Advances in Animal and Veterinary Sciences
Research Article
Using Hop Cones, Vitamin E, Methionine, Choline and Carnitine for Treatment of Subclinical Ketosis in Transition Dairy Cows
Ihor Vudmaska1*, Iryna Petrukh1, Sergii Sachko1, Vasyl Vlizlo2, Yurii Kosenko2, Mariia Kozak1, Anna Petruk3
1Institute of Animal Biology of NAAS, 38 V. Stus Str., Lviv, 79034, Ukraine; 2State Scientific-Research Control Institute of Veterinary Medicinal Products and Feed Additives, 11 Donetska Str., Lviv, 79019, Ukraine; 3Stepan Gzhytskyj National University of Veterinary Medicine and Biotechnologies, 50 Pekarska Str., Lviv, 79010, Ukraine.
Abstract | The aim of this study was to determine the effectiveness of feed additive that contains hop cones, vitamin E, choline, methionine and carnitine in the treatment of subclinical ketosis in transition dairy cows. Twenty multiparous dairy cows with a milk yield > 5000 kg in the previous lactation were used after calving in this experiment. The cows were divided into two groups: control - healthy cow (n = 10) and experimental – cows with subclinical ketosis (n = 10). The subclinical ketosis cows received a feed additive containing ground pellets of hop cones - 20 g, alpha-tocopherol - 3 g, rumen protected choline - 20 g, methionine - 20 g and L-carnitine - 1 g per head per day for 20 days. Blood samples were collected from the tail (coccygeal) vein before morning feeding on 1st and 20th days of experiment. Levels of glucose and β-hydroxybutyrate ketone were determined in blood. Insulin and cortisol were measured in the plasma. Total protein, albumin, urea nitrogen, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in serum. In the urine, ketone bodies were detected. The results obtained showed that the subclinical ketosis cows before treatment had a β-hydroxybutyrate level decreased by 36% and also glucose level was lower than healthy ones. After treatment, levels of glucose, insulin and cortisol increased by 31 %, 22 % and 15% respectively in subclinical ketosis animals. The feed additive also has a hepatoprotective properties as serum albumin level increased, while urea nitrogen level decreased in comparison to the healthy cows. However, these changes are superimposed on the natural physiological changes that occur in the blood parameters during the first few weeks after calving, as the healthy cows that did not receive feed additives showed unidirectional changes but less pronounced. On conclusion, the proposed feed additive can be used in the treatment and prevention of subclinical ketosis.
Keywords | Cows, Ketosis, Treatment, Feed Additive.
Received | October 12, 2020; Accepted | October 20, 2020; Published | December 10, 2020
*Correspondence | Ihor Vudmaska, Institute of Animal Biology of NAAS, 38 V. Stus Str., Lviv, 79034, Ukraine; Email: [email protected]
Citation | Vudmaska I, Petrukh I, Sachko S, Vlizlo V, Kosenko Y, Kozak M, Petruk A (2021). Using hop cones, vitamin E, methionine, choline and carnitine for treatment of subclinical ketosis in transition dairy cows. Adv. Anim. Vet. Sci. 9(1): 55-62.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.55.62
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Vudmaska et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The main causes and pathogenesis of ketosis in dairy cows are associated both with energy deficiency after calving and increased gluconeogenesis with the intensive release of fatty acids from adipose tissue and active formation of ketone bodies in the phase of intensive lactation. Endocrine system of cows suffering from ketosis undergoes considerable exertion. Its compensatory mechanisms tries to equalize the energy deficit, which appear in the lowered blood glucose level and increased gluconeogenesis through reducing the insulin, IGF-1 and leptin levels and increasing cortisol level (Vlizlo et al., 2018).
