Journal of Animal Health and Production
Research Article
Journal of Animal Health and Production 1 (3): 29 – 31Effect of Moxifloxacin (Mofoi™) in Broilers
Tarun Kumar1*, Devan Arora2, Davinder Singh2
- Bovian healthcare Pvt. Ltd, SSR Corporate Park, Faridabad, India;
- Department of Veterinary Public Health and Epidemiology, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, India
*Corresponding author:[email protected]
ARTICLE CITATION:
Kumar T, Arora D, Singh D (2013). Effect of moxifloxacin (Mofoi TM) in broilers. J. Anim. Health Prod. 1 (3): 29 – 31.
Received: 2013–07–29, Revised: 2013–08–18, Accepted: 2013–08–21
The electronic version of this article is the complete one and can be found online at
(http://nexusacademicpublishers.com/table_contents_detail/11/72/html)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Multiple antibiotic resistance patterns in the poultry sector are making most of the bacterial disease outbreaks unmanageable for poultry consultants/ practitioners. Moxifloxacin possess broad spectrum antibacterial activity against Gram–positive and Gram–negative bacteria, anaerobes and atypical organisms. Salmonella isolates from broilers showed maximum sensitivity (86.6 %) for Moxifloxacin whereas sensitivity against E.coli was 80%. Moxifloxacin effectively and successfully treated outbreaks of complicated chronic disease in broiler flocks with the dose of 5 mg/kg body weight in 3 days.
INTRODUCTION
Quinolones are the class of antibiotics that are widely used in poultry medicine, but development of resistance is a limiting factor in treating commonly prevalent bacterial infections in poultry. Fluoroquinolones possess wide spectrum of bactericidal activity, good tissue distribution, low plasma protein binding and relatively low MICs (minimum inhibitory concentrations) against susceptible target microorganisms. Since the introduction of the first quinolone in 1962, structural modifications have resulted in the production of numerous agents in second–, third–, and fourth–generation fluoroquinolones. Use of moxifloxacin and gatifloxacin was approved in December 1999 by the US Food and Drug Administration (FDA) (Spreng et al., 1995; Brown, 1996). Moxifloxacin is a novel 4th generation fluoroquinolone with a methoxy group in the C–8 position and C–7 side chain. It has a broad spectrum of antibacterial activity against Gram–positive and Gram–negative bacteria, anaerobes and atypical organisms such as Mycoplasma and Chlamydia spp. (Kowalski et al., 2003). Pharmacokinetic studies of moxifloxacin in animals have been studied e.g. in horses (Gardner et al., 2004), rabbits (Carceles et al., 2006), lactating goats (Carceles et al., 2007), lactating ewes (Goudah, 2008) and broilers (Goudah, 2009). A study was conducted to know the sensitivity of Moxifloxacin against Salmonella and E.coli isolates as well a field trial was conducted in a severely infected poultry flock.
MATERIALS AND METHODS
Bacterial isolates
In the present study, a total of 30 isolates including 15 isolates of Salmonella spp. and 15 E. coli isolates were analyzed for antibiotic resistance pattern. The fresh bacterial isolates were kindly provided by the Department of Veterinary Public Health and Epidemiology, Hisar (Haryana), India. All the isolates were previously isolated from broilers and were conformed as E. coli and Salmonella by bio–typing and serotyping.
Antimicrobial susceptibility testing
Antibiotic sensitivity test was performed as per the disc diffusion method (Bauer et al. 1996). Five different antibiotic discs namely Moxifloxacin M (5 mcg), Gentamicin G (10 mcg), Enrofloxacin Ex (10 mcg), Ciprofloxacin Cf (10 mcg) and Cloxacillin Cx (10 mcg) were used. Results were recorded using antibiotic zone scale and interpreted as resistant, intermediate and sensitive based on values given in zone size interpretative chart.
