Advances in Animal and Veterinary Sciences
Research Article
Enhanced Effect of Resveratrol on Hepatocellular Carcinoma of Rats Treated with 5-Fluorouracil
Amany E. Nofal1*, Reda M. Fayyad2
1Zoology Department, Faculty of Sciences, Menoufia University, Egypt; 2Pharmacology Department, Faculty of Medicine Al-Azhar University, Egypt.
Abstract | Toxicity and resistance to chemotherapeutic drugs remain major obstacles to successful therapy. We researched the ameliorative impacts of combining natural agent-like resveratrol with a chemotherapeutic drug-like 5-fluorouracil to improve its efficacy and reduce its side effect severity. Our results indicated that RES and 5-FU had a therapeutic impact against Diethylnitrosamine-induced HCC, and the best result was gained when both had been used together, they retain normal liver structure and functions also decreased the cytotoxic impact of 5-FU. RES remarkably reduced most of the effects induced by DEN, as they suppressed the significant increased relative liver weight, the hepatic nodules number and their relative volumes, the raised levels of serum liver function tests, and increased the depression of T. protein, albumin, globulin, liver homogenates antioxidants indicating their antioxidant effect. Furthermore, it showed alterations in the expression of the levels of some inflammatory cytokines in serum, and immunoreactivities (Bax, CK18, and CA19-9) in the liver sections of the HCC group when compared with other groups. Dual-treatment of the phytochemical substance and the antitumor drug had an observantly therapeutic and immune-modulatory efficacy against HCC as they together have less toxicity than pharmaceutical agents.
Keywords | Chemotherapeutic Drug, Hepatocellular Carcinoma, Inflammatory Cytokines, Resveratrol, 5-Fluorouracil
Received | June 09, 2021; Accepted | July 08, 2021; Published | October 01, 2021
*Correspondence | Amany E. Nofal, Zoology Department, Faculty of Science, Menoufia University, Egypt; Email: [email protected]
Citation | Nofal AE, Fayyad RM (2021). Enhanced effect of resveratrol on hepatocellular carcinoma of rats treated with 5-fluorouracil. Adv. Anim. Vet. Sci. 9(11): 1978-1988.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1978.1988
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Nofal and Fayyad. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Hepatocellular carcinoma (HCC) represents approximately 90% of primary liver cancers (Galle et al., 2018). Diethylnitrosamine (DEN) is found in foods such as smoked or dried fish, cheese, soybeans, cured meat, and groundwater (El-Tabl et al., 2019). DEN is a potent liver carcinogen in experimental animals that initiate the stages of carcinogenesis throughout a time of promoting cell proliferation that goes together with the liver cells’ necrosis and destroys DNA integrity (Shahat et al., 2015). HCC in experimental animals is either induced by oral, intravenous, or intraperitoneal administration with diethylnitrosamine, the dose and duration of the carcinogen administration influenced the occurrence and the latent period of the liver tumor (Khazaei et al., 2018). 5-fluoropyrimidine-2, 4(1H,3H)-dione (Fluorouracil) (5-FU) has been used for years as one of the first-line chemotherapy for the treatment of cancers (Zhang et al., 2008). 5-FU treatment resulted in a high incidence of toxicity (Lee et al., 2015). Therapeutic doses of 5-FU in tumor treatment led to many toxic and side effects produced by DNA damage that in turn led to stimulation of apoptosis (Grem, 2000). Natural extracts had been used to augment the efficacy of 5-FU in chemotherapy, lessen their side effects as well as reduce the duration of treatment, and the anticipated dose (Grem, 2000). Resveratrol (RES) is a polyphenol substance found in by various plants such as grapes, peanuts, plums, and red wine has used in the treatment of obesity, and inflammation (Chachay et al., 2011). RES has a possible effect against a number of diseases, among them its ability to achieve both chemo-preventive and therapeutic effects against some cancers (Aggarwal et al., 2004). Resveratrol directly induces apoptosis via diverse mechanisms in different cancer stages (Singh et al., 2013). RES is very useful in the protection against the development of liver fibrosis and cirrhosis (Hong et al., 2010), in addition to, attenuation of hepatic steatosis and inflammation by regulation of autophagy and inhibition of lipid accumulation (Ji et al., 2015). Our results looked into the benefits of combining resveratrol with 5-FU to improve its efficacy and reduce its side effect severity. Adikwu et al. (2019) reported that RES had a therapeutic benefit in nephrotoxicity in rats caused by 5-FU, which further elucidates the protective impact of RES on normal cells. El-Oraby et al. (2020) reported the synergistic impacts of resveratrol with 5-fluorouracil against oral squamous cell carcinoma in rats.