Ruminants are predisposed to ketosis due to the peculiarity of their digestion; in which short-chain fatty acids (e.g. acetic, propionic, butyric, etc.) are formed in the rumen from carbohydrates that enhance glucose deficiency (Flythe et al., 2017). Disturbances in the diet composition and structure, in particular the high protein level and shortage of carbohydrates lead to a fermentation imbalance in the rumen. This decreased glucogenic propionic acid formation and increased ammonia formation in the rumen from the degradable protein, especially soluble one (Flythe et al., 2017). Ammonia inhibits the tricarboxylic acid cycle reactions and causes ketogenesis via binding alpha-ketoglutaric acid. As a result, acetic acid utilization is destroyed and acetoacetic and beta-hydroxybutyric acid are formed, which enhances the development of ketosis (Simonov and Vlizlo, 2015). On the background of decreased liver function due to fatty degeneration that accompanies ketosis, some of the ammonia remains in the blood, causing body intoxication (Simonov and Vlizlo, 2013a,b).
Most treatment ways for ketosis are focused mainly on the regulation of glucose and fatty acid metabolism, with ignorance to the ammonia intoxication. Although, ionophore antibiotics added to cattle diets reduced ruminal ammonia content (Mullins et al., 2012). They selectively affect gram-positive bacteria to which hyper-ammonia producing bacteria (HAB) belong. Ionophores also inhibit the activity of lactic acid bacteria (LAB), thereby stimulating the precursor of glucose - propionic acid formation (Compton et al., 2015). However, the fibrolytic bacteria activity is somewhat inhibited in the rumen, which reduces the formation of acetate and decreases the milk fat content.
Hop cones contain a number of biologically active components similar in action to ionophore as prenylated flavonoids: lupulone, humulone and their derivatives (Weiskirchen et al., 2015; Flythe et al., 2017). Consequently, hop cones and their preparations can be considered as a potential substitute for ionophore antibiotics (Flythe, 2009). In addition, hop cones contain other active substances with a wide range of regulatory action as phenols, essential oils and resins that have antimicrobial, antioxidant, phytoestrogens and insulin-stimulating effects (Karabin et al., 2016).
Both animal and rumen bacteria need vitamin E as an active antioxidant. Rumen microorganisms require vitamin E more than animals (Hernández-Mendo et al., 2017). The toxicity of tocopherol is very low so its addition to the ruminants diet in higher amount can stimulate the rumen cellulolytic bacteria and compensates the negative effect of ionophores (Sachko and Vudmaska, 2019).
Carnitine helps in the transfer of long-chain fatty acids from cytoplasm to mitochondria, acetyl-CoA from peroxisomes into the cytoplasm and regulates the acyl-CoA / CoA-SH ratio (Ringseis et al., 2018). Under condition of excess fatty acids entrance to the liver, carnitine enhances its oxidation, thus, reducing the accumulation of triacylglycerols in the hepatocytes (Meyer et al., 2020). Choline is a component of phosphatidylcholine, and known as hepato-protector (Jayaprakash et al., 2016). Choline is also involved in the regulation of insulin secretion and considered as an acetylcholine precursor (Shahsavari et al., 2016; Humer et al., 2019). Methionine an essential amino acid that are also widely used as hepato-protector (Zhou et al., 2016). Metabolism of methionine and choline are closely interrelated and are often considered interchangeable in terms of hepatoprotection but recently reviewed that the compounds of both have different effects on liver and milk production of cows (Zhou et al., 2016). Since rumen bacteria destroy much of the feed choline, methionine and carnitine, so ruminants must receive them in a rumen-protected form. (Jayaprakash et al., 2016; Shahsavari et al., 2016; Zhou et al., 2016; Pirestani and Aghakhani, 2018).
The aim of this study was to determine the effectiveness of feed additive that contains hop cones and vitamin E to protect from degradation by rumen bacteria, in addition to choline, methionine and carnitine for normalization of metabolism in the treatment of subclinical ketosis in transition dairy cows.
MATERIALS AND METHODS
Animals and Feeding
Twenty fresh Holstein dairy cows in second and third parity weighing 550 to 600 kg, with an average milk yield in previous lactation about 5-6 thousand kg were used into experiment from calving till 20th day in milk. Rapid tests were performed to determine the ketone bodies content in urine and β-hydroxybutyrate level in blood for diagnosis of ketosis. Cows were then divided into two groups: control – with blood β-hydroxybutyrate concentration in the range of 0.2-1.1 mmol/L (n = 10) and experimental - suffering from subclinical ketosis with blood β-hydroxybutyrate concentration in the range of 1.3-2.2 mmol/L (n = 10). The cows were fed diet meets their nutrient requirements (Table 1).
