Journal of Animal Health and Production
Research Article
Hepatocyte Nuclear Factor 4-α, Glucocorticoid Receptor and Heat Shock Protein 70 mRNA Expression during Embryonic Development in Chickens
Abdelkareem Abdallah Ahmed1*, Hassan Hussein Musa2, Amal Zakaria Sifaldin1, Taha Hussein Musa 3, Jaafar Sulieman Fedail4
1Department of Physiology and Biochemistry, Faculty of Veterinary Sciences, University of Nyala, Nyala, Sudan; 2Faculty of Medical Laboratory Sciences, University of Khartoum, Khartoum, Sudan; 3Key Laboratory of Environmental Medicine, Ministry of Education, School of Public Health, Southeast University, Nanjing 210009, Jiangsu, China; 4Department of Biology, Faculty of Education, University of Nyala, Nyala, Sudan.
Abstract | Glucocorticoids (GCs) play a vital role during embryonic development. Hepatocyte nuclear factor 4 alpha (HNF4α) is a transcription factor that has been shown to be crucial for hepatocyte differentiation and development of the liver. Here we aimed to disclose the differences in embryonic expression of hepatic HNF4-α, glucocorticoid receptor (GR) and heat shock protein 70 (HSP70) between slow and fast growing broiler chickens embryos. Fast-growing chicken and slow-growing chicken eggs were incubated and liver samples from the embryonic development at embryo 10 (E10), (E14), (E18) and day 1 post hatch (D1) were collected. Quantitative PCR used to measure hepatic mRNA expression of GR, HSP70 and HNF4-α. Hepatic mRNA expression of HNF4-α was significantly higher in fast-growing chicken embryos at all the embryonic stages investigated. However, hepatic GR mRNA expression was significantly higher in fast-growing chicken embryos only on E10. Hepatic mRNA expression of HSP70 was significantly (P<0.05) higher in fast-growing chicken embryos at E18 and D1 posthatch stages. Our data may provide the first evidence that hepatic expression of HNF4-α, GR and HSP70 differs between fast-growing and slow-growing chickens embryos, which may account to some extent for the breed disparities in embryonic development.
Keywords | Chicken embryo, GR, HSP70, HNF4-α, Liver
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | May 23, 2015; Revised | June 11, 2015; Accepted | June 15, 2015; Published | June 23, 2015
*Correspondence | Abdelkareem Abdallah Ahmed, University of Nyala, Nyala, Sudan; Email: [email protected]
Citation | Ahmed AA, Musa HH, Sifaldin AZ, Musa TH, Fedail JF (2015). Hepatocyte nuclear factor 4-α, glucocorticoid receptor and heat shock protein 70 mRNA expression during embryonic development in chickens. J. Anim. Health Prod. 3(3): 54-58.
DOI | http://dx.doi.org/10.14737/journal.jahp/2015/3.3.54.58
ISSN | 2308–2801
Copyright © 2015 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Avian eggs contain a variety of maternal derived steroid hormones, which mediate maternal influences on offspring phenotype (Gil, 2008; Groothuis et al., 2005). Glucocorticoids (GCs) are known to play vital roles in embryonic development and mediate maternal effects in mammals (Gmelin et al., 1985) and birds (Spencer et al., 2009). GCs exert their effects by binding to glucocorticoid receptor (GR) and mineralocorticoid receptors (MR) that belong to the steroid receptor family. GR and MR are present in almost every cell of the body; regulate expression and functions of many genes that controlling a variety of biological functions (Shohami et al., 1995). In birds, the CORT is the principal GCs, and its metabolism occurs mostly in liver (Kucka et al., 2006). Cloning and characterization of GR in pituitary and tissues during chick embryonic development have been reported (Kwok et al., 2007; Porter et al., 2007). However, the relevant information between breeds is scare.
