Advances in Animal and Veterinary Sciences
Research Article
Determination of Tildipirosin Residues in Different Rabbit Tissues using Hplc Method
Sherief S. Abd Elhafeez1*, Ahmed A. Said2, Abd Elaleem F. Abd Elaleem2, Sameh M. El-Nabtity2, Maha S. Abd Elhafeez3
1Memphis for Pharmaceutical and Chemical Industries Company, Amerya, Cairo, 11753, Egypt; 2Pharmacology, Department, Faculty of Veterinary Medicine, Zagazig, University, 44511, Egypt; 3Department of Chemistry, Toxicology and Feed Deficiency, Animal Health Research Institute, Agricultural Research Center, 12611, Dokki, Giza, Egypt.
Abstract | Bacterial respiratory diseases cause economic casualties in rabbit industry. Tildipirosin injection characterized by rapid onset and prolonged effect and the single injection manner can reduce stress from overuse. The study was intended to examine tissue depletion of tildipirosin macrolide antibiotic in 21 New-Zealand rabbits after single intramuscular injection at a dosage of 4 mg kg-1 b.wt. Three rabbits were massacred on the 1st, 3rd, 5th, 7th, 9th, 15th, and 21st day after injection of tildipirosin. Specimens from muscle, kidneys, liver, and blood were occupied from massacred rabbits and analyzed for tildipirosin residues using simple, accurate and validated HPLC assay. Tildipirosin was extremely concentrated in kidneys followed by liver, whereas small amounts were found in the muscle. Tildipirosin remained within detectable limit till the 7th day in muscle, 9th day in serum and liver and till the 15th day in kidneys after drug administration. According to the MRLs settled by regulatory organizations and EMA, medicated rabbits with tildipirosin must not be slaughtered before 4 days from drug injection to be safe for human consumption.
Keywords | Tildipirosin, Residues, Rabbits, HPLC.
Received | February 06, 2021; Accepted | March 17, 2021; Published | June 15, 2021
*Correspondence | Sherief S Abd Elhafeez, Memphis for Pharmaceutical and Chemical Industries Company, Amerya, Cairo, 11753, Egypt; Email: [email protected]
Citation | Abd Elhafeez SS, Said AA, Abd Elaleem EF, El-Nabtity SM, Abd Elhafeez MS (2021). Determination of tildipirosin residues in different rabbit tissues using hplc method. Adv. Anim. Vet. Sci. 9(7): 1040-1044.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.1040.1044
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abd Elhafeez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Macrolides are valuable antibacterial medicines that are mostly used in veterinary practice for treatment of bacterial infections. Tildipirosin is a new semisynthetic antibiotic derivative of macrolide which is utterly used in veterinary medicine in the therapy of respiratory infections. In Europe, Tildipirosin is actually legalized to evade and medicate the respiratory infections in pigs and cattle triggered by diverse bacteria as Pasteurella multocida, Haemophilus parasuis and Actinobacillus pleuropneumoniae. Tildipirosin is administered as a single dose injection and the anticipated optimal clinical dose is 4 mg/kg b.wt (European Medicines Agency EMA, 2013).
A lot of regulatory agencies all over the world as European Medicines Agencies (EMA) and Codex Alimentarius Commission (CAC) have created and imposed MRLs/PLs to ensure the limited existence of antibiotic residues in foods of animal origin and also restricting the employment of barred veterinary medicines (EMA, 2011; CAC, 2017). Consequently, the Joint FAO/WHO Expert Committee on Food Additives and Contaminants (JECFA) amended the MRLs/PLs in foods for veterinary drug residues, at its 40th Conference of the CAC (CAC, 2017).
HPLC is one of the greatest prevailing techniques in analytical chemistry with the capability for separation, identification, and quantification of analytes exists in food of animal origin. It extremely used day by day in chemical residue analysis field (Kebede et al., 2014) and this technique consider an automatic process with extraordinary specificity, accuracy, precision, and rapid results (Kivrak et al., 2016).