The experimental cows with subclinical ketosis received feed supplement containing ground pellets (type 90) of Ukrainian variety hop cones «Slovianka» (α-acids - 5 %, β-acids - 7 %, cohumulone - 25 %) - 20 g, α-tocopherol acetate - 3 g (Rovimix E50, DSM - 6 g), choline chloride - 20 g (Sta-Chol, Bioscreen Technologies - 50 g), methionine - 20 g (Pro-Met, Bioscreen Technologies - 40 g) and L-carnitine - 1 g (Carnipass, Lonza Ltd - 5 g) per head per day for 20 days.
Blood Sampling and Biochemical Measures
Blood samples were collected from the tail (coccygeal) vein before morning feeding on 1st and 20th days of the experiment.
Table 1: Ingredient and chemical composition of the diet
| Item | % as DM basis |
| Ingredients | |
| Corn silage | 30,6 |
| Grass-legume hay | 28,7 |
| Barley grain | 8,7 |
| Wheat grain | 8,2 |
| Soybean meal | 16,8 |
| Molasses | 5,0 |
| Salt | 0,6 |
|
Mineral premixa |
1,3 |
| Composition | |
| DM intake, kg/d | 15,7 |
| ME, Mcal/kg | 2,5 |
|
NEL, Mcal/kg |
1,6 |
| CP, % | 18,3 |
| RDP, % | 11,2 |
| RUP, % | 7,1 |
|
CF, % |
16,5 |
| NDF, % | 35,3 |
| ADF, % | 19,9 |
| EE, % | 4,7 |
| Ca, % | 0,53 |
| P, % | 0,34 |
| Ash, % | 7,4 |
| Vit D, thousand IU/day | 12,2 |
| Vit E, g/day | 1,25 |
aThe premix consisted of:
Limestone, monocalcium phosphate, sulphates of magnesium, iron, zinc,
copper, cobalt, manganese; potassium iodate, sodium selenite.
Premix contained per kilogram:
Ca - 175 g, P - 3 g, Mg - 70 g, Fe - 4000 mg, Zn - 3420 mg, Cu - 485 mg,
Co - 15 mg, I - 75 mg, Mn - 1430 mg, Se - 15 mg.
To obtain plasma, blood samples were collected in plastic tubes with the addition of heparin. For serum, blood was taken in test tubes without anticoagulant. The tubes were immediately placed in a thermos with ice. After 2 hours, the blood for plasma and serum was centrifuged at 2,000 g for 15 min.
Glucose and β-hydroxybutyrate levels were determined in whole blood immediately after sampling by CareSens Dual meter (i-SENS, Inc.) using test strips CareSens PRO for glucose and KetoSens for ketone. Insulin and cortisol were measured in plasma with immunoassay analyzer StatFax using ELISA diagnostic test kits (DRG) test kits. Concentrations of total protein, albumin, urea nitrogen, and activities of alanine transaminase (ALT) and aspartate transaminase (AST) in the serum were analyzed with diagnostic kits (Human diagnostics) using biochemical analyzer Humalyzer 2000.
Statistical Analysis
Data were expressed as means ± SEM. The statistical analyses was performed by t-test, comparing the blood parameters of cows of each group at the beginning and end of the experiment. Difference between means were considered significant at P ≤ 0.05.