Stressful condition increased synthesis of a group of stress related proteins that belonging to the heat shock protein (HSP) families. HSP classified into 6 families (HSP 10 to 100) based on their molecular weight (van Eden et al., 2005). Heat shock proteins carry out essential housekeeping functions and are molecular chaperones which are vital for the survival of cells. Among these families, Hsp-70 is necessary for protein folding cell and translocation (Mayer and Bukau, 2005). The HSP70 is expressed in both normal and is highly stress-inducible cells, and it has been extensively studied as stress biomarker (El Golli-Bennour and Bacha, 2011). Stress responses are start by activating corticotropin-releasing factor (Shekhar et al., 2005), which recruits endocrine, immune, and neural systems (Kamimura et al., 2013). The type and level of stress determines the outcome, but constant responses are stimulated by of the hypothalamic pituitary adrenal axis. The ontogeny of temperature-regulated heat shock protein 70 synthesis in preimplantation bovine embryos has been reported (Edwards et al., 1997). Upto date, the breed differences of HSP70 chicken embryo is not reported.
Hepatocyte nuclear factor 4α (HNF-4α) is a liver-enriched transcription factor which, plays a crucial role in the tissue-specific expression of a variety of genes (Sladek et al., 1990). In addition, HNF-4α has a critical role in the adult liver, where it controls a number of genes involved in stress response such as endoplasmic reticulum stress-dependent gene (Arensdorf et al., 2013) It has been implicated the function of HNF-4α in early endodermal development (Duncan, 2000). The absence of HNF-4α in mouse embryos resulted in embryonic death before completing gastrulation due to the dysfunction of visceral endoderm system (Chen et al., 1994). In gene expression patterns, the role of HNF-4α was found to be vital for subsequent steps of hepatocyte differentiation (Li et al., 2000). In humans, heterozygous mutations of HNF-4α were strongly associated with so called maturity onset diabetes of the young 1 (Yamagata, 1996). However, the expression of HNF-4α during embryonic development in chicken and its association with egg deposition of CORT between slow and fast growing broiler chickens has not been investigated. Therefore, the objectives of this study were to investigate the expression of HNF-4α and GR and HSP70 mRNA in the developing embryos of slow and fast growing chickens.
Materials and Methods
Breeder Eggs, Incubation and Tissue Sampling
Forty fertile eggs of slow-growing chicken (n=20) and the fast-growing chicken (n=20) were incubated at 37.5 ± 0.5°C and 60% relative humidity following standard settings for automatic turning and ventilation. The eggs were put side by side and distributed equally on different shelves in the incubator so as to minimize possible variations which may occur during the incubation period. The first day of incubation was assigned as embryonic day 1 (E1) and the day of hatching was assigned as day 1 (D1). On E10, E14, E18 and D1, liver samples (n= 10 per each breed) were collected. Liver samples were quickly frozen in liquid nitrogen then stored at −80°C for RNA extraction.
RNA Extraction and mRNA Quantification
The liver samples were ground with pestle and mortar in liquid N2 and a portion of approximately 50 mg was used for the RNA extraction using the TRIzol total RNA kit (Invitrogen, Biotechnology Co, Ltd, Carlsbad, CA, USA) according to the manufacturer’s instructions. To ensure that total RNA preparations were free of genomic DNA contamination, two methods were used. First, the total RNAs were treated with 10 U DNase I (RNase Free, D2215, Takara, Japan) for 30 min at 37°C, and were purified according to the manufacturer’s protocol. Second, the primers for the reference gene (β-actin) were designed to span an intron, ensuring that any genomic DNA contamination can be reported easily by an extra product in the melting curves for real-time PCR. Real-time PCR was performed in an Mx3000P (Stratagene, USA) according to our previous publication (Ahmed et al., 2014). Mock RT and No Template Controls (NTC) were included to monitor the possible contamination of genomic and environmental DNA at the RT and PCR steps. A pooled sample made by mixing equal quantities of the RT products (cDNA) from all the samples was used for optimizing the PCR conditions and tailoring the standard curves for each target gene, and melting curves were performed to insure a single specific PCR product for each gene. The PCR products were sequenced to validate the identity of the amplicons. Primers specific for the HNF4α and GR (Table 1) were synthesized by Geneary, Shanghai, China. Chicken β-actin was used as a reference gene for normalization purposes. The mRNA quantification was based on threshold cycles (Ct) rather than band densities in the quantitative real-time PCR. The method of 2−ΔΔCt was used to analyze the real-time PCR data (Livak and Schmittgen, 2001).
