Advances in Animal and Veterinary Sciences
Research Article
Blueberry Extract (Vaccinium corymbosum) Attenuates Tnf-α Expression and Renal Inflammatory Cell Counts in Rats Models of Acute Kidney Injury
Ahmad Fauzi*, Dian Vidiastuti, Aldila Noviatri, Muhammad Yusuf Rizal
Faculty of Veterinary Medicine University of Brawijaya, Malang, East Java, Indonesia 65151.
Abstract | Acute kidney injury is a loss of kidney function characterized by decreased kidney function, decreased glomerular filtration, and increased levels of urea and creatinine. Blueberry extract has a high anthocyanin content which can act as an antioxidant and anti-inflammatory, making it a potential candidate for natural therapy for acute kidney injury. The purpose of this research was to determine the role of blueberry extract on TNF-α expression and the number of inflammatory cells in acute kidney injury. Rats (Rattus norvegicus) male Wistar stock aged 6-8 weeks, body weight between 200-250 grams, 20 rats were divided into 5 groups consisting of a negative control group (NC), namely rats without treatment, a positive control group (PC) namely rats induced acute kidney injury by glycerol 50%, and therapy groups T1, T2, T3 induced acute kidney injury and were given blueberry extract at doses of 500 mg/kg, 1000 mg/kg, and 1500 mg/kg respectively. The Blueberry extract was given orally 1 x 24 hours for 14 days. At the end of the research, the rats were euthanized, and the rats’ kidneys were taken for the measurement of TNF-α expression using immunohistochemical staining and the number of inflammatory cells using hematoxylin-eosin staining. Analysis of quantitative data on TNF-α expression and the inflammatory cells count using the One Way ANOVA test with a significance value of P<0.05. The results showed that the extract of Blueberry could reduce TNF-α expression (P<0.05) and the number of inflammatory cells (P<0.05) in the kidney with the best dose of 1500 mg/kg. The conclusion is the blueberry extract has a therapeutic effect on acute kidney injury based on TNF-α expression and the number of inflammatory cells.
Keywords | Acute kidney injury, Blueberry, TNF-α, Inflammatory cell, Renal
Received | March 12, 2021; Accepted | March 17, 2021; Published | June 15, 2021
*Correspondence | Ahmad Fauzi, Faculty of Veterinary Medicine University of Brawijaya, Malang, East Java, Indonesia 65151; Email: [email protected]
Citation | Fauzi A, Vidiastuti D, Noviatri A, Rizal MY (2021). Blueberry extract (vaccinium corymbosum) attenuates tnf-α expression and renal inflammatory cell counts in rats models of acute kidney injury. Adv. Anim. Vet. Sci. 9(7): 1087-1094.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.1087.1094
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Fauzi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Kidney failure due to acute kidney injury (AKI) shows a higher risk of death compared to kidney failure due to diabetes or other causes (Shah et al., 2020). Acute kidney injury is initiated by tubular cell damage, in which cell necrosis occurs followed by an inflammatory cell response such as leukocytes and expression of proinflammatory cytokines and chemokines (Sato and Yanagita, 2018). Early diagnosis and the drugs were chosen are the key to successful treatment. Several drugs agent like NSAID, gen
tamicin, and cisplatin have been reported to be nephrotoxic to the kidney and triggering the acute tubular necrosis (Bensman, A., 2019; Fauzi et al., 2020; Huang et al., 2020; Xiang et al., 2019) so that these nephrotoxic drugs should be avoided as much as possible, to prevent further AKI disease. Challenges in the targeted treatment of AKI are very important in veterinary medicine today (Gao et al., 2020). Treatment efforts are still being developed, which is by exploring drug supplementation that can reduce the risk of inflammation and injury to the kidneys.
Reduction of inflammation in tubular injury had an important role in improving the condition of acute kidney injury. Blueberry had been considered to have a neuroprotective effect on Alzheimer’s disease (Ma, H 2018), an antioxidant and anti-inflammatory effect in diabetic retinopathy (Huang et al., 2018), and an antioxidant effect in AKI (Nair et al., 2014). blueberries and cranberries were known for having high levels of antioxidants like anthocyanins, resveratrol, proanthocyanidins, flavonoids, and polyphenols (Wang et al., 2019; Jurikova, 2019). Anthocyanins and polyphenol of blueberry have been reported can inhibit pro-inflammatory molecules and products of oxidative stress (DelBo et al., 2016). Therefore, this research is very important to research to determine the anti-inflammatory effect by observing the TNF-α expression and renal inflammatory cell count in animal models of acute kidney injury and finding the effective dosage of blueberries.