RESULTS
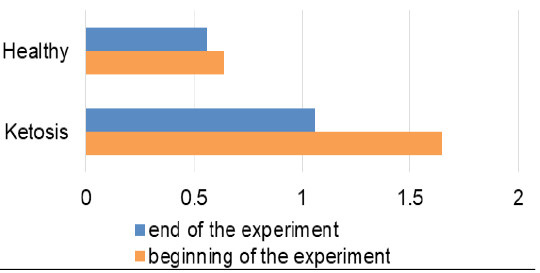
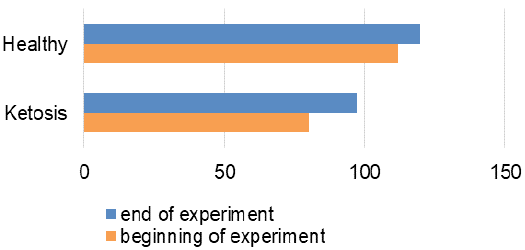
The obtained experimental results are presented as figures 1-9 and summarized in Table 2. The blood β-hydroxybutyrate concentration was 2.6 times higher in subclinical ketotic cows (1.65 ± 0.09 vs. 0.64 ± 0.10 mmol /L, p <0.001) than healthy ones (Figure 1). Blood glucose level of experimental group was 15% lower (2.05 ± 0.08 vs. 2.42 ± 0.13 mmol/L, p <0.01) than healthy ones (Figure 2), and also beyond the physiological range (2.2 mmol/L). This was accompanied with lower insulin concentration in plasma 29% (80.0 ± 3.25 vs. 111.9 ± 4.59 pmol/L, p <0.01) than healthy cows (Figure 3) and higher cortisol concentration 30% (115.1 ± 4.59 vs. 88.4 ± 2.97 nmol/L, p <0.05; Figure 4).
Feeding ketotic cows with the feed additive for 20 days led to a decrease of ketone bodies level which was clear by the absence of ketonuria in the qualitative urine analysis. Similarly, the β-hydroxybutyrate level in blood was lowered 36% (Figure 1) than before feeding the feed supplement (1.06 ± 0.12 vs. 1.65 ± 0.09 mmol /L, p <0.001). Although it did not reach the level of the healthy cows (0.56 ± 0.07 mmol /L), that was nevertheless lower than the maximum allowable value for healthy cows (1.2 mmol/L).
A favorable prognostic indicator that showed an improvement in energy supply of the ketotic cows was the glucose level in blood after treatment (Figure 2) with the feed additive increased to 2.69 ± 0.12 mmol/L, which is higher 31% (p <0.001) than before the feed supplement and did not differ from the healthy ones (2.87 ± 0.08 mmol/L). Such changes in β-hydroxybutyrate and glucose levels indicate normalization of the metabolic state and recovery of the subclinical ketotic cows.
The recovery of ketotic cows is confirmed by the hormonal status. Plasma insulin content was increased by 22% (97.2 ± 2.94 pmol/L, p <0.001), in comparison to before the feed supplement (Figure 3). Cortisol concentration (Figure 4) also decreased from 115.1 ± 4.59 nmol /L to 97.7 ± 2.82 nmol /L after feed additive (p <0.01). Such hormonal changes indicate inhibition of lipolysis and proteolysis.
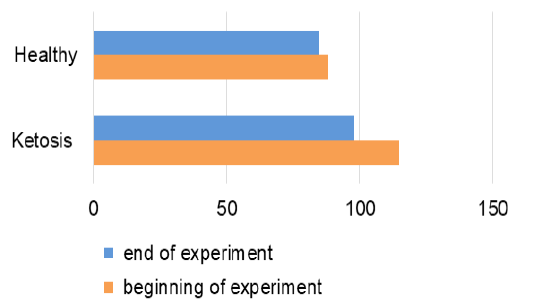
Total protein level in serum of ketotic cows before supplementation was 16% (Figure 5) lower than the healthy ones (65.7 ± 1.88 vs. 73.8 ± 2.0 g / L, p <0.05). These hypoproteinemia could be due to the hypoalbuminemia (Figure 6), in which the serum albumin level was (28.1 ± 0.24 vs. 34.1 ± 0.78 g / L, p <0.01) lower 23% than healthy cows. Protein metabolism factors could also confirm the positive effect of the feed additive on liver function, in which after feeding the supplement the total protein level was increased to 76.6 ± 1.23 g /L (p <0.05), that was equal to its rate in the healthy cows (76.1 ± 1.01 g /L). These increase in total protein could be attributed to the increase in the albumin level after feeding with the feed additive which was higher 18% (33.2 ± 1.03 g /L, p <0.01) than before feeding supplement. Such changes indicate an improvement in the protein-synthesizing function of the liver. Therefore, the feed additive used in the experiment has hepatoprotective properties.