Statistical Analysis
The relative quantitative data of gene expression were analysed by Two-way ANOVA using SPSS 16.0 for Windows followed by a least-significant difference (LSD) test for individual comparisons. Values of mRNA abundance are expressed as the fold change relative to the average value of one group. The data were expressed as mean ± SEM. A P-value < 0.05 was considered significant.
Results
Ontogeny of HNF4α, GR and HSP70 in Fast Growing and Slow Growing Chicken Embryos Liver
In the present study, we reported the ontogeny of HNF-4α, GR and HSP70. The HNF-4α mRNA expression was significantly (P<0.05) higher in fast-growing chicken embryos liver at all the embryonic stages investigated except E10 (Figure 1A). In contrast, GR mRNA expression was significantly (P<0.05) higher in fast-growing chicken embryos liver at E10 but not on other stages (Figure 1B). However, the HSP70 mRNA expression was significantly (P<0.05) higher in fast-growing chicken embryos liver at E18 and D1 (Figure 1C).
Table 1: Primers sequences used in Real-time PCR
|
Target genes |
Gen Bank accession number |
PCR products (bp) |
Primer sequences |
|
β-actin |
L08165 |
300 |
F : 5’- TGCGTGACATCAAGGAGAAG -3’ R : 5’- TGCCAGGGTACATTGTGGTA -3’ |
|
GR |
DQ227738 |
102 |
F : 5’- CTTCCATCCGCCCTTCA -3’ R : 5’- TCGCATCTGTTTCACCC -3’ |
|
HNF4α |
AY700581.1 |
116 |
F: 5’- GAGTGGGCCAAGTACATCCC -3’ R: 5’- TCGTTCCCTAGCAGCAAGAC -3’ |
|
HSP70 |
AY178443.1 |
230 |
F: 5’- GCGAGTGGCTGACTGACCA-3’ R: 5’-AAGTATGATGACCCCACA -3’ |
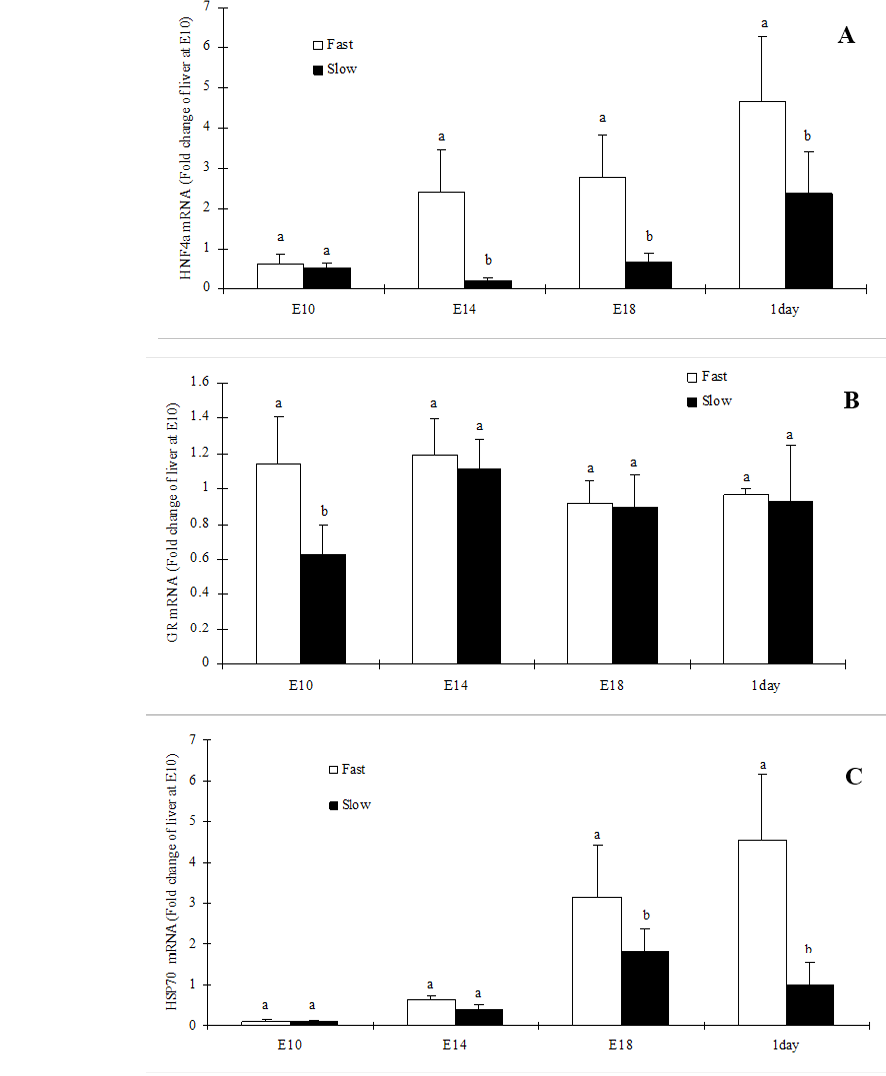
Figure 1: Hepatic expression of HNF4α (A), GR (B) and HSP70 (C) mRNA in slow-growing and fast-growing broiler breeders’ embryos
Values are mean ± SEM; n=6/group; Different superscript letters on the bars indicate significantly different mean values at P<0.05
Discussion
