liver damage, while Oral administration of Lactobacillus acidophilus (OXP) significantly reduced the ALT and AST levels in serum of mice (Lin et al., 2018). Thermally treated oil can convert fatty acid configuration from cis to trans isomers. Trans isomers increases the incidence of hypertension and through on the broad line lead to cardiovascular disease (Bryk et al., 2011). We confirmed the decrease of cardiac biomarker AST,which is decreased in OXO group in compare to OXP & control group. As well as Haque et al. (2017) confirmed that decreased levels of transaminase enzymes ALT and AST indicated less or no liver damage while treated group with the oxidized oil (OXO) caused nephrotoxicity and hepato-toxicity seen by increased level of liver and kidney functions. nephrotoxicity and hepatotoxicity seen by increased level of liver and kidney functions. Therefore increased AST/ALT ratio predictive of the progression of fibrosis especially with AST elevated levels in non -alcoholic fatty liver diseases (NAFLD). (Botros and Sikria, 2013). Senanayake et al. (2015) found that mathematical transaminase ratio of AST/ALT increased with age especially in ALT in control and OXP treatment groups due to high metabolic demand by hepatocyte than OXO treated due to liver damage. Only ALT levels predict the progression to metabolic syndrome. While in the OXO treated group (Figure 6) the AST/ALT ratio had lower due to liver damage and it worth noting that there weren’t any general accepted reference of De Ritis ratio in birds.
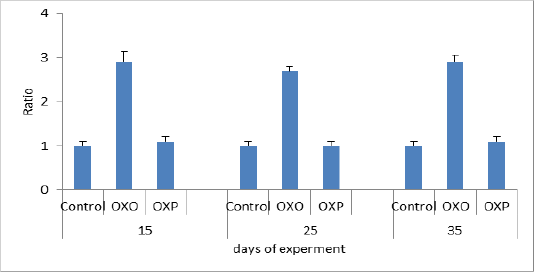
Figure 4: ALT ratio in different groups across experiment. P value and the significance was measured (** p < 0.01 vs. OXO).
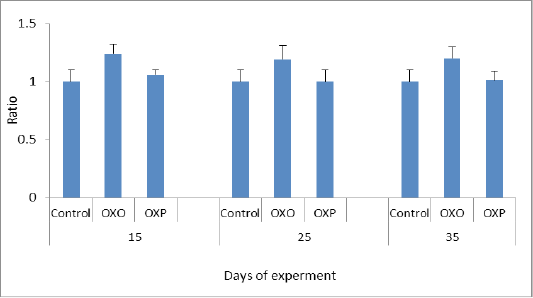
Figure 5: AST ratio in different groups across experiment. P value and the significance was measured (* p < 0.05 vs. OXO).
Most antioxidants are heat labile and thermal heated oil more prone to oxidation leading to disturbance of endogenous antioxidants of living organisms & couldn’t fight the oxidative stress. The mechanism of antioxidant system is to combine with free radicals, and so inactivated them thus increment of the free radicals intracellular concentration and increase the TOC (Mielnik et al., 2003). So increasing MDA levels in our study related to increase oxidative stress of OXO group in compared to OXP & control groups of quail may be triggerd by free radicals (Aytekin et al., 2015, Esgalhado et al., 2015). Also feeding oxidized oil lower the efficiency of antioxidant enzymes system and so decrease (TOC) & lower the capacity of scavenging free radicals, causing metabolic oxidative stress in quail consumed ration supplemented with oxidized oil (OXO) group in countrary to OXP & control groups. (Liang et al., 2015) (Figure 8).
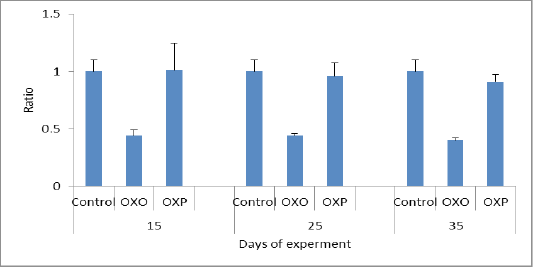
Figure 6: De rittis coefficient
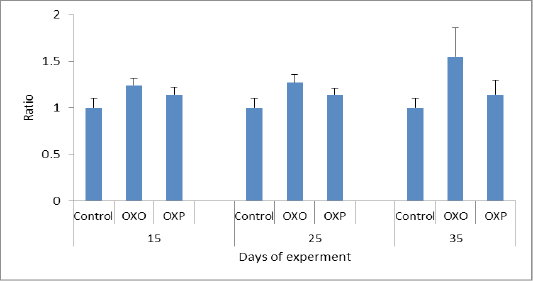
Figure 7: MDA ratio in different groups across the experiment. MDA P value and the significance was measured (* p < 0.05 vs. OXO).
