Advances in Animal and Veterinary Sciences
Short Communication
Native Breed of Chicken Entertains Different B cell Target Antigens than Popular Breeds for Protection against Pathogenic E. coli
Sudip Nandi1, Siddhartha Narayan Joardar1*, Indranil Samanta1, Bithi Roy1, Pradip Kumar Das2, Tapas Kumar Sar3, Seikh Sahanawaz Alam1
1Department of Veterinary Microbiology; 2Department of Veterinary Physiology (RKVY Laboratory); 3Department of Veterinary Pharmacology, Faculty of Veterinary and Animal Sciences, West Bengal University of Animal and Fishery Sciences, 37, Kshudiram Bose Sarani, Post Office- Belgachia, Kolkata-700037, West Bengal, India.
Abstract | To determine the B-cell target antigens of virulent E. coli that might be exploited to protect itself, serological responses were assessed in one of the best-adapted exotic breeds of backyard system (Rhode Island Red, RIR), one native breed (Haringhata Black, HB), and one commercial strain (broiler) upon experimental inoculation of virulent E. coli. Variation in detection of B cell target antigens of E. coli was observed in HB, RIR and broiler birds by Western blotting. It is concluded that B cell target antigens (78, 66, 43, 29, 5 kDa) are different in native birds against virulent E. coli that might be the driving factor of disease resistance as opposed to RIR and broiler birds where clinical symptoms were imminent.
Keywords | Broiler, Disease resistance, E. coli, Haringhata black, Poultry, Rhode Island Red
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 27, 2016; Accepted | June 12, 2016; Published | June 16, 2016
*Correspondence | SN Joardar, Department of Veterinary Microbiology, Faculty of Veterinary and Animal Sciences, West Bengal University of Animal and Fishery Sciences, 37, Kshudiram Bose Sarani, Post Office- Belgachia, Kolkata-700037, West Bengal, India; Email: [email protected]
Citation | Nandi S, Joardar SN, Samanta I, Roy B, Das PK, Sar TK, Alam SS (2016). Native breed of chicken entertains different B cell target antigens than popular breeds for protection against pathogenic E. coli. Adv. Anim. Vet. Sci. 4(6): 311-314.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.6.311.314
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Nandi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Infectious diseases are the major challenge in poultry rearing. Preventive health management through vaccination is universally recommended practice to meet this challenge, although, cases of vaccine failure and disease outbreak are also common (Dhama et al., 2013). Hence identifying disease resistance breeds/strains in poultry is most lucrative approach. The poultry and farm animals are considered as major reservoir of E. coli and Salmonella throughout the world without any clinical syndrome (Girlich et al., 2007; Carattoli, 2008; Watson et al., 2012; Samanta et al., 2014a). However, mode of disease resistance generated in native breeds of poultry against E. coli and underlying immunological mechanism is not yet known (Samanta et al., 2014b). Therefore, it is wise to assess the basic immunological reactivity in native breeds of poultry to explore their disease resistance pattern against pathogenic E. coli. With this background, the present study was conducted to assess handling of B-cell antigens of pathogenic E. coli for protection upon experimental inoculation of live bacteria in one native breed (Haringhata Black, HB), one of best adapted exotic breed of backyard system (Rhode Island Red, RIR), and one commercial strain (broiler) in West Bengal, a major backyard and native breed rearing state in India.
Materials and Methods
Experimental Birds
Rhode Island Red, Haringhata black and Broiler birds (35 days old) were used as experimental birds procured from the University Farm at Mohanpur, Nadia. Each variety of birds was divided into two groups (control group & sensitized group) and each group contained 6 birds.
Experimental Design
The experimental birds were offered normal feed (Amrit feedTM, India) containing maize, soyabean, ground nut cake, mineral mixture, vitamin up to the end of the experiment with ad libitum feed and water. The period of observation was on 14th day post inoculation. The Institute Ethics Committee of West Bengal University of Animal and Fishery Sciences, India approved this study.
Sensitization of Experimental Birds
After 7 days of acclimatization in experimental cages of the Department, all the birds of one group (sensitized) of each variety were inoculated (I/P) with virulent field isolate of ESBL producing E. coli [strain- SK3, serogroup - O62, genotype- bla TEM (+ve), bla CTX-M (-ve), bla SHV (-ve)] bacteria (109 CFU/ml dose). The field isolate (SK-3) was obtained from a local broiler (29 days old) which was suffering from diarrhoea, fever, roughened feather and was un-responding to higher group of cephalosporin antibiotics. Another group of birds were kept as control. The birds were maintained for observation up to 14 days. Neither the sensitized group nor the control group of birds in the present study for each category (HB, RIR, Broiler) received any kind of antibiotic during the study period.