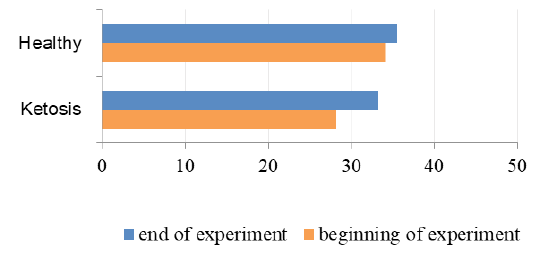
Figure 6: The contents of albumin in serum (g/L).
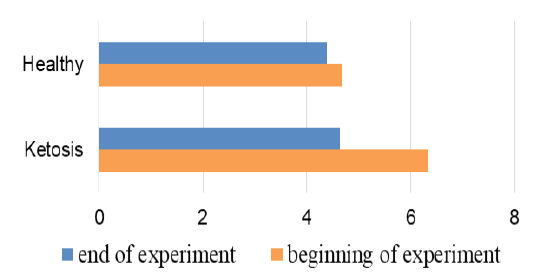
The important criterion of nitrogen metabolism is the blood urea concentration. Before treatment with feed supplement the serum urea level was higher in subclinical ketotic cows than healthy ones (6.33 ± 0.37 vs. 4.67 ± 0.20 mmol /L, p <0.001, Figure 7). After supplementation the urea nitrogen level was decreased to 4.64 ± 0.22 mmol /L (p <0.05) and did not differ from the control group (4.39 ± 0.25 mmol /L).
The normalization of the structure of liver cells were evidenced by levels of liver enzyme markers in serum (Figure 8 and 9). The AST level before treatment in the serum of ketotic cows was higher 25% (1247,28 ± 83.32 vs. 935.17 ± 50.81 nkat /L, p <0.01) than in the serum of control healthy group. After supplementation the AST level decreased 30% (p <0.01) to 877,21 ± 56.92 nkat /L, and did not differed from control ones (935.17 ± 50.81 nkat /L). Such changes of AST activity indicate a positive effect of the feed additive on the restoration of liver function. Changes of ALT activity are similar, but less expressed.
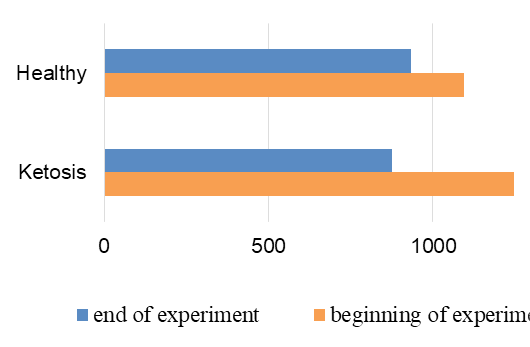
Figure 8: AST activity in serum; nkat/L
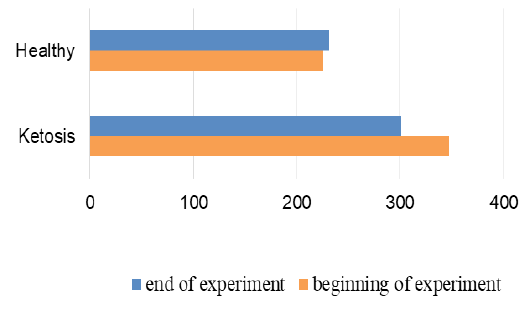
Figure 9: ALT activity in serum; nkat/L
DISCUSSION
In the high yielding transition dairy cows, liver is in a state of extreme metabolic load, which is very often evident by fatty degeneration in the hepatocytes. At the postpartum period, cow’s liver has three main metabolic problems: glucose synthesis, oxidation and excretion of fatty acids as a part of VLDL and transformation of ammonia into urea. Violation of one of these problems potentiates the deviation of others. As ammonia intoxication inhibits glucose synthesis and fatty acid metabolism, steatosis inhibits glucose and urea synthesis, and intensive gluconeogenesis reduces liver’s ability to metabolize ammonia and fatty acids (West, 1997; Bobe et al., 2004). Thus, liver functions are suppressed and subclinical ketosis occurs that can progress to clinical form under adverse conditions. The prevention and treatment of ketosis require comprehensive simultaneous regulation of all these metabolic processes in cows’ liver.