MATERIALS AND METHODS
Chemicals
DEN, Carbon tetrachloride (CCl4), Dimethyl sulfoxide (DMSO), RES, and 5-FU were purchased from the corporation of Sigma-Aldrich (St. Louis, MO, USA). The 5-FU with chemical formula C4H3FN2O2 and molecular weight 130.078 g/mol was supplied as a bottle of a colorless, water-soluble solution at 10 mg/ml concentration. RES (3,4′,5-trihydroxy-trans-stilbene, C14H12O3, Molar mass 228.2 g/mol), with the purity of 99% and 20-μM stock solution, was prepared in 0.2% DMSO; first, it dissolved in 0.2% DMSO then diluted with normal saline solution) (Lee et al., 2015; Soliman et al., 2018).
Ethics statement
The animal ethics committee in accordance with the animal care guidelines approved this experiment (Approval No. MUFS/ F/ HI/ 1/ 20).
Experimental design
60 adult male Sprague-Dawley rats, weighing 165-175 g were obtained from the Institute of Ophthalmic Disease Research, Cairo, Egypt, and acclimated for 7 days in specific pathogen-free cages at moderated temperature (24-25°C) in the Animal House of the Zoology Department, Faculty of Science, Menoufia University throughout the experimental work. The rats had been maintained in good ventilation and a photoperiod of light/ dark cycle. All rats have received a standard laboratory diet, and water was available ad libitum during the experiment.
Rats were randomly allocated into the following groups: 10 rats were used as a negative untreated control group. 10 rats were orally given 0.2% solution of DMSO (the solvent of resveratrol) through a gastric tube for 2 months equivalent to those of the resveratrol (Soliman et al., 2018). 10 rats were intraperitoneally injected with a single dose of freshly prepared DEN (200 mg/kg b.w), 2 weeks later, they were subcutaneously injected with CCl4 once weekly (3 mL/kg b.w) for 6 weeks to enhance DEN carcinogenicity as a model for liver cancer (Aboubakr et al., 2017; El-Tabl et al., 2019). 30 rats received DEN as in the previous group, and then treated either with 5-FU or RES or both of them as following; 10 HCC animals were intraperitoneally injected by 5-Fluorouracil (12.5mg/kg, 3 days weekly (day after day) for 2 months) (Soliman et al., 2018), 10 HCC-rats were orally administrated resveratrol dissolved in DMSO (200 μg/kg b.w daily for 2 months) (Soliman et al., 2018; El-Oraby et al., 2020), and 10 HCC rats treated with both 5-FU and RES (6.25 mg 5-FU/kg (ip) on days 1, 3, and 5 of the week for 2 months, and in the same period, rats were daily oral given 100μg RES/kg).
Morphological and macroscopic examinations
Rats from each group were weighed to distinguish their body weight. Control and treated groups were dissected and livers were instantly excised, weighed. Livers surfaces were examined in 2-mm cross-sections searching for obvious neoplastic hepatic nodules (grayish-white patches), which were easily distinguished from surrounding normal non-nodular liver tissue (reddish-brown). The number of nodules was counted and detected their diameter using a digital caliper to measure the approximated spheres of nodules in two perpendicular planes to the nearest mm to detect the average diameter for each nodule (Aboubakr et al., 2017). Liver weight percentage was calculated using the Equation 1, while estimated nodular volume (V) by using the Equation 2.
Liver weight percentage = Liver weight (g) ∕ Bodyweight (g) × 100 …(1)
Estimated nodular volume V = 4/3 × r3 ….(2)
r is the half of the average diameter (mm), and the nodular volume was determined as a relative nodular volume.