Antigen
The somatic soluble antigen of the E. coli isolate (SK3) was prepared using an ultrasonicator (Hielscher Ultrasonics GmBH, Germany). Ultra-sonication was done on ice at 150W with repeating duty cycles and 0.5 sec pulse pressure for two min with 30 sec interval (five times). The soluble sonicated extract was centrifuged at 10,000 g for 30 min at 4°C and the supernatant was collected as antigen (Choi et al, 1989). The somatic soluble antigen was kept at -20ºC for further use.
Protein Estimation
Protein concentration of somatic soluble antigen was estimated using commercially available protein estimation kit (Merck Biosciences, India). The absorbance was measured at 660 nm by UV-VIS Spectrophotometer (TechComp, Taiwan).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The crude somatic soluble bacterial antigen was analyzed by one-dimensional sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) in vertical slab gel electrophoresis instrument (AE-8450) with power pack (ATTO Corporation, Japan) as described earlier (Alam et al., 2014). About 40 µg of protein was loaded in each lane. The molecular weights of the resolved polypeptides were determined by medium range protein markers (PMW–M, Merck Biosciences, India) containing phosphorylase (97.4 kDa), bovine serum albumin (66 kDa), ova albumin (43 kDa), carbonic anhydrase (29 kDa), soabean trypsin inhibitor (20.1 kDa) and lysozyme (14.3 kDa). The gel was analyzed by Gel Documentation System (UVP, UK).
Western Blotting
The polypeptides were separated by 12.5% SDS-PAGE (Laemmli, 1970) and were transferred to nitrocellulose filter paper, NCP (Immobilon- NC, Sigma-Adrich, USA) from the gel as per Towbin et al. (1979) with slight modification (Svoboda et al., 1985).
Detection of Immunodominant Peptides by Western Blotting
NCP was kept in blocking buffer (5% skimmed milk powder in phosphate buffer solution, pH 7.4, PBS) for overnight at 4ºC. NCP was then washed with washing buffer (PBS-Tween 20). The NCP was incubated for two hr with pooled serum (n=3) from experimental birds (inoculated and control) of each variety collected at 14 DPI. The sera were diluted with blocking buffer (1:20). After washing, the NCP was incubated with rabbit anti-chicken horse radish peroxidase conjugate (Genei, India) for two hrs. The NCP was rinsed with substrate solution (H202 and DAB tablet, Sigma-Aldrich, USA) for 2 min and dipped into distilled water to stop the reaction. Lastly, it was dried up and preserved.

Figure 1: Assessment of polypeptide profile of ESBL-producing E. coli O62 somatic antigen by SDS-PAGE followed by Commassie brilliant blue R-250 staining
Lane S: Sample protein; Lane M: Standard molecular weight marker
RESULTS
Protein Content
The estimated protein of the soluble somatic antigen of the local E. coli isolate (SK-3) was 2.3 mg/ml.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
To assess polypeptide profile of the crude somatic antigen, SDS-PAGE analysis was performed. The crude sonicated protein of local E. coli isolate (SK-3) revealed 23 peptide bands ranging from 5 kDa to 205 kDa (Figure 1). Out of which, 15 major bands of 5, 6.5, 17.5, 23, 26, 27, 29, 41, 43, 54, 66, 78, 91, 138, 158 kDa and eight minor bands of 14.3, 20.1, 31, 36, 38.5, 97.4, 117.5, 179, 205 kDa were observed.