Field study 1
Flock History
As per the farmer, vaccination was done according to standard recommendations in a flock of 17,000. Birds start showing signs and symptoms at the age of 5 day with 1 % mortality daily. The symptoms were like decrease in feed intake and tracheal rales. No treatment was given till the age of 8 days. On 8th day farmer started treatment and the birds were injected with Gentamycin + Amikacin + Multivitamin and Liver extract. The treatment was followed for 5 days but there was no control in the mortality. On 15th day farmer treated the birds with another combination i.e. Gentamycin+ Amikacin+ Tylosin & liver extract. Treatment was continued for two days, but the affected flock showed no improvement and the mortality was increasing at rapid and daily mortality of the flock was 300 birds per day. On 17th day, Sulphonamide and Chloramphenicol, was started and given for two days but again the story continues and the farmer was losing the flock at rapid pace. On 19th day another unique combination of Amikacin+ lincomycin+ Ceftriaxone was injected by the farmer to the affected flock and continued till 20th day but the affected flock showed no improvement. Till date farmer lost 60 % of its flock. On next day farmer approached the veterinarian and after doing postmortem the disease was diagnosed as complicated chronic respiratory disease (CCRD). Farmer was advised to stop all the medication immediately and to treat the affected flock with new antibiotic in the market i.e. Moxifloxacin (MofoiTM) with the dose of 5 mg/kg body weight orally.
Field study 2
Another field study was conducted in three broiler farms located in the area of Sonipat, Haryana (India) with flock strength of 20000, 12000 and 10000. The birds started showing clinical signs like loss of appetite, tendency to huddle together, open mouth breathing and conjunctivitis at the age of 13 days. Mortality started at the age of 14th day. Almost 50% of the flock was affected. As per the farmer no treatment was given to the flock.
RESULTS
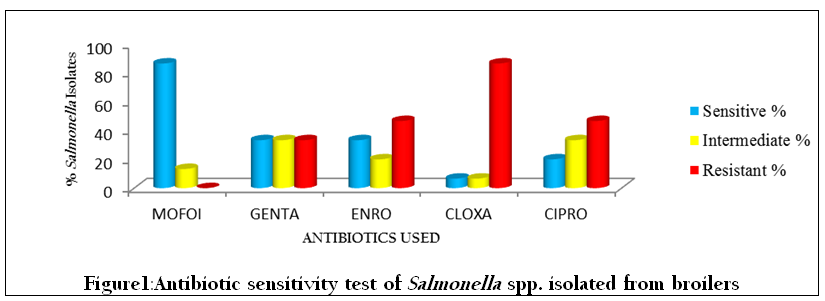
For Salmonella spp. isolated from broilers, maximum sensitivity (86.6 %) was shown by Moxifloxacin, followed by Gentamicin (33.3%), Enrofloxacin (33.3%), Ciprofloxacin (20%) and Cloxacillin (6.6%). None of the isolates were resistant to Moxifloxacin whereas Cloxacillin showed maximum resistance (86.6%), followed by Enrofloxacin (46.6%), Ciprofloxacin (46.6%) and Gentamicin (33.3%), (Table 1, Figure 1).
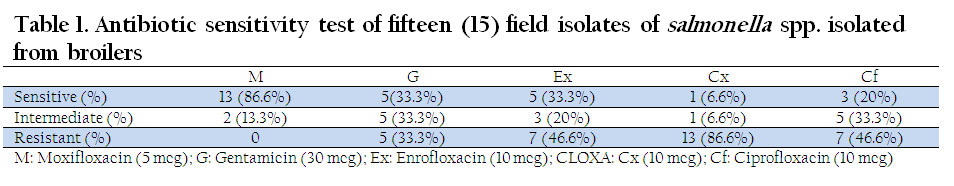
Table 1: Antibiotic sensitivity test of fifteen (15) field isolates of salmonella spp. isolated from broilers
The E. coli strains isolated from broiler outbreaks when analyzed for antibiotic sensitivity test, it was revealed that Enrofloxacin, Ciprofloxacin and Cloxacillin were resistant to all the isolates whereas Gentamicin was resistant to 86.6% of the isolates. Moxifloxacin showed maximum sensitivity (80%) and none of the isolate was resistant to Moxifloxacin (Table 2).
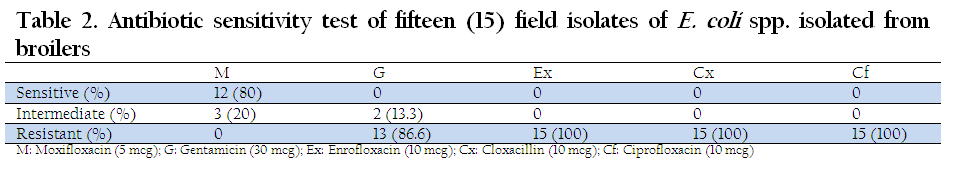
Table 2: Antibiotic sensitivity test of fifteen (15) field isolates of E. coli spp. isolated from broi
Field study 1
Disease was diagnosed as complicated chronic respiratory disease (CCRD) on the basis of postmortem lesions. At the age of 21st day Moxifloxacin (MofoiTM) was given @ 5mg/kg body weight orally. The mortality was reduced from 500 birds per day to 106 birds. The treatment was continued to next two days. On 23rd day the mortality was reduced to 52 birds and the birds started putting on weight.