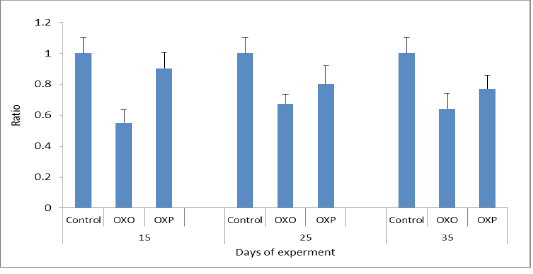
Figure 8: TAC ratio in different groups across the experiment. P value and the significance was measured (** p < 0.01 vs. OXO).
Tan et al. (2018) had been referred to effect of lipid per-oxidation caused by oxidized oil in ration which known via the elevated levels of thiobarbituric acid reactive substances (MDA) in the internal organs which causing vigorous damage to these organs and the inflammatory changes which occurs and lower the ability to counteract the free radicals. Our study agreed with such hypothesis as we proven that; the quail fed only on the oxidized oils (OXO) showed remarkable and significant decrease in the antioxidant capacity in compare with the control group and the group which fed on probiotics plus oxidized oils (OXP) Figure 7. Thus MDA level measured in which considered as maker of oxidative stress-induced liver injury and oral administration of lactobacillus species inhibited increase of MDA level in serum or liver of oxidized oil induced oxidative stress mice (Lin et al., 2018).
Conclusions
The probiotics especially lactobacillius spp have a significant effect in reducing oxidative stress. Probiotics must be provided in daily diets in order to preserve liver vitality. In this study we proven the efficacy of using the probiotic lactobacillus spp as a prophylactic feed additives to counteract the ROS generation and the cellular damage which induced by the free radicals produced by the oxidized oils in the ration.
A further work still needed to declare the actual mechanism by which the probiotic lactobacillus blocking the ROS generation via molecular analysis ROS/ antioxidant pathways and its gene regulation especially its role in regulation of the NRF2 gene which regulates the expression of antioxidant proteins that protect against oxidative damage triggered by ROS generation.
acknowledgements
The authors acknowledged Dr. Ebed M. Ahmed for his assistance in the analyzing of data.
conflict of interest
The authors declared that there is no conflict of interest.
authors contribution
Enas Nosser, was raring the experimental animals, collecting and analyzing the samples. Marwa Khalifa designed the experiment, time and type of samples, provided the probiotic strains and doses, both authors were sharing in writing, revision, and analyzing the data of the manuscript. All authors have delegated to Marwa Khalifa to undertake responsibility for publication and correspondence to the AAVS journal. All authors approved the final version of the manuscript for publication.
REFERENCES
Featuring
-
The Effects of Replacing Fish Meal with Skipjack Tuna Offal Meal on the Growth Performance and Carcase Quality OF Local Chickens
Srisukmawati Zainudin, Hartutik, Edhy Sudjarwo, Osfar Sjofjan
Adv. Anim. Vet. Sci., Vol. 13, Iss. 1, pp. 43-50
-
Antibiotic Resistance in Lactic Acid Bacteria Isolated from Vietnamese Fermented Foods
Trang Nguyen Phan, Loc Bao Nguyen, Thi Anh Ngoc Tong
Adv. Anim. Vet. Sci., Vol. 13, Iss. 1, pp. 36-42
-
Impact of Fig-Enriched Tris-Albumin Extender on the Quality of Boer Buck Spermatozoa
Lalu Ahmad Zaenuri, Rodiah Rodiah
Adv. Anim. Vet. Sci., Vol. 13, Iss. 1, pp. 26-35
-
Understanding Smallholder Beef Production: Cost Structures, Market Channels, and Pathways to Enhanced Profitability
Wiranut Thannithi, Payungsuk Intawicha, Nattamaporn Kongmuang, Wilasinee Inyawilert, Attapol Tiantong, Sureeporn Saengwong
Adv. Anim. Vet. Sci., Vol. 13, Iss. 1, pp. 16-25
Subscribe Today
Receive free updates on new articles, opportunities and benefits

© 2024 ResearchersLinks. All rights Reserved. ResearchersLinks is a member of CrossRef, CrossMark, iThenticate.