MATERIALS AND METHODS
Experimental Model
As many as 20 White Rats (Rattus norvegicus) Wistar Stock aged 6-8 weeks with an average 150-250 grams of body weight were divided into 5 treatment groups, each cage was contained 4 rats consisting negative control group (NC) which contain untreated rats, the positive control group (PC) was induced by 50% glycerol, and the T1, T2, T3 therapy groups were induced by 50% glycerol and treated with the ethanol blueberry extract at dosage 500 mg/kg BW, 1000 mg/kg BW, dan 1500 mg/kg BW, and blueberry extract was given to rats orally once a day for 14 days. Rats were given standard feed and ad-libitum water during treatment (Scudamore, 2014). The whole model has complied with the guidelines declared by the institutional animal care and use committee with the registration number No. 1132/KEP/UB.
Animal Model of Acute Kidney Injury
The animal models of acute kidney injury were achieved by injecting 50% glycerol at single dosage 10mL/kg BW intramuscularly, where the injection was divided into the bicep femoris muscle of the right leg and left leg (Singh, 2012). The 50% glycerol was made by diluting the glycerol with 1:1 aqua pro injection in the falcon tube. The solution was then homogenized with a vortex mixer for 5 minutes. According to Fauzi et al. (2016a) and Korrapati et al. (2012) distribution of 50% glycerol at a dose (10 mL/kg) intramuscularly to rats can cause renal dysfunction, ischemia in renal cells, and nephrotoxicity in tubules which are known as acute tubular necrosis. Glycerol induction was given to the positive control group and the therapy group (T1, T2, and T3).
Determination of BUN and Creatinine Serum
The determination of blood urea nitrogen (BUN) and creatinine levels was analyzed using a photometer Horiba Pentra C2000 Japan. BUN and creatinine levels were tested by enzymatic reaction following the manufacturer’s instructions. The normal value of BUN levels in rats was 13.9 - 28.3 mg/dL and the normal value of creatinine levels was 0.30 - 1.00 mg/dL (Weiss and Wardrop, 2011).
Blueberry extract preparations
Blueberries are obtained from local markets in Malang, East Java, Indonesia. The blueberries were tested for determination, extraction, and phytochemical at UPT Materia Medica Batu Indonesia. The maceration method is used in the extraction of blueberries, with 70% ethanol solvent (Barnes et al., 2009). The blueberry extract was started to be given to the rats a day after injection of 50% glycerol.
Kidney Histopathology preparation and examination
At the end of the research, The rats’ kidneys were collected and were put in 10% formaldehyde for histopathological preparation. The kidneys were sliced into 2×1×0.5 cm and were rinsed with distilled water. Then the sliced tissue was put inside a tissue cassette for fixation with 10% formaldehyde. Then the tissues proceed to be dehydrated with increasing levels of alcohol from 70%, 80%, 90%, 95%, and 100%. After the dehydration process was completed, the clearing process was done by dipping samples into xylol 1, 2, and 3. Then, the infiltration process was preceded by immersing the tissues to liquid paraffin 1 and 2, followed by the embedding process, which is the process of embedding the tissue to the paraffin block by pouring liquid paraffin into the paraffin mold. The tissue was put before the liquid paraffin. After the paraffin hardened, the paraffin blocks were cut using a microtome, The renal paraffin block was sliced within 3-4µm the thickness of sectioning (Chong et al., 2012). Kidney tissue was stained by Hematoxylin-eosin staining, with 400x magnification measurement on BX51 Olympus microscope.
Calculation of the inflammatory cells number
The number of inflammatory cells was analyzed by observing the histopathology of the kidneys in the glomerular and tubular sections with Hematoxylin Eosin (HE) staining. Inflammatory cells counted are neutrophil cells, macrophage cells, and lymphocytes. Histological observation refers to the research conducted by Ysebaert et al. (2000), in which the inflammatory cells were counted in 5 visual fields with the unit cell yield/mm2 in 1000x magnification. Calculation of the number of inflammatory cells of kidney tissue using the Image J program (Symmetry Software, NIH. Gov) (Liu et al., 2017).