Blood collection and tissue preparation
Overnight fasted rats were sacrificed, blood samples were obtained from the inferior vena cava, left to clot, and centrifuged at 3000 RPM for 10 min for sera biochemical analyses (stored at -20 oC). The livers were excised, small pieces of them were fixed in 10% neutral formalin, dehydrated, washed, embedded, cut for 4-5 μm paraffin sections using a rotary microtome (Leica, Model Rm 2125, Germany), and installed on clean glass slides without the use of any adhesive medium for the histopathological, and immunohistochemical studies. The remaining liver tissues were homogenized with ice-cold phosphate buffer (pH 7.4) by using a glass Potter-Elvehjem homogenizer fitted with a Teflon Plunger. The homogenates were centrifuged at 700 × g for 10 min, then at 9000 × g at 4 oC for 20 min for purification from the cellular debris and nuclei. The resulting supernatants were kept in a deep freezer at -20 oC till performed further analysis (Alekseeva et al., 2017).
Histological studies
To assay the histopathological changes in the liver tissue, liver paraffin sections were stained with Hematoxylin and Eosin (H & E) (Bancroft and Gamble, 2008).
Immunohistochemical assessments (IHC)
Cell apoptosis and tumor areas were determined by immunohistochemical examinations of a pro-apoptotic mitochondrial membrane protein (Bax), Cytokeratin 18 (CK18), Cancer antigen (CA19-9). Deparaffinized liver sections were incubated with suitable primary antibodies using the routine indirect avidin-biotin immunolabeling- peroxidase method, then counterstained by Mayer’s hematoxylin, cleared in xylene, and mounted in DPX (Hsu and Raine, 1981).
Image analysis
The light microscope (Olympus BX 41, Japan) was used to examine the liver section slides, and Olympus digital camera was used for Photography. Analysis for the Digital images was achieved quantitatively by using a semi-quantitative scoring system (Image J software, Java-based application for analyzing images) for immunohistochemical reactions based on measure the colored stained percentage per the field in ten randomly photographed high-power fields at a magnification of x400. The positive immunostaining reactions of Bax, CK18, CA19-9 that appeared as brown-stained were analyzed by measuring the areas percentage of positive staining cells (Cotoi et al., 2012).
Biochemical parameters
Measurements of liver functions
Aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) levels were measured in serum as parameters for liver functions according to the methods of Gella et al. (1985). Also, total protein and albumin were determined using Spinreact kits (Spain) according to Doumas et al. (1971). The serum globulin level was measured as the result of obtained serum total protein concentration minus the obtained serum albumin concentration (Salem et al., 2011).
Determination of oxidative stress and antioxidant enzymes in liver homogenates
The lipid peroxidation, and the activities of the antioxidant enzymes, were measured in the homogenized liver supernatants using commercial kits (Bio-Diagnostics Co, Egypt) following the steps of the manufacturer’s guide. Protein content was determined for the assessment of antioxidant enzymes followed the methods of Lowry et al. (1951). Superoxide dismutase activity (SOD, Sigma 19, 160) was measured according to Marklund and Marklund (1974). Catalase activity (CAT, Sigma CAT100) as the antioxidant enzyme was analyzed corresponding to the procedure of Aebi (1974). The activity of glutathione peroxidase (GSH) was measured corresponding to the procedure of Richardson and Murphy (1975). Lipid peroxidation (LPO) was essentially assayed via measuring malondialdehyde (MDA) in the homogenized liver supernatants by using the method of Ohkawa et al. (1979).
Determination of selective inflammatory cytokine and tumor marker levels
The hepatic tumor necrosis factor-alpha (TNF-α) and alpha-fetoprotein (AFP) concentrations were measured in liver homogenates. TNF-α was determined by using a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kits specifically for rats, using a specific monoclonal antibody for rat TNF-α kits (Sigma Aldrich, USA) corresponding to the manufacturer’s protocol. Also, AFP concentration was measured with specific rat AFP-ELISA kits (LSBio, Seattle, USA) corresponding to the manufacturer’s protocol (Belanger et al., 1973).