Field study 2
On post–mortem examination fibrinous pericarditis and peri–hepatitis was observed along with severe tracheitis and air sacculitis. Depending on clinical signs and postmortem lesions the disease was diagnosed as complicated chronic respiratory disease (CCRD). At the age of 15 days treatment was started with Moxifloxacin (MofoiTM) @ 5mg/kg body weight. First dose of MofoiTM was given @ 5mg/kg body weight through parenteral route i.e. intramuscular, followed by oral therapy in the morning 2–3 hours drinking water at the same dose for two more days. Within 12 hours of first dose mortality was reduced to 10% and after completion of 3 day treatment there was no mortality in the flock and birds feed intake resumed to normal.
DISCUSSION
The pharmacokinetic studies has been studied extensively in animals but to our knowledge this is the first report from India showing the antimicrobial sensitivity pattern of moxifloxacin in poultry as well the successful therapeutic field trials of moxifloxacin in broilers affected with complicated chronic respiratory disease. In the present investigation it was revealed that moxifloxacin showed high degree of sensitivity against Salmonella as well as E. coli isolates. It was observed that most of the isolates were resistant to the antibiotics that continue to be used in the field for a long time, it may be because of the indiscriminate use of various antibiotics by the farmers. The similar findings were observed by Oteo et al., (2005); Kumar et al., (2012) and Joshi et al., (2012). Moxifloxacin exerts potent bactericidal activity against a wide range of pathogens and in addition possesses pharmacokinetic properties that result in high concentrations in the circulation and tissues, thereby providing adequate bactericidal concentrations at the foci of infection (Balfour and Wiseman, 1999). Mycoplasma infections have been shown to reduce the performance of commercial broilers, egg–laying hens, dairy cows and goats, leading to significant economic loss (Viscione et al., 2008). In the present field trial studies it was observed that moxifloxacin was able to control the mycoplasma and E.coli infections very effectively and at very short span of time. The present study was supported by the findings of Hamamoto et al., (2001); Goudah (2008) and Li et al., (2012) as they proposed moxifloxacin as promising antimycoplasmal agent. Clinical efficacy of moxifloxacin showing high systemic bioavailability, extensive distribution to pulmonary tissues and fluids, and a low incidence of gastrointestinal side effects in humans was also demonstrated (Ball, 2000; Blondeau and Hansen, 2001), proving it to be an efficient drug to combat most of the bacterial infections. With the growing clinical need because of existence of multidrug existence pattern, a new antimicrobial drug, moxifloxacin, a novel 8–methoxy quinolone, has been introduced into the poultry medicine to combat most of the existing poultry bacterial challenges.
REFERENCES
Balfour JA and Wiseman LR (1999). Moxifloxacin. Drugs. 57:363 – 73.
PMid:10193688
Ball B (2000). Moxifloxacin (Avelox) an 8–methoxyquinolone antibacterial with enhanced potency. International Journal of Clinical Practice. 54: 329 – 332.
PMid:10954961
Bauer AW, Kirby WMM, Sherries JC and Truck M (1996). Antibiotic susceptibility testing by standardized single disc method. American Journal of Clinical Pathology. 45: 493.
Blondeau JM and Hansen GT (2001). Moxifloxacin: a review of the microbiological, pharmacological, clinical and safety features. Expert Opinion on Pharmacothery. 2: 317 – 335
http://dx.doi.org/10.1517/14656566.2.2.317
PMid:11336589
Brown SA (1996). Fluorquinolones in animal health. Journal of Veterinary Pharmacology and Therapeutics. 19: 1 – 14.
http://dx.doi.org/10.1111/j.1365-2885.1996.tb00001.x
PMid:8992019
Carceles CM, Serrano JM, Marin P, Escudero E and Fernandez–Varon E (2006). Pharmacokinetics of moxifloxacin in rabbits after intravenous, subcutaneous and a long acting poloxamer 407 gel formulation administration. Journal of Veterinary Medicine. 53: 300 – 304.