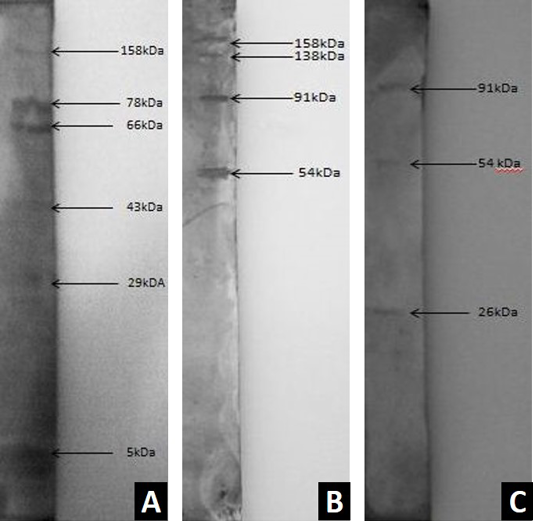
Figure 2: E. coli
A) Western blot analysis of ESBL-producing E. coli O62 somatic antigen using Haringhata black antiserum; B) Western blot analysis of ESBL-producing E. coli O62 somatic antigen using RIR antiserum; C) Western blot analysis of ESBL-producing E. coli O62 somatic antigen using Broiler antiserum
Detection of Immunodominant Peptides by Western Blotting
Western blot analysis of somatic soluble protein of the isolate (SK-3) was done with the experimental sera obtained from sensitized HB, RIR and broiler birds after inoculating the isolate. The polypeptide bands of crude protein of E. coli were found to be reactive at 158, 78, 66, 43, 29, 5 kDa against HB serum (Figure 2A). But in case of RIR serum, four immunoreactive bands were detected having molecular weights of 158, 138, 91 and 54 kDa (Figure 2B). In broiler birds, the crude protein showed immunoreactive polypeptide bands of 91, 54 and 26 kDa in Western blot (Figure 2C).
Discussion
The present study was aimed to detect B-cell antigens in three breeds of poultry viz. Haringhata Black, a native breed of India, Rhode Island Red; a backyard breed and a commercial strain (broiler) upon experimental inoculation of pathogenic E. coli isolate. The study indicated the comparative role of serological activities in three different poultry breeds by which they could resist pathogenic E. coli. The E. coli strain (SK 3) was selected for experimental inoculation because it was isolated from a local diseased broiler. Further, the isolate possessed bla TEM gene which is one of the major ESBL genes produced by E. coli (bla TEM, bla SHV, bla CTX-M) and the bacteria of reservoir poultry chiefly harbours bla TEM (Olsen et al., 2014). The broiler birds inoculated with pathogenic E. coli showed the clinical syndrome such as high fever, roughened feather and diarrhea within 9 days. In RIR bird, intensity of fever and diarrhea was less than the broiler birds. HB chickens didn’t show any observable clinical sign and symptom. Probably the higher resistance capacity of the HB birds was responsible for the development of no clinical manifestation.
The polypeptide profile of the somatic antigen and immunodominant protein profile of the challenge bacterium was ascertained. Varied as well as similar B cell target antigens of E. coli isolate (SK-3) was observed in HB (158, 78, 66, 43, 29, 5 kDa), RIR (158, 138, 91 and 54 kDa) and broiler birds (91, 54 and 26 kDa) by Western blot. This might be due to variation in species or strain of the birds used for the study. Previous report shows that the sonicated soluble protein of E. coli possessed bands of 77, 55, 52, 46, 42, 40, 37, 30, 26, 23, 19 and 12 kDa on SDS PAGE analysis (Malik et al., 1999) which is different from the present finding. Further, Mukherjee (2006) reported that 97, 58, 38 kDa bands of E. coli O44 were immunogenic as assessed by Western blot. Previously, on Western blot analysis two immune reactive bands were detected having molecular weight of 28 kDa and 39 kDa in O39 serotype of E. coli (Mitra, 2007). This variation might be due to different serotype used and the process of preparation of crude protein. Western blot was successfully used to determine serological responsiveness against pathogenic E. coli (Hopkins et al., 1995). Unique profile of B cell target antigens as observed in HB serum (78, 66, 43, 29, 5 kDa) should be given due importance as these might be the cause of protection against pathogenic E. coli through humoral immune responses. However, further studies are needed to establish this notion.
Thus the present investigation could detect varied humoral immune responses in the poultry breeds used in the study upon exposure to pathogenic E. coli by responding against different B cell antigens of E. coli. This type of response variation might be due to variation in their individual breed characteristics that encompass the disease resistance pattern of the breeds. This anticipation is applicable to the native breed Haringhata Black as B cell target antigens (78, 66, 43, 29, 5 kDa) are different in native birds against virulent E. coli that might be the driving factor of disease resistance as opposed to RIR and broiler birds where clinical symptoms were discernible.
Acknowledgements
This work was supported by the grant (BT/164/NE/TBP/2011) of Twinning Programm for NE, sponsored by The Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi. Authors are thankful to the Vice Chancellor, West Bengal University of Animal and Fishery Sciences, Kolkata for providing necessary infrastructure ficilities. No financial interests or benefits are arising from the direct applications of the present study.
Conflict of Interests
The authors declare that they have no competing interests.
Authors’ Contribution
Data were collected by SN, BR and SA while it were analyzed by IS, PKD and TS. The entire work was done under the supervision of SNJ. Manuscript was prepared by SN and correction /modifications were done by SNJ.
References