Statistical analysis
The resulted data were subjected to statistical significance tests using one-way analysis of variance (ANOVA) pairwise between different studied groups, followed by Tukey HSD multiple range tests (Abdi and Williams, 2010) to compare different groups for abnormally distributed quantitative variables, as the data of the treated groups were compared with each other and with the control group. The differences in the obtained results had considered significant at P < 0.05.
RESULTS and Discussion
Morphological and macroscopic examinations
It is clearly demonstrated from data represented graphically in Figure 1 that rats administered orally by DMSO for 2 months did not show evidence of any abnormal changes in all morphological, and macroscopic examinations compared to the normal control group. The relative liver weight of DEN-induced hepatic cancer model rats was significantly higher (5.93 ± 0.4) than the normal control group (3.31 ± 0.06; p < 0.05), whereas the treatments either with 5-FU, RES, or both together exerted a significant reducing effect compared to HCC group (5.49 ± 0.1, 4.39 ± 0.2, 4.89 ± 0.8, respectively; p < 0.05). Moreover, co-administration of RES with 5-FU suppressed body weight loss and basically prevented excess absolute liver weight induced by DEN. Consequently, the relative liver weights are pulled to the normal value. The body weight, liver weights, and average of different groups are shown in Figure 1, and were significantly different as compared to those of the control group and with each other (P < 0.05).
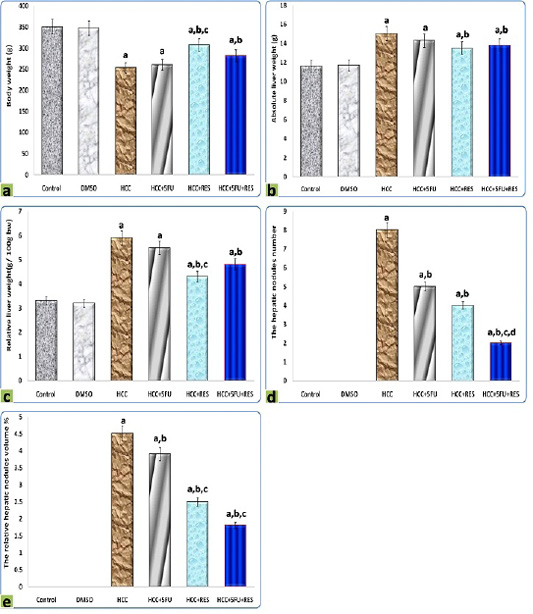
Figure 1: Graphic diagrams showing (a) body weight, (b) liver weight, (c) relative liver weight, (d) the hepatic nodules number, and (e) the relative hepatic nodules volumes of rats treated with different treatments. The significance by using one-way ANOVA followed by Tukey-HSD test for j multiple comparisons. a: Refers to significant change when compared to control rats; b: Refers to significant change when compared to HCC rats; c: Refers to significant change when compared to HCC+5-FU rats; d: Refers to significant change when compared to HCC+RES rats.
The hepatic nodules number and relative volumes were significantly reduced after RES (4 and 2.5% respectively) compared to HCC-group (8 and 4.5% respectively), moderate decrements in them were observed at 5-FU group (5 and 3.9% respectively), on the other hand, these decreases were significantly (p < 0.05) enhanced by combined administration of 5-FU with RES (2 and 1.8% respectively) (Figure 1).
Histological examination
The microscopic examination of liver sections of the control and DMSO groups displayed the normal histological organization of hepatic lobules and sinusoidal architecture, as well as the hepatic parenchymal cells, were normal with small uniform nuclei in granulated cytoplasm, radially arranged around the central vein without any evidence of tumor, inflammation, or cell injury (Figure 2a, b). The hepatic tissue of rats that received DEN showed loss of the normal lobular structure of the liver and the absence of portal tracts, the presence of tumor cell ballooning with dense and pale stained cells, irregular polymorphic and hyperchromatic hepatocytes were arranged in irregular 2-3 cell-thick plates. Also, several large basophilic nuclei with prominent centrally situated nucleoli were observed. Hemorrhage with edema in the dilated sinusoidal areas, inflammatory cell infiltration with very fine fibroblastic cell proliferation was observed around a congested blood vessel. Moreover, a population of larger hepatic cells was apparently either empty or vacuolated with a large number of fat droplets or showed focal tumor necrosis (Figure 2c, d).