Immunohistochemistry of TNF–α Expression
TNF-α expression was determined in Kidney specimens that were fixed with formaldehyde and embedded in paraffin. Paraffin blocks were prepared serially as thick as 4 μm from each representative paraffin block on Poly-L-Lysine coated slides and stained for TNF-alpha with a rabbit polyclonal anti-TNF-α (1:100, Bioss, Boston, MA, USA) using the Histofine Simple Stain kit (Nichirei Corporation) following the manufacturer’s instructions. All parts of the tissue were evaluated with a light microscope (BX53, Olympus, Tokyo, Japan) and a microscope camera (XC10 Olympus, Tokyo, Japan). Observation of the TNF–α expression using Immunohistochemistry by observing 5 fields of view with 400x magnification then assessment of the average of the percentage area. Calculation of TNF-alpha expression of kidney tissue using the Image J program to measurement percentage of area expression of immunoratio (Symmetry Software, NIH. Gov) (Liu et al., 2017).
Data Analysis
The TNF-α expression and the number of inflammatory cells were analyzed quantitatively with SPSS for Windows 8 software with Analysis of Variant Analysis (ANOVA) and Tukey post hoc test with a significance value of P <0.05.
RESULTS AND DISCUSSION
Acute Kidney injury is a crisis with a sudden decrease in kidney function due to an increase in metabolites production such as urea and creatinine. The following Table 1 summarizes the results of BUN levels and serum creatinine levels after induced by 50% glycerol.
Based on Table 1, there was an increase in BUN and creatinine levels in the positive control group with values of 65.45 mg/dL and 1.6 mg/dL when compared to the negative control group. The injection of 50% glycerol can induce rhabdomyolysis and the incidence of acute kidney injury (Fauzi et al., 2016b). The normal value of BUN levels in white rats was 13,9 – 28,3 mg/dL, and the normal creatinine levels were 0.30 to 1.00 mg/dL. The increased levels of BUN and creatinine indicate abnormalities in function in the kidneys due to injury to nephron, especially in the renal tubules, so that the filtration and reabsorption functions fail and result in retention of serum BUN and creatinine in the blood (Sharawy et al., 2018).
Blueberry Attenuates Tnf-Α Expression In Kidney Rats Model Of Acute Kidney Injury
TNF-α expression in rat kidneys from each treatment group was shown in Figure 1.
Table 1: The Level of BUN and Creatinine in acute kidney injury rats’ model
| Parameter | Group | |
| Negative Control (mg/dL) | Positive Control (mg/dL) | |
| BUN | 23.08 | 65.45 |
| Creatinine | 0.5 | 1.60 |
The results showed that the TNF-α expression in each treatment group was indicated by the difference in the percentage of brown color shown in the expression area formed. In the negative control group (NC), TNF-α expression was less than the positive control group (PC) and (T1), (T2), and (T3) decreased TNF-α expression in a gradient from the positive control group (Figure 1).
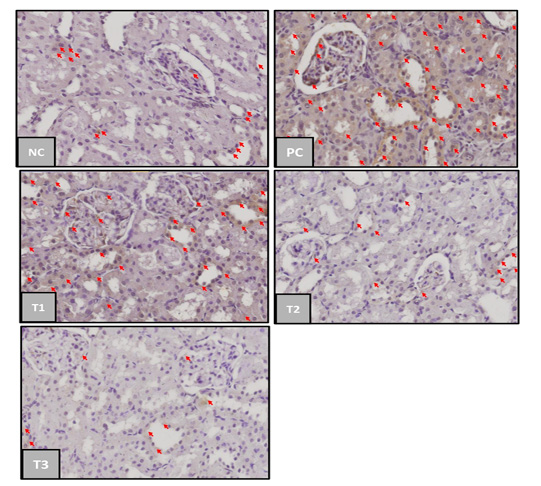
Figure 1: TNF-α-expression in kidney of acute kidney injury (AKI). Negative control group (NC); Positive Control Group (PC); blueberries extract on therapy group with dosage of 500 mg/kg (T1); dosage 1000 mg/kg (T2); dosage 1500 mg/kg (T3); the red arrow indicated TNF–α expression. (immunochemistry staining, magnification 400x).