http://dx.doi.org/10.1111/j.1439-0442.2006.00827.x
PMid:16901274
Carceles CM, Villamayor L, Escudero E, Marin P and Fernandez–Varon E (2007). Pharmacokinetics and milk penetration of moxifloxacin after intramuscular administration to lactating goats. The Veterinary Journal. 173: 452 – 455.
http://dx.doi.org/10.1016/j.tvjl.2005.11.003
PMid:16377219
Gardner SY, Davis JL, Jones SL, Lafevers, DH, Hoskins MS, Mcarver EM and Papich MG (2004). Moxifloxacin pharmacokinetics in horses and disposition into phagocytes after oral dosing. Journal of Veterinary Pharmacology and Therapeutics. 27: 57 – 60.
http://dx.doi.org/10.1046/j.0140-7783.2003.00529.x
PMid:14995968
Goudah A (2008). Pharmacokinetics and tissue residues of moxifloxacin in broiler chickens. British Poultry Science. 50:2, 251 – 258.
http://dx.doi.org/10.1080/00071660802710108
PMid:19373726
Goudah A. (2009). Disposition kinetics of moxifloxacin in lactating ewes. The Veterinary Journal.78: 282 – 287.
http://dx.doi.org/10.1016/j.tvjl.2007.08.007
PMid:17900948
Hamamoto K, Shimizu T, Fujimoto N, Zhang Y and Arai S (2001). In Vitro Activities of Moxifloxacin and Other Fluoroquinolones against Mycoplasma pneumonia. Antimicrob. agents Chemother. 45 (6): 1908 –1910.
http://dx.doi.org/10.1128/AAC.45.6.1908-1910.2001
PMid:11353651 PMCid:PMC90571
Joshi S, Singh R and Singh SP (2012). Antibiotic resistance profile of Escherichia coli isolates from Colibacillosis in and around Pantnagar, India. Vet. World. 5 (7): 405 – 408.
http://dx.doi.org/10.5455/vetworld.2012.405-408
Kowalski RP, Dhaliwal DK, Karenchak LM, Romanowski EG, Mah FS, Ritterband DC and Gordon YJ (2003). Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. American Journal of Ophthalmology. 136: 500 – 505.
http://dx.doi.org/10.1016/S0002-9394(03)00294-0
Kumar T, Mahajan NK and Rakha NK (2012). Isolation and prevalence of Salmonella serovars from poultry in different parts of Haryana, India. Indian Journal of Animal Sciences. 82 (6): 557 – 560.
Li L, Shen W, Zhang K, Tang X, Guo N, Xing M, Liu L, Yuan P, Shi Q, Liang J, Shen F and Yu L (2012). In–vitro Antimycoplasmal Activity of Triclosan in Combination with Fluoroquinolones against Five mycoplasma Species. Iranian Journal of Pharmaceutical Research. 11 (4): 1111 – 1119.
PMid:24250544 PMCid:PMC3813145
Oteo J, Lazaro E, De Abajo FJ, Baquero F, Campos J and Spanish members of EARSS (2005). Antimicrobial–resistant invasive Escherichia coli, Spain. Emerg. Infect. Dis. 11: 546 – 53.
http://dx.doi.org/10.3201/eid1104.040699
PMid:15829192 PMCid:PMC3320321
Spreng M, Deleforge J, Thomas E, Boisrame B and Drugeon H (1995). Antibacterial activity of marbofloxacin. A new fluoroquinolone for veterinary use against canine and feline isolates. Journal of Veterinary Pharmacology and Therapeutics. 18: 284 – 289.
http://dx.doi.org/10.1111/j.1365-2885.1995.tb00592.x
PMid:8583541
Viscione KA, Branton SL, Vance AM, Gerard PD, Whitmarsh SK and Peebles ED (2008). Effects of 6/85–strain Mycoplasma gallisepticum inoculation alone at ten weeks of age or in conjunction with F–strain Mycoplasma gallisepticum inoculation overlays at twenty–two or forty–five weeks of age on the performance of commercial egg–laying hens. Poult. Sci. 87: 588 – 93.
http://dx.doi.org/10.3382/ps.2008-00009
http://dx.doi.org/10.3382/ps.2007-00424
PMid:18281589