Advances in Animal and Veterinary Sciences
Research Article
Morphological Structure of the Lymphatic System of Sheep Abomasum
Aigul Idrisovna Gazizova*, Nurgul Barlybayevna Akhmetzhanova, Aigul Serkeshovna Tozhybaeva, Leila Mazhitovna Murzabekova, Asiya Serikovna Bekenova
S. Seifullin Kazakh Agro Technical university, Zhenis Avenue, 62, Astana, 010011, Republic of Kazakhstan
Abstract | The research was aimed to apply classical research methodologies in studying the structure of the abomasum lymphatic bed. The roots of the lymphatic bed are the lymphatic capillaries, which make a fine-meshed network in the mucosa of the abomasum. Lymphatic capillaries anastomose with each other, forming loops of various shapes. The largest ones are found in the middle third of the side walls of the abomasum body. The wall of lymphatic postcapillaries consists of endothelial cells, in which postcapillaries are located. The lymphatic system plays an important role in maintaining the immune resistance of the organism. Characteristic of studying the lymphatic system of the abomasum has been given. During the preparation, it was found that the lymphatic postcapillaries are in topographical proximity to venules and arterioles. Studying the sinuosity of the vessels, the authors have not found any regularity. The vessels have a coefficient of sinuosity, which changes regardless of their order and periods of postnatal ontogenesis. This is the evidence of the fact that they may be both nearly straight and sinuous.
Keywords | Lymphatic system, Abomasum, Lymph nodes, Lymph
Received | June 12, 2019; Accepted | August 30, 2019; Published | October 15, 2019
*Correspondence | Aigul Idrisovna Gazizova, S. Seifullin Kazakh Agro Technical university, Zhenis Avenue, 62, Astana, 010011, Republic of Kazakhstan; Email: [email protected]
Citation | Gazizova AI, Akhmetzhanova NB, Tozhybaeva AS, Murzabekova LM, Bekenova AS (2019). Morphological structure of the lymphatic system of sheep abomasum. Adv. Anim. Vet. Sci. 7(s1): 15-20.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s1.15.20
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Gazizova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In the modern morphology, one of the objectives is studying the morphological and physiological features of animal organism systems, clarification of their adaptive capacities, and resistance to external and internal environmental factors (Sapin, 1998).
The structure and function of organs may be understood precisely only with the knowledge of macro and microarchitectonics of the lymphatic bed. The importance of the latter in the life activity of the organism in the conditions of the norm and pathology is very high. Lymph is involved in fluid balance in the tissues, together with the venous system it ensures drainage of organs, and is the mechanism of propagation of many pathological processes (Putalova et al., 2000; Gazizova et al., 2006).
One of the most urgent tasks in the field of morphological studies is the clarification of the age-specific and adaptive abilities of the organism structures. Currently, many researchers pay great attention to studying the lymphatic system due to development of immunology and the demand of practical medicine and veterinary, which consider administering medications with the lymph more efficient than with traditional methods (Putalova et al., 2000; Borisov, 1997).
An important criterion for determining harmlessness of meat and slaughter products is the state of the lymphatic system. It has been found that lymph nodes and the vessels are involved in many infectious diseases of animals, particularly in the pathological process, whereby specific changes occur in them. Studying the topography of the lymphatic vessels, regional nodes and the links between them and other tissues allows correct understanding of the ways of infection spreading and the metastatic spread of malignant tumors in animals. It also allows reasonable veterinary-sanitary examination of carcasses and organs and determining rational approaches to surgical diseases (Sapin and Borziak, 1982).
Disorders in the lymphatic system and inadequacy of its functions affect the development and the outcome of several diseases. Correction of disorders that occur in the lymphatic system in case of various diseases, as well as optimization of its inadequate functions, are important principles of general therapy. Correct insight into the structure and functions of the lymphatic system is important for understanding the metabolic processes, organism reactions to infections, patterns of malignant tumors’ spreading (Gazizova and Ahmetzhanova, 2008).
The lymphatic system of the gastrointestinal tract is of particular interest due to many reasons. However, we have not found in the literature any special full-fledged research devoted to studying the lymphatic bed of the gastrointestinal tract of small ruminants, sheep and goats in particular, except for fragment works about the lymphatic bed in some organs of the digestive tract (Gazizova et al., 2006).
MATERIALS AND METHODS
To study the lymphatic bed in the gastrointestinal tract of sheep, we have used 35 organs obtained from clinically healthy sheep of two age groups – pubescence and maturity. The obtained material was studied by the following methods: interstitial injection, specimen preparation, making total preparations, morphometry, clarification of preparations, making histologic sections.