Regarding bacterial respiratory diseases, it can cause economic casualties in rabbit industry such as rhinitis caused by P. multocida (Soriano-Vargas et al., 2012). The parenteral medication of antibiotics appears to be an ideal alternative scheme as most oral therapies destroy the normal intestinal flora (Carman & Borriello, 1983) so tildipirosin was injected for its rapid onset, prolonged effect and the single injection manner can reduce stress from overuse (EMA, 2013). The intent of this research was to study tissue depletion of tildipirosin in rabbits after solo intramuscular injection.
MATERIAL AND METHOD
Chemicals
Zuprevo® (4% tildipirosin) was obtained from MSD Animal Health Company, Egypt. Water, acetonitrile and methanol were ultrapure HPLC grade obtained from Fisher Scientific. Ammonium acetate and Orthophosphoric acid were procured from Merck Specialties Pvt. Ltd., India.
Tildipirosin standard (purity of 98%) was supplied by Clear synth Co. Stock solution (1 mg/ml) was prepared by dissolving 10.2 mg in 10 ml methanol, this solution stable for 1 month in amber glass at -20oC. The stock solution was diluted with purified water to obtain the fortification solution at a concentration of 10 ppm, which was freshly prepared.
Apparatus
HPLC apparatus involved Agilent Series 1200 quaternary gradient pump, Series 1200 autosampler, Series 1200 UV-Vis detector, and HPLC 2D- Chemstation software. The chromatographic column was a reversed-phase column (C18, 4.6 mm, 250 mm, 5 µm, Agilent Co.). Solid-phase extraction (SPE) cartridges (Bond Elut C18, 500 mg/3 mL) were used to clarify tissue matrixes.
Experimental design and sample collection
To obtain data regarding tissue distribution and residues of tildipirosin in rabbits, twenty-five healthy New-Zealand rabbits (2- 2.5 kg b.wt) were used, fed on drug-free feed and given water ad labtum for two weeks “accommodation period”. All animals were kept under proper hygienic conditions and housed in batteries in the faculty of Veterinary Medicine, Zagazig University. (Figure 1)
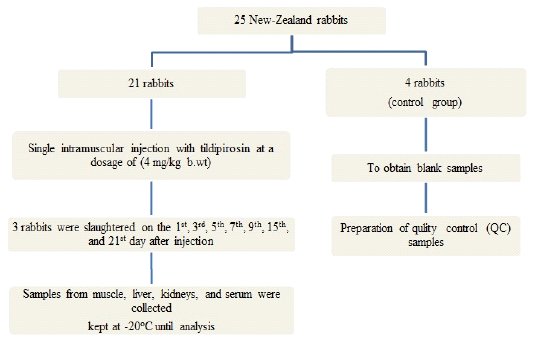
Figure 1: Flow chart of experimental design
Standard curve preparation
The calibration curve was created by fortifying blank rabbit tissues (muscle, liver, kidney) and blank serum with various volumes of fortification solution to yield a concentration range of 200-8000 ppb (calibration samples) and spike blank tissues to prepare quality control (QC) samples at 200, 400 and 800 ppb for muscle, 1000, 2000 and 4000 ppb for liver, 1500, 3000 and 6000 ppb for kidneys. Serum QC levels specified at a low level of 0.2 ppm, moderate level 2 ppm, and high level 6 ppm.
Sample preparation
At the time of analysis, incompletely melt icy tissues at room temperature (23oC) for half an hour and merge in a food processor for ~30 seconds at extraordinary speed to gain an even paste-like constancy.