Table 2: Significant decrease of TNF-α expression in acute kidney injury shown in blueberry therapy groups
| Group |
TNF–α Expression (% area) Mean ± SD |
| Negative Control (NC) |
28.48 ± 11.95a |
| Positive Control (PC) |
65.35 ± 9.67b |
| Blueberry 500 mg/kg (T1) |
52.43 ± 12.58ab |
| Blueberry 1000 mg/kg (T2) |
48.28 ± 17.35ab |
| Blueberry 1500 mg/kg (T3) |
33.46 ± 12.77a |
Direction: note a, b dan c indicated significant differences (p<0.05)
Based on Table 2. the positive control group had a significant difference of TNF–α expression mean (p<0,05) compared to the negative control group. Blueberry extract therapy groups on treatments 1 and 2 had no significant difference (p>0.05) compared to the positive and negative control group, while the treatment 3 group has a significant difference compared to the positive control group. These results showed that blueberry therapy at a dosage 1500mg/kg was able to provide a significant therapeutic effect on the incidence of acute kidney injury. TNF-α can appear in normal conditions in small amounts as an immune response produced by macrophages in the phagocytosis process of dead cells and antigens that enter the body (Chu, 2013).
The positive control group had the highest mean TNF-α expression which had 65.35%, it was due to acute kidney injury after 50% glycerol induction and not given any therapy to relieve the inflammation. 50% glycerol induction will cause rhabdomyolysis conditions thereby increasing the production of nephrotoxic myoglobin. Myoglobin induces oxidative stress and lipid peroxidation of proximal tubular cells, then inducing the release of a series of mediators, including the pro-inflammatory cytokine TNF-α. The more inflammation in the tissue, the higher the TNF-α level (Wu, et. al., 2017).
The Blueberry extract however only presented a slight decrease in TNF-α expression for T1 and T2 groups compared to T3 group marked with a different notation from positive control group (PC). The average TNF-α expression between treatment groups decreased in proportion to the increase in the therapeutic dose of blueberry extract. This is in line with the research of Nair et al. (2014) stated that there was a decrease in the production of proinflammatory cytokines TNF-α in the kidneys in animal models of metabolic syndrome that had been given blueberry extract therapy. The average value of TNF-α expression in treatment group 1 (dose 500 mg/kg) was 52.43%, treatment group 2 (dose 1000 mg/kg) was 48.28%, and treatment group 3 (dose 1500 mg/kg) was 33.46%. When the three therapy groups were compared to the effect of reducing TNF-α expression, the treatment group 3 (dose 1500 mg/kg) was the best dose of blueberry fruit extract in reducing TNF-α expression as an anti-inflammatory in rats model of acute kidney injury.
The decrease in TNF-α expression was due to the blueberry extract therapy containing high amounts of anthocyanins and flavonoids. Based on the results of phytochemical testers, our blueberry extract contains an anthocyanin content of 150.98 ppm. Blueberry extract anthocyanins are reported to act as anti-inflammatory and antioxidants in diabetic retinopathy (Huang et al., 2018). Anthocyanins are also reported to have antioxidant, anti-inflammatory, anti-carcinogenic, and protective effects from heart damage and improvement of the microcirculation (Ghosh, 2007). Flavonoids can reduce the expression of pro-inflammatory cytokines/chemokines including TNF-α and inhibit the activity of NF-Kß and stimulate cell regeneration in necrotic tubules. Anthocyanin acts as decreasing the lipid inflammatory perox enzyme so that it can suppress the production of pro-inflammatory cytokines TNF-α (Nicoletti et al., 2015).