Therefore, the aim of the current study was to determine the effect of a complex feed supplement that could affect different aspects of metabolism in rumen and liver. The hypothesis of this study assumed that the obtained effects are not only a consequence of the direct actions of the supplement components, but also the result of improving the physiological and metabolic conditions of the cows.
Previous studies indicated the effect of hop cones and its extract on the enzymatic processes in rumen and its similarity in action to ionophores antibiotics (Karabin et al., 2016; Flythe et al., 2017). Ionophores antibiotics and hops β-acids (lupulone) inhibit the activity of Gram-positive bacteria in the rumen. The gram-negative bacteria are not sensitive to ionophores (Karabin et al., 2016).
The hyper-ammonia-producing bacteria (HAB) the main source of ammonia in the rumen. HAB belong to the gram-positive species, which are inhibited by ionophores and hops beta acids (Eschenlauer et al., 2002; Flythe et al., 2009; Flythe et al., 2017). Inhibition of ammonia production has a positive effect on metabolism in cows, as it protects protein from degradation in the rumen, preventing animal intoxication with ammonia and reduces the metabolic loading of liver (Bobe et al., 2004).
The inhibition by β-acids occurs as result of the decrease in lactic acid production so lower lactic rumen acidosis danger. The gram-negative propionate-producing bacteria are not sensitive to ionophores. Thus, proportion of propionate in volatile fatty acids composition increased when hop cones are fed (Wang et al., 2010), which is important for glucose synthesis in liver. However, from the negative effect of β-acids and ionophore the reduction in the formation of acetic acid in the rumen due to inhibition of the cellulolytic bacteria activity.
Lavrenčič et al. (2018) fed growing beef cattle 6 g and 11 g of hop DM/kg diet and reported an increase in the blood glucose level and a decrease in the non-esterified fatty acid
Table 2: Blood metabolites of the cows
| Healthy | Ketosis | |||
| Beginning of the experiment | End of the experiment | Beginning of the experiment | End of the experiment | |
|
β-Hydroxybutyrate, mmol /L |
0.64±0.10 | 0.56±0.07 | 1.65±0.09 |
1.06±0.12*** |
| Glucose ,mmol /L | 2.42±0.13 | 2.87±0.08 | 2.05±0.08 |
2.69±0.12*** |
| Insulin, pmol/L | 111.9±4.59 | 120.03±5.28 | 80.0±3.20 |
97.2±2.94*** |
| Cortisol, nmol /L | 88.4±2.97 | 84.93±1.82 | 115.1±4.6 |
97.7±2.80** |
| Total protein, g/L | 73.8±2.01 | 76.1±1.01 | 65.7±1.88 |
76.6±1.23* |
| Albumin, g/L | 34.1±0.78 | 35.5±0.36 | 28.1±0.24 |
33.2±1.03** |
| Urea nitrogen, mmol /L | 4.67±0.20 | 4.39±0.25 | 6.33±0.37 |
4.64±0.22 * |
| AST, nkat /L |
1095,5±68.24 |
935.2±50.81 | 1247.28±83.32 |
877.21±56.92** |
| ALT, nkat /L | 226.53±25.88 | 231.36±17.23 | 347.44±20.01 |
301.24±26.51 |
Notes: Parameter measurements: β-Hydroxybutyrate and glucose - whole blood; insulin and cortisol - in blood plasma; protein, urea AST and ALT - in blood serum. The differences are statistically significant compared to beginning of the experiment: * – p<0.05; ** – p<0.01; *** – p<0.001.