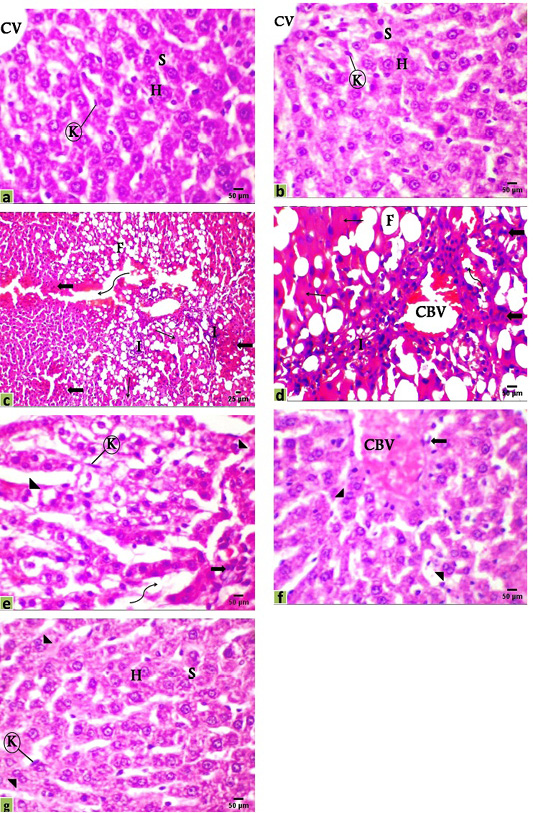
Figure 2: Photomicrographs of rats liver sections stained with H&E showing; (a) Control, (b) DMSO, (c and d) HCC model, (e) HCC+5-FU, (f) HCC+RES, and (g) HCC+5-FU+RES. Hepatocytes (H), sinusoid (S), Kupffer cell (K), central vein (CV), congested blood vessel (CBV), connective tissue infiltration with very fine fibroblastic cell proliferation (I), fat droplets (F), variable irregular shaped-dense and pale staining malignant cell (thick arrow), necrosis (thin arrow), edema in sinusoid (irregular arrow), binucleated cell (arrowhead), Scale bar = 50µm for all photo except (c) it’s scale bar = 25µm.
Liver sections from HCC-rats treated with RES or 5-FU or both of them showed various improvements with some reversible changes but generally less than those indicated in the HCC-group. The hepatic tissue of HCC treated with 5-FU showed a significantly decreased number of steatotic cells and the rearrangement of normal parenchyma with some vesiculated and frequently binucleated liver cells associated with activated Kupffer cells as signs of the regeneration of hepatic tissue. On the other hand, edema in dilated sinusoids, infiltration of a small number of inflammatory cells, and tumor cells were observed (Figure 2e). Moreover, the hepatic tissue of HCC-rats treated with RES showed a significant improvement and a remarkable reduction in the histological alterations with congestion of the central vein and a few numbers of tumor and inflammatory cells, necrosis is observed seldomly and tissue architecture appeared normal not far away off control rats with a number of binucleated cells indicating the regeneration of hepatic tissue (Figure 2f). Additionally, most of the hepatocytes were relatively well preserved after co-treatment of RES with 5-FU showed nearly normal hepatic structure with a marked attenuation in the severity of the liver injury and most cells appeared with well-defined outlines, contained finely granular and acidophilic cytoplasm with binucleated cells (Figure 2g).