The mechanism of action of blueberry compounds has been reported to vary in various cell systems. Blueberry polyphenols act as an anti-inflammatory in brain microglial cells by developing several inflammatory cytokines, TNF-a, LPS, nitric oxide, and IL1b (Ma H et al., 2018a; Ma L et al., 2018; Lau et al., 2007). Some of the compounds contained in the most abundant blueberries include the conjugates of delphinidin, malvidin, and cyanide, which exhibit strong anti-tumorigenic and anti-inflammatory effects (Pereira et al., 2017). Anthocyanin-delphinidin compounds work through various pathways such as caspase activation and cytokine inhibition (Haseeb et al., 2013), including Delphinidin inhibits NF-kB and COX-2 activation induced by IL-1b human chondrocyte expression. IL-1b and NF-kB are major mediators of inflammation and disease progression. The combination of cyanidin, malvidin, peonidin, petunidin, and delphinidin (chloride form) suppresses the growth of aggressive non-small cell lung cancer and reduces the expression of NO, PGE2, IL-8, iNOS, and COX-2 on the lower thrusters of NSAIDs, acids. 5-aminosalicylate (Serra et al., 2013).
Blueberry Attenuates Renal Inflammatory Cell Count In Kidney Rats Model Of Acute Kidney Injury
The results of therapeutic effect from blueberry extract on the number of inflammatory cells based on histopathology of rats’ kidney (Rattus norvegicus) in the acute kidney injury (AKI) model is illustrated in (Figure 2).
The histopathology of the kidney showed that there were inflammatory cells in all groups. Macrophages have a single large nucleus and an irregular cytoplasm. Microscopically, it can be seen that in the positive control group there were a lot of inflammatory cells compared to the negative control group (NC). Also, in the positive group (PC) there were structural abnormalities of the kidney such as erythrocyte deposits which were a sign of congestion and hemorrhage, eosinophilic masses, tubular epithelial necrosis, widening of the distal tubular lumen, and glomerular necrosis. According to Fauzi et al. (2016b) in rats injection of 50% glycerol can cause acute tubular necrosis with morphological characteristics such as tubular cell necrosis, dilation of the tubular lumen, and swelling of the proximal tubular cells with loss of villi. These toxic substances accumulate in the kidneys, causing tubular epithelial cells and capillary endothelium to experience oxidative injury from the formation of excessive reactive oxygen species (ROS) so that tubular epithelial cells are damaged and acute tubular necrosis. This event will trigger an increase in the number of inflammatory cells in the tissues, especially the tubules (Siahaan et al., 2016).
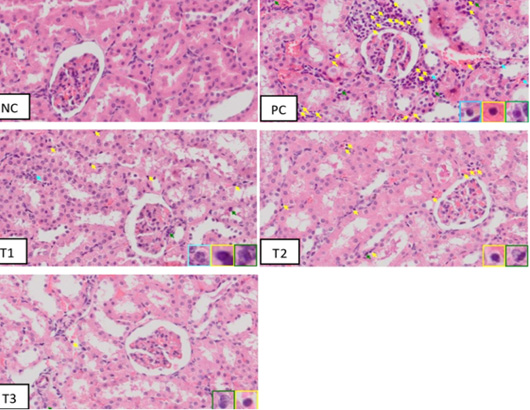
Figure 2: Inflammatory cells on histopathology of Acute Kidney Injury, Negative Control Group (NC) Positive Control Group (PC); Blueberries extract therapy group dosage 500 mg/kg (T1); dosage 1000 mg/kg (T2); dosage 1500 mg/kg (T3). Note: light blue arrow indicated neutrophils inflammatory cells, green arrow indicated macrophage inflammatory cells, and yellow arrow indicated lymphocyte inflammatory cells. (HE staining; magnification 400x)
The treatment group Figure 2 (T1, T2, and T3) indicated a gradual decrease in the number of inflammatory cells and tubular damage compare with positive group. Regeneration of tubular epithelial cells occurs because renal tubular epithelial cells are stable cells, which are capable of dividing rapidly in response to injury but resting in normal circumstances (Siahaan et al., 2016). The following Table 3 summarizes the calculation result from induced rat kidney inflammation cells by 50% glycerol.