-s concentration, whereas, β-hydroxybutyrate and urea concentration remained unchanged. In this current study there was an increase in blood glucose, and a decrease in hydroxybutyrate and urea after supplementation with hop cones. This can be explained by the peculiarities of metabolism in transition dairy cows, which are manifested in significant disorders of carbohydrate, lipid and protein metabolism. In addition, the supplement used contained hepatoprotective components that affect liver function, including ketogenesis and urea formation. In a previous study, a feed supplement containing hop cones and large doses of vitamin E was used and a similar changes only after calving was obtained, whereas after a month the effect of the supplement has not been noticed (Vudmaska et al., 2019a, 2019b). However, Focant et al. (2019) reported that hops had no effect on urinary N excretion, CH4 emission, milk production, and milk composition of dairy cows.
Methionine, choline and carnitine are well known hepato-protectors that are widely used for the prevention and treatment of liver dysfunction, including hepatic steatosis. Carnitine transports fatty acids to mitochondria for further beta-oxidation (Ringseis et al., 2018; Meyer et al., 2020). Choline and methionine are involved in the synthesis of phosphatidylcholine, promote the formation of very low-density lipoproteins and the excretion of triacylglycerols from the liver (Jayaprakash et al., 2016; Shahsavari et al., 2016; Zhou et al., 2016). However, none of these compounds has a pronounced effect on ketosis-related biochemical parameters in the blood of clinically healthy cattle (Shahsavari et al., 2016; Zhou et al., 2016; Ringseis et al., 2018; Humer et al., 2019; Meyer et al., 2020). Ketosis is predominantly accompanied by steatosis (West, 1997; Bobe et al., 2004), so improving liver function promotes better metabolism of glucose, urea, and other metabolic pathways. This may partly explain the changes in blood parameters obtained in this study.
Vitamin E fed in large doses regulates rumen fermentation by affecting the digestibility of dry and organic matter, fiber digestion, the formation of volatile fatty acids and lactate, and biohydrogenation of unsaturated fatty acids (Naziroğlu et al., 2002; Pottier et al., 2006; Wei et al., 2015; Hernández-Mendo et al., 2017).
Transition period and ketosis are often accompanied by oxidative stress. The supplement used in this study contained three components with antioxidant properties: vitamin E, hop cones (Karabin et al., 2016) and methionine (Zhou et al., 2016). Thus, prevention of oxidative stress through these antioxidant compounds could be one of the positive factors in the treatment of metabolic disorders.
CONCLUSION
Supplementing cows suffering from subclinical ketosis with diet with feed additive that includes hop cones, vitamin E, methionine, choline and carnitine, could help to normalize carbohydrate and protein metabolism through normalization of insulin and cortisol secretion, reducing β-hydroxybutyrate concentration and increasing blood glucose concentration. Also, these feed additive can led to an increase in the total protein and albumin levels and decrease transaminases (AST, ALT) activities, which are signs of improved liver structure and function. Thus, the proposed feed additive can be used for the treatment and prevention of ketosis and steatosis in dairy cows.
acknowledgements
The research was funded by scientific program of National Academy of Agrarian Sciences of Ukraine.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
authors contribution
Ihor Vudmaska: the author of the idea, designed the research, prepared the introduction and discussion sections, and revised the manuscript. Iryna Petrukh: examined the contents of hormones, participated in writing the results section. Sergii Sachko: conducted the experiment, examined the total protein, albumin, urea, glucose, and β-hydroxybutyrate concentrations, prepared materials and methods section, and participated in writing the results section. Vasyl Vlizlo: participated in writing the introduction and results sections. Yurii Kosenko: performed data analysis. Mariia Kozak: examined enzymatic activities, participated in writing the results section. Anna Petruk: prepared abstract, analyzed data statistically, took a part in preparation of discussion. All authors approved the manuscript.
REFERENCES