Glucocorticoids (GCs) are known to play critical roles in embryonic development and maternal programming both in mammals and birds (Gmelin et al., 1985; Spencer et al., 2009). Heat shock proteins (HSPs), a molecular chaperones play a critical role in the protection of cells from extreme pathological, physiological, and environmental conditions (Kiang and Tsokos, 1998). HSPs increase glucocorticoid receptor (GR) activity in cells and implicate cross-talk between the heat shock and GR signal pathways. In the present study, fast-growing chicken embryo livers expressed high HSP70 mRNA than that of slow-growing broiler chicken embryo. Moreover, the fast-growing chicken embryo livers expressed high abundant HSP70 mRNA than that of slow-growing broiler chicken embryo. Based on our findings, we postulate that the differences in GR and HSP70 mRNA expression during the embryonic development may have a role in the embryonic development of the chick liver. Moreover, the differences in GR and HSP70 between these two chicken breeds during the embryonic development may explain the differences in stress response in life later.
Hepatocyte nuclear factor 4 alpha (HNF4α) is crucial for the organization and maintenance of liver-specific gene expression. Here we reported for the first time that hepatic expression of HNF4α was significantly abundant in fast growing broiler chicken embryos livers than that of slow growing breed. This could be due to the fact that HNF4α play a critical role in liver morphogenesis (Chen et al., 1994; Zaret, 2000; Zaret, 2002) differentiation (Costa et al., 2003) and metabolism (Gonzalez, 2008). Previous studies reported that dexamethasone (Synthetic GCs) mediated up-regulation of human CYP2A6 involves the glucocorticoid receptor and increased binding of HNF4α to the Proximal Promoter (Onica et al., 2008). However, the precise cross-talk between HSP, GR and HNF4α is currently unclear. Further studies are required to clarify the cross-talk between those stress and differentiation related genes.
Acknowledgments
The authors are highly grateful to Professor Zhao and Shi Fangxiong at the Nanjing Agricultural University for their support to conduct this research.
Conflict of interests
There exisit not any conflict of interest exists.
Authors’ contributions
For creating this article, Abdelkareem, Jaafar and Amal conducted the experiment while Taha and Hassan wrote and revised the manuscript.
References