Based on the results of Table 3, which showed that the positive control group had significantly different results (p <0.05) from the negative control group, T2 group and T3 group. The T1 group of blueberry extract (dose 500 mg/kg) did not have a reduced effect on inflammatory cell infiltration when compared to the positive control group. Group T2 blueberry extract (dose 1000 mg/kg) and group T3 blueberry extract (dose 1500 mg/kg) with significantly different results against the positive control group. Based on the calculation of the number of inflammatory cells, it can be concluded that blueberry extract therapy at a dose of 1500 mg / kg was able to provide a significant therapeutic effect with results that were close to normal. The negative control group still showed inflammatory cells. This is because inflammatory cells are cells that are normally found in tissues as a form of natural immunity in the body in low numbers even though there is no tissue damage. The low number of inflammatory cells in normal tissue functions in the initial self-defense when there is injury or inflammation (Permata, 2019).
Table 3: The inflammatory cells indicated gradual decrease due to blueberry extract dose
| Groups |
Inflammatory cells (cell/mm2) Mean ± SD |
| Negative Control (NC) |
6 ± 4a |
| Positive Control (PC) |
73 ± 10c |
| Blueberry 500 mg/kg (T1) |
65 ± 16bc |
| Blueberry 1000 mg/kg (T2) |
51 ± 11b |
| Blueberry 1500 mg/kg (T3) |
19 ± 2a |
Direction: note a, b dan c indicated that there were significant differences (p<0.05)
The positive control group had a large mean number of inflammatory cells, which were 73 cells/mm2, the increase in the number of inflammatory cells in the positive control group was an inflammatory response due to acute kidney injury. Intramuscular injection of 50% glycerol causes rhabdomyolysis, which can increase nephrotoxic incidence due to myoglobin deposition in the tubules and glomerulus of the kidney (Korrapati et al., 2012). Myoglobin induces oxidative stress and lipid peroxidation in proximal tubular cells, triggering the release of a series of mediators, including cytokines followed by activation of inflammatory cell leukocytes (Wu et. al., 2017). The number of inflammatory cells will increase along with the amount of tissue damage (Permata, 2019).
The average number of inflammatory cells between the treatment groups decreased in proportion to the increase in the therapeutic dose of blueberry extract. Treatment Group T3 (dose 1500 mg/kg) was the best dose to reduce the number of inflammatory cells in rat’s acute renal failure models. The decrease in the number of inflammatory cells in the treatment group was the result of blueberry therapy which contains anthocyanins. Antocyanins play an important role in reducing the risk of various diseases with antioxidant properties, which can bind free radicals, and has an anti-inflammatory effect by inhibiting cyclooxygenase (COX) and lipoxygenase activity (Pereira et al., 2017). Based on the results of phytochemical testers, our blueberry extract contains an anthocyanin content of 150.98 ppm which has shown the high anthocyanin content in blueberry. Anthocyanins are also reported to have antioxidant, anti-inflammatory, anti-carcinogenic, and protective effects from heart damage and improvement of the microcirculation (Ghosh, 2007). Blueberry and other berry extracts exhibit anti-inflammatory and antioxidant effects in a rat model (Nardi et al., 2016). Several studies have reported that blueberry extract has better antioxidant activity than individual compounds (Van Breda et al., 2018, Van Breda et al., 2019). Several studies in experimental animals have shown that the dietary addition of blueberries or administration of blueberry extract slowed down neurodegeneration due to inflammation and oxidative stress (Subash et al., 2014). Consumption of blueberry extract can play a role in reducing pain and inflammatory responses in osteoarthritis patients (Du C et al., 2019). Various benefits and positive effects of the pterostilbene constituent blueberry were reported to protect the cornea from the inflammation (Li J et al., 2016). Furthermore, clinical studies in animals and humans have shown beneficial effects of blueberries on various metabolic diseases such as diabetes and cardiovascular disease (Kalt et al., 2019).
CONCLUSION
This research concludes that the treatment of the blueberry extract (Vaccinium corymbosum) can reduce the expression of TNF-α expression (P<0.05) and renal inflammatory cell (P<0.05) in animal models of acute renal injury with the best dose of 1500 mg/kg.
ACKNOWLEDGMENTS
The authors are thankful to the Institute of Research and Community Service IRCS-LPPM, Universitas Brawijaya for providing the research funding and to the Faculty of Veterinary Medicine, Universitas Brawijaya which has provided the facilities to carry out the research and also to Chilyah Said Basalamah who contributed to help editing the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTION
All the authors contributed equally during this study and approved the final version of the manuscript.
REFERENCES