The architectonics of the lymphatic bed of the abomasum was studied after it was filled with colored masses. For this, we used the method of injection through the lymph node with a 0.5% solution of methylene blue and a 50% solution of black and blue ink, gelatin-based ink, indication with orange cadmium.
Disposable syringes with a capacity of 1-2 ml were used for the injection of colored masses. In addition, we proposed a method for injecting the lymphatic bed (provisional application No. 2008/1176.1).
The preparation of macro and micro slides of the lymphatic bed was conducted as follows. After being frozen for 24-30 hours, the frozen organ was removed from the refrigerating chamber and subjected to defrosting. Defrosting was carried out as follows: the organs removed from the refrigerating chamber were left for 1-2 hours at room temperature.
Next, the defrosted organs were placed in the sink and left under a stream of cold water for 5-6 hours until the complete disappearance of ice and the formation of the soft consistency of the organ.
After the preparation, the organ was placed in a tray and spread. Regional lymph nodes were found and then along the edge of the tray, not on the organ itself, warm water was poured in a thin stream, the temperature of which was 37-40ºC. The cooled water in the tray was changed 3-4 times before the injections.
Injections were carried out under pressure through regional lymph nodes of the organs. A mixture consisting of 1% methylene blue and black ink in the ratio 1:0.5 was used as the injection mass.
The injection mass moves through the lymph nodes via the vessels of organs faster and better, if during injections, a gentle massage with a cotton swab dipped in warm water is performed. Lymphatic vessels, filled through regional lymph nodes, begin to clearly stand out in different layers of the stomach of ruminants.
The method of detecting lymphatic vessels 24 hours after the slaughter of animals is characterized by the fact that when the studied objects are kept in the refrigerating chamber, biochemical processes take place in the organs and the lymph settles on the wall of the lymphatic vessels, allowing for the better advancement of the injection mass.
The proposed method allows one to achieve accurate filling of the lymphatic bed with sufficient contrast and to study the structure of the wall of the lymphatic capillaries.
After the filling of the lymphatic bed, the organ was placed for 7-14 days in 10% formalin solution for fixation. Then it was washed in cold running water for 12-20 hours, after which we proceeded to the dissection of lymph vessels and nodes.
Dissection was performed using eye scalpels, scissors, surgical and anatomical tweezers and dissecting needles under a microscope. For the most complete study of the histotopography of the lymphatic bed, as well as its relation to the blood vessels, histologic sections were prepared from various parts of the organs and regional lymph nodes. The histological material was embedded in paraffin blocks using various methods.
Longitudinal and transverse sections were prepared from the obtained blocks. The finished sections were stained with hematoxylin and eosin.
To obtain the total preparation of the lymphatic vessel, first, using a microscope, we prepared the desired lymphatic vessel with the help of an eye scalpel, tweezers, scissors and dissecting needles and fixed it in 7-10% formalin solution for 12-24 hours. Then, using binocular magnifying glasses, we cleared the vessel from connective tissue and dissected it longitudinally (small vessels, postcapillaries and capillaries were not dissected). After that, the preparation was additionally fixed, washed in running water for 20-30 minutes, clarified in methyl salicylate and put through four portions of xylene (5-10 minutes in each portion). The finished product was spread on the slide with endothelium upwards and fixed with polystyrene or Canada balsam.
RESULTS
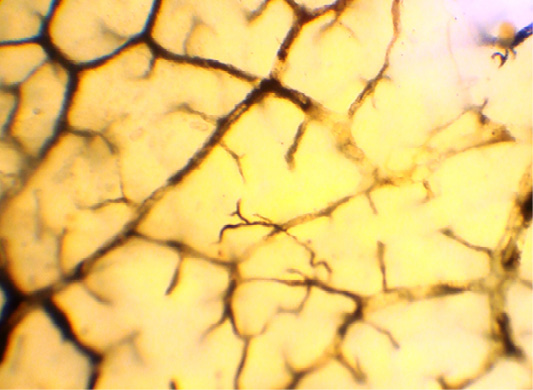
By their relation to the organ, lymphatic vessels are divided into intra- and extraorganic, and by diameter–into small, medium and large, with certain peculiarities of the wall structure (Figure 1).