The extraction was completed according to Rose et al. (2013) with little alterations. One gram of homogenized tissue (0.5 ml serum) was mixed with 2 ml of Acetonitrile for 30 min. Centrifugation at 3300 xg/10 min at 10oC using high-speed cooling centrifuge. The supernatant was transferred to a polypropylene tube and the extraction was repeated again with 2 ml of Acetonitrile and mix for 1 minute; then centrifugation again. The supernatant transferred to polypropylene tube and the combined supernatants were evaporated till complete dryness using nitrogen evaporator water bath at 45oC. The dried residues were reconstituted with 3 ml of 0.05 M ammonium acetate buffer then applied to pre-activated Solid Phase Extraction (SPE) cartridge by 2 mL methanol and 2 mL of 50 mM ammonium acetate buffer. The analyte was eluted with 2 ml of methanol slowly. Evaporation of 1 ml of elute under nitrogen steam at 45oC. Re-dissolving with 0.5 ml (tissue) and 0.25 ml (serum) of (50 mM ammonium acetate buffer: methanol) (50: 50).
| Parameter | Serum | Muscle | Liver | Kidney | Acceptance criteria | |
| Retention time | 1.409 | |||||
| Range (ppb) | 200- 8000 | |||||
| Slope | 0.5655 | 0.5137 | 0.5638 | 0.5685 | ||
| Intercept | -1.2174 | 5.2828 | -13.671 |
3.1331 |
||
|
Correlation coefficient (R2) |
0.9998 | 0.9996 | 0.9999 | 0.9997 | ≥ 0.99 | |
| LOD (ppb) | 6.67 | 8.3 | 11.67 | 10.86 | ||
| LOQ (ppb) | 20 | 25 | 35 | 33 | ||
| Recovery (%) | 92.8-96 | 90.7-97.4 | 87-89 | 96.5-98 |
75-110 |
|
| Intra-day precision (CV %) | 0.15 | 0.38 | 0.66 | 0.45 | ≤ 1% | |
| Inter-day precision (CV %) | 0.68 | 0.66 | 0.87 | 0.92 | ≤ 2% | |
| Pooled robustness (CV %) | 1.23 | 1.18 | 1.21 | 1.62 | ≤ 6% | |
| SST | Tailing factor (TF) | 1.04±0.02 | 1.08±0.01 | 1.04±0.02 | 1.04±0.02 | ≤ 2 |
| Symmetry | 0.93±0.01 | 0.91±0.01 | 0.93±0.01 | 0.93±0.01 | ||
| Theoretical plate (N) | 5250±50 | 5200±70 | 5254±50 | 5303±20 |
N ˃ 2000 |
|
Table 2: Tildipirosin concentrations (ppb) in serum, muscle, liver and kidneys after single intramuscular inoculation in healthy rabbits (n=3)
|
1st |
3rd |
5th |
7th |
9th |
15th |
21th |
|
| Serum | 40.3±1.5 | 25.3±0.6 | 14.3±0.6 | 9.9±0.2 | 7.3±0.3 | nd | nd |
| Muscle | 492.3±6.8 | 216.7±15.3 | 35.3±1.5 | 12.3±1.2 | nd | nd | nd |
| Liver | 1330±70 | 956.7±75.7 | 296.7±15.3 | 188±9.2 | 80.7±4 | nd | nd |
| Kidney | 3317.3±77 | 2837.3±116 | 1030±60.8 | 406.3±21.2 | 249.7±19.6 | 108.7±7.1 | nd |
Chromatographic parameters
Injection volume: 50 µl, Flow rate: 0.8 ml/min., Column temperature: 35oC, Wave length: 289 nm and the mobile phase: 0.02 M ammonium acetate: methanol (40:60) where pH adjusted to 3.5 by phosphoric acid.
Method validation
The analytical method was validated according to USP 34-NF 38 (2019). Linearity & range, intra-day precision & inter-day precision, recovery, limits of detection and quantification (LOD & LOQ), robustness, system suitability test (SST) and specificity were determined using fortified samples and QC samples.
Statistical Analysis
The obtained results were statistically evaluated using Microsoft excel 2010 (Neyeloff et al., 2012).
RESULTS and DISCUSSION
Method validation
The results of method validation summarized in Table (1) showed that the developed method for analysis is accurate, precise, robust and sensitive due to its low detection limits.