The intraorganic lymphatic bed of the gastrointestinal tract of sheep is represented by the capillaries that are located in all layers of the organ, postcapillaries, and vessels of the first, second and third-orders. Small lymphatic capillaries transform into postcapillaries, in which the valve apparatus is already present, and the wall consists of endothelial cells. Anatomically postcapillaries are located in the submucous coat, but in the studies, we discovered these capillaries between the layers of the muscle membrane.
The fine-meshed lymphatic network of capillaries of the mucous membrane develops into a large-meshed network of the submucous coat through numerous capillaries perforating the muscular plate of the mucous membrane vertically or at an angle. The peculiarity of a lymphatic vessel wall structure is the presence of smooth muscle cells (myocytes).
Capillaries have an irregular diameter in various parts of the stomach submucous coat. The largest diameter of capillaries is in the region of the lesser curvature of the stomach in the pyloric area. The smallest diameter of lymphatic capillaries is on the side walls of the stomach body, and the smallest diameter was observed in the area of the bottom. Anastomosing with each other, lymphatic capillaries form polygonal, rhomboid, triangular, elongated, or sometimes more rounded loops.
In the muscular layer of the multichamber stomach, including abomasum, we identified three layers of the lymphatic capillaries’ network located in three layers. It means that in the circular muscle layer, lymphatic capillaries are located in various planes (Figure 2).
The loops of this network have an elongated shape, and are placed parallel to the muscle bundles of this layer, and transversely to the long axis of the organ. The densest network of lymphatic capillaries was found in the distal half part of the abomasum body, and in the area of the pylorus. The rarest network of lymphatic capillaries in the circular muscle layer was found at the bottom of the abomasum, where this layer was much less developed.
Lymphatic capillaries of the longitudinal muscle layer are located in the connective tissues’ interlayers between bundles of the muscle fibers. Capillaries of this layer are interconnected to form rectangular networks, polygonal and irregular networks are found more rarely. In the abomasum, the densest and the bulkiest network of lymphatic capillaries is found in the pyloric section and in the area of the lesser curvature of the stomach. With age, the size of loops of lymphatic capillaries, and the width of the capillaries increase. Lymphatic capillaries of the longitudinal muscle layer form postcapillaries, which flow into the lymphatic bed of the serous membrane.
Lymphatic capillaries in the intermuscular layer interconnect to form networks of various forms. Lymphatic capillaries of the serous membrane are located in the connective tissues, adhering to the longitudinal muscle layer; by interconnecting, they form in the serous membrane a network of capillaries, the loops of which have an oval or elongated polygonal shape. Longitudinal axes of these loops on the side walls of the abomasum are elongated along its axis.
During the research, we found that the width of the lymphatic capillaries increased in the ontogenesis. The density of the capillary network, the size, and the shape of the capillary cells depend on the type of the tissue and the anatomical part of the studied organ of the abomasum. As a result of the study, it has been found that the mucous and the submucous membranes of the pyloric part and the lesser curvature of the abomasum of sheep have much more tightly spaced lymphatic capillaries than other parts of this organ.
Lymphatic postcapillaries are formed from merging several lymphatic capillaries. Walls of lymphatic postcapillaries, same as in lymphatic capillaries, consist of endothelial cells, however, unlike capillaries, they have valves that determine directed lymph flow. When a lymphatic postcapillary turns into a lymphatic vessel, the wall becomes more complicated: connective tissues and myocytes appear in it.
Lymphatic postcapillaries are contained in the submucous membrane between layers of the muscle coat and the serous coat of abomasum.
In the submucous coat of the abomasum, lymphatic postcapillaries are located between lymphatic capillaries. The number of valves varies from 2 to 5. Lymphatic vessels are formed by merging 2 to 5 lymphatic postcapillaries.
Lymphatic postcapillaries of the muscle coat of abomasum are in the connective tissues between its layers. Here the number of valves reaches 3 to 7, or even 8. Lymph outflow from these plexuses is directed toward the serous membrane to a greater extent, and insignificantly – to the side of the submucous coat (Figure 3).
In the serous membrane, the number of valves in the lymphatic postcapillaries ranges from 5 to 15. Plexuses of lymphatic postcapillaries are located closer to the longitudinal muscle layer; 3–5 postcapillaries merge to form a first order lymphatic vessel of the serous membrane.
Lymphatic postcapillaries in the abomasum differ from lymphatic capillaries by the presence of valves, which are in fact folds of the endothelium. By merging, lymphatic postcapillaries form lymphangions.
A lymphangion is a section of a lymphatic vessel between two valves. Alternating of narrowing and expanding sections gives the lymphatic vessel a peculiar beaded appearance and distinguishes it from blood vessels. In a lymphangion, the valve and the wall are distinguished. Three parts have been identified in the wall of lymphangion: the muscular cuff, the wall of the valve sinus, and the area of valve attachment. The structure of the walls of intraorganic lymphangions in the abomasum of ruminants becomes more complex toward extraorganic vessels. The valves of lymphangion are its key elements that determine the direction of lymphatic drainage from the organ to the thoracic duct and prevent lymph backflow.
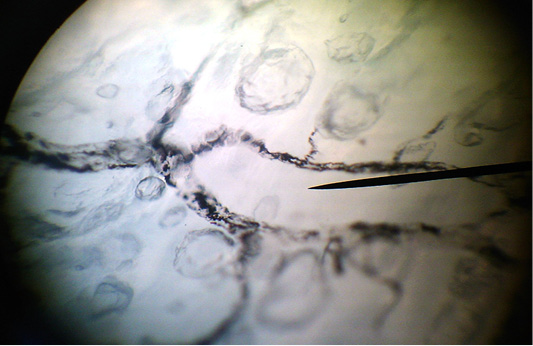
Figure 3: The clarified total preparation. Lymphocapillary net of the longitudinal muscular layer of the abomasum.
The number of lymphangions in a lymphatic vessel varies widely and depends on the type of the lymphatic vessel, its length and the age of the animal.
The presence of smooth muscle cells called myocytes in the wall is a characteristic feature of the lymphatic vessels. The lymphatic vessels in the abomasum of sheep are located in the submucous coat and the serous membrane. On the contours of these vessels, nodes are clearly visible, which correspond to the valves’ locations. The submucous coat contains only lymphatic vessels of the first order, which have been formed by merging of 2–5 capillaries. Anastomosing between themselves, lymphatic vessels form submucous plexuses of lymphatic vessels that are located in deeper layers of the submucous coat than the network of lymphatic capillaries. During the research, we have found that the width of first-order lymphatic vessels in various parts of the submucous coat of abomasum varies.
The largest loops formed by the first order lymphatic vessels were found in the arch of the abomasum, and the smallest ones–in the folds of the submucous coat, in the pyloric part, and on the side walls of abomasum body adjacent to the lesser curvature of the organ. The shape of the loops in the plexuses of first-order lymphatic vessels is most often oval and polygonal. Passing the muscle coat at an angle, first order lymph vessels accept its lymphatic channel and flow into lymphatic vessels of the serous membrane close to the smaller and large curvature of the abomasum.
Lymphatic vessels in the serous membrane were divided into three orders. Vessels of the first order, merging in the quantities from 2 to 5, form second-order lymphatic vessels. In turn, 2–3 second-order lymph vessels form third-order vessels, which reach the small and big curvatures. Next, third-order lymphatic vessels turn to extraorganic vessels (the vessels of the fourth order) that reach the regional lymph nodes.
As a result of studying intraorganic lymphatic vessels of the gastrointestinal tract, including abomasum of small ruminants, it should be noted that abomasum wall, which is thick, rich in glands has an intricate lymphatic system. Formation of intraorganic lymphatic vessels starts in the submucous coat and ends in the serous membrane. We have found that the width of intraorganic lymphatic vessels increases in the proximal direction, and is directly proportional to the age of the animal.
At the same time, some small lymph vessels usually located near the capillaries in networks do not always have clear signs of lymph vessels. Depending on the localization (above or below superficial fascia), lymphatic vessels are divided into superficial and deep ones.
Lymphatic vessels located in the areas with well-developed fatty tissue have a greater number of valves, compared to the vessels in other areas. The valves are intended for ensuring the centripetal direction of lymph flow through a lymphatic vessel and preventing backflow.
Extraorganic afferent lymphatic vessels of the abomasum are formed near the edges of the organ from intraorganic third-order lymphatic vessels in the serous network. Afferent extraorganic lymphatic vessels formed near the lesser curvature follow between the layers of the lesser omentum to the regional lymphatic nodes of the lesser curvature (cardiac part, midpart, and pyloric part), as well as the lymph nodes in the omasum and the forestomach. Lymphatic vessels formed near the greater curvature, passing between the layers of the greater omentum, proceed to the lymphatic nodes in the fundal part of the abomasum. Afferent lymphatic vessels follow the course of blood vessels or cross them.
In the cardiac part, 1–2 afferent lymphatic vessels in this part of abomasum are directed to the lymphatic nodes in the forestomach.
From the midpart of the right wall in the region of curvature, 7 to 15 afferent lymphatic vessels flow into the right midpart group of lymphatic vessels.