Tildipirosin chromatograms either in serum or different tissues were demonstrated at a specific retention time 1.409 with no intervention between peaks of any matrix impurities and the intended peak as showed in Figure (2).
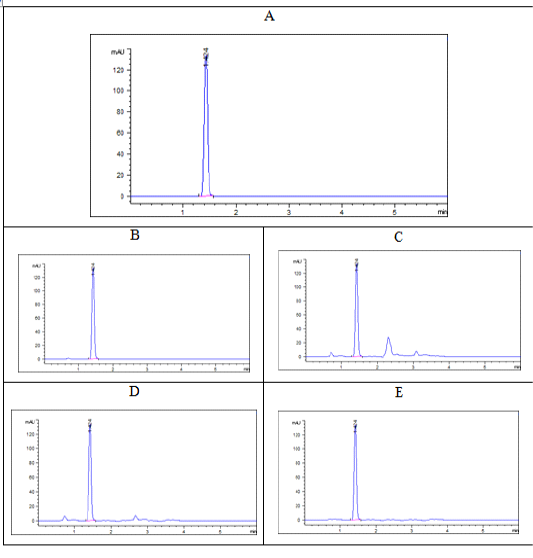
Figure 2: Chromatograms of tildipirosin at a concentration of 1000 ppb (A: pure standard, B: serum, C: liver, D: kidney, E: muscle) at retention time 1.04 min.
Tissue residues of tildipirosin
One of the basic characteristics of macrolides is its high distribution and concentration in tissues with significant accumulation in phagocytic cells so remain for long time in tissues after its plasma concentration declined (Galecio et al., 2020) and this appear clearly during our study on tildipirosin that has an extended and strong antibacterial action, high concentration in diverse tissues and high bioavailability (Lei et al., 2018; Lombardi et al., 2011).
The withdrawal time awareness of any antimicrobial considers an important and essential issue aim to reduce the probability of antimicrobial resistance and hazards from these residues (Boucher et al., 2017; Drusano et al., 2016). Hopefully, this is the first study discussing tissue depletion of tildipirosin in rabbits.
In this study, single intramuscular injection of tildipirosin at dosage of 4 mg kg-1 b.wt in rabbits clarified that kidneys and liver contain the highest drug concentrations (3317.3±77 and 1330±70 ppb, respectively) while the lowermost concentrations were detected in muscle (492.3±6.8 ppb) on the 1st day after tildipirosin injection as shown in Table 2. These results agreed with EMA (2013) that mentioned that the highest concentrations of tildipirosin were present in kidneys (8600 ppb) followed by liver (5524 ppb) and the lowest concentrations were present in fat (460 ppb) and muscle (324 ppb).
Tildipirosin remained within detectable limit till the 7th day in muscle, 9th day in serum and liver and up to 15th day in kidneys after drug administration (Table 2).
The MRLs legalized by EMA for tildipirosin in caprine tissues are 400, 2000 and 3000 ppb in muscle, liver and kidneys; respectively (EMA, 2013) and the recommended withdrawal time is four days for rabbits to be safe for human consumption.
CONCLUSION
After single IM administrations of tildipirosin (4 mg/kg b.wt), it was greatly concentrated in kidneys followed by liver while the lower amounts were found in muscle. Based on the MRLs established by regulatory agency EMA, medicated rabbits with tildipirosin should not be slaughtered before four days from drug injection to be safe for human consumption.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
ACKNOWLEDGMENT
My sincere gratitude and deepest thanks to the staff of Central Lab at the Faculty of Veterinary Medicine, Zagazig University and the staff of Chemistry Department, Animal Health Research Institute for their support and cooperation.
authors contribution
Sherief S. Abd Elhafeez contributed experimental design and wrote the article, while Maha S. Abd Elhafeez contributed analytical procedure and statistical analysis, Sameh M. El-Nabtity contributed critical reviews of the article and Ahmed A. Said and Abd Elaleem F. Abd Elaleem served as scientific advisor.
REFERENCES