From the pyloric part of the right wall in the area of lesser curvature, 1–6 afferent lymphatic vessels originate, which are interrupted in the right pyloric group of lymphatic nodes.
From the cardial part of the lesser curvature area of the abomasum, 2–3 afferent lymphatic vessels originate, which join the left-side cardiac group of lymph nodes.
During the research, it has been found that from the median part of the abomasum wall, 1–2 afferent lymphatic vessels in 15% of cases fall into the median right-sided lymphatic nodes.
From the pyloric part of the lesser curvature area, 1–2 afferent vessels originate, which spread to the lymphatic nodes in the duodenum at the beginning of the right gastro-omental, and then the gastro-duodenal arteries.
From the cardiac part of the left wall in the area of large curvature, 1–3 afferent lymphatic vessel originate, which enter the lymphatic nodes in abomasum – forestomach – omasum.
Afferent lymphatic vessels (1–4) in the fundal part of the left wall of abomasum are accompanied by short branches of the left gastro-omental artery and vein, merging together to flow into the fundal lymphatic node.
From the cardial part of the greater curvature area of the abomasum, 1–4 afferent lymphatic vessels originate, which join the left-side cardiac group of lymph nodes. In addition, in 65% of cases, 1–3 lymphatic vessels from this part extend to the cranial nodes in the omasum.
From the middle of the left wall in the area of lesser curvature, 4 to 8 afferent lymphatic vessels originate, which flow into the left median group of lymphatic nodes.
During the research, it has been found that from this part of the abomasum wall, 1–2 afferent lymphatic vessels in 15% of cases fall into the median right-sided lymphatic nodes. From the caudal segment of this part, in 7% of cases, 1–3 afferent vessels extend to the lymph node above the pyloric stomach region.
From the pyloric part of the left wall in the area of lesser curvature, 1–2 afferent lymphatic vessels originate, which extend to the left-hand pyloric group of lymphatic nodes. It has been found that in 15% of cases, 1–2 afferent lymphatic vessels extend to the lymphatic nodes in the duodenum along the flow first in the right gastro-omental, and then the gastro-duodenal arteries. Along with that, in 13% of cases, 1–3 afferent lymphatic vessels depart from this segment and flow into the lymphatic node above the pyloric stomach region.
From the pyloric part of the right wall in the area of the greater curvature of the abomasum, perpendicular to the edge of the organ, 1–2 afferent lymphatic vessels depart, which are interrupted in the lymphatic node below the pyloric stomach region. During the research, we have found that in 45% of cases, 1–2 afferent lymphatic vessels extend to the lymphatic nodes in the duodenum along the flow of the right gastro-omental artery and veins.
Outgoing extraorganic efferent lymphatic vessels are different from afferent lymphatic vessels. The number of efferent lymphatic vessels outgoing from a lymphatic node is several times less than the number of afferent ones.
Efferent lymphatic vessels from the lymphatic nodes of the lesser curvature extend in various directions parallel to the lesser curvature between layers of the omentum accompanying first the right and then the left gastric artery.
DISCUSSION
Thus, the research has shown that a network of afferent lymphatic vessels in the area of the lesser curvature of the abomasum is the most developed, compared to the greater curvature.
On their way to the regional lymph nodes, collectors (afferent vessels) merge into larger vessels. The predominant lymph outflow from the walls of the abomasum is toward lesser curvature, mostly to the middle lymph nodes. The number of these vessels on each side of the abomasum ranges within 4–8 in sheep, and within 5–15 in newborns to a single lymph node.
From the cardiac part of the abomasum wall, 3–5 lymphatic vessels on each side extend to the cardiac lymph nodes, and 1–2 lymphatic vessels (in 70% of cases) extend to the lymphatic node in the omasum, and one lymphatic vessel (in 15% of cases) falls into the lymph node of the forestomach.
The width of afferent lymphatic vessels is usually less than the width of efferent lymphatic vessels.
CONCLUSION
The results of studying the lymphatic bed of sheep abomasum make the morphological basis for determining the ways of metastasis spread of tumor cells and lymphatic nodes’ involvement. In studying the pathogenesis of abomasum diseases, methods of treatment and surgical interventions on this organ, one should consider the close anatomic-topographic relations between the lymphatic bed in the abomasum and adjacent organs (rumen, forestomach, omasum, duodenum, liver, greater and lesser omenta).
Conflict of interest
Authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTION
All authors contributed equally.
REFERENCES