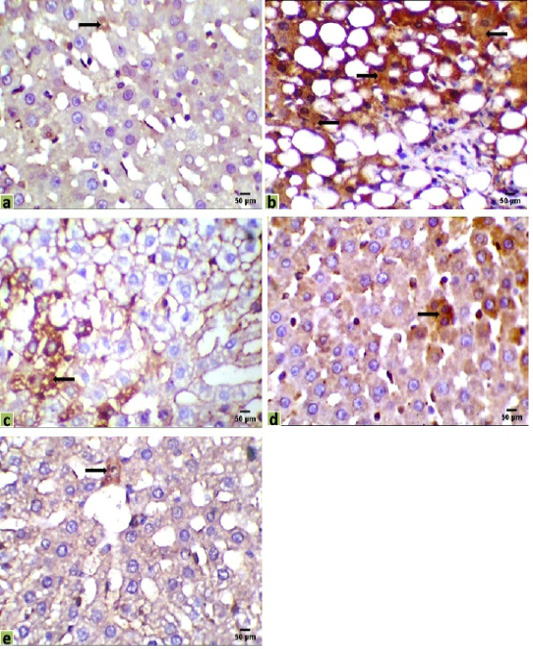
Figure 3: Immunohistochemical photomicrographs of rats liver sections showing immuno-expressions of Bax in various groups (arrows); (a) Control, (b) HCC model, (c) HCC+5-FU, (d) HCC+RES, and (e) HCC+5-FU+RES, Scale bar = 50µm.
Immunohistochemical observations
Liver sections were immunohistochemically exposed to Bax, CK18, and CA19-9 antibodies to evaluate cancer initiation, and their positive immunoreactivities have seemed like brown expressions in different locations of the liver tissues indicated the results of various therapies. Liver immunostaining of cancer model rats showed a strong brown focal cytoplasmic staining to Bax and CK18 in hepatocytes, while a positive cytoplasmic immunostain for CA19-9 in intrahepatic bile duct epithelium, and a negative adjacent intervening hepatocyte were observed (Figures 3, 4 and 5).
The immunohistochemical examinations of liver sections displayed that there were a few positive immunoreactivity Bax in the hepatic tissue of the control group (0.098%), indicating the presence of a normal life cycle of the cells. Positive immunostaining for Bax was significantly increased in the hepatic tissue of the HCC group (35.090%) compared with the control group. Mild positive expressions of Bax in liver sections were observed in HCC-rat treated with 5-FU or RES alone (15.165%, 9.003%, respectively), while a few expressions of Bax were recorded in sections of the co-treated group (5-FU and RES together, 1.782%) (Figure 3).
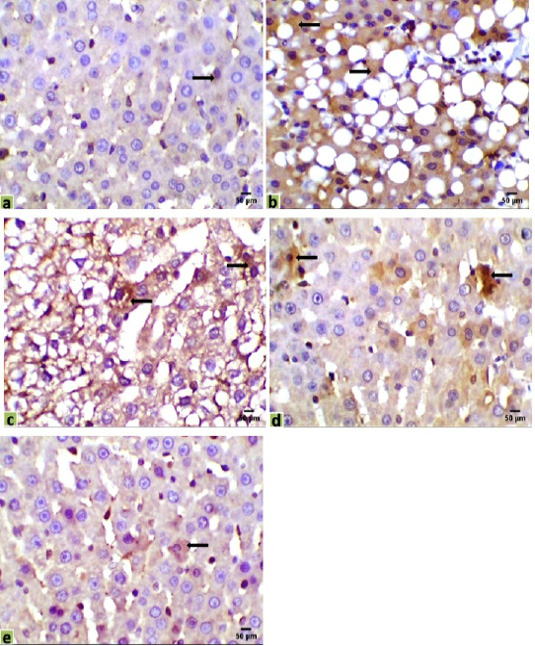
Figure 4: Immunohistochemical photomicrographs of rats liver sections showing CK18 expression in various groups (arrows); (a) untreated control, (b) HCC model, (c) HCC+5-FU, (d) HCC+RES, and (e) HCC+5-FU+RES, Scale bar = 50µm.
Immunohistochemical observations of the liver tissues of HCC animals showed a significant increase of the immunostaining CK18 (31.043%) in mostly all the hepatocytes, compared with the very low expression in the control liver sections (0.329%). Moderate positive expressions of CK18 were observed in some hepatocytes of the mono-treated HCC-rat with 5-FU or RES (18.584%, 14.6%, respectively). However, a few expressions of CK18 were recorded in a very few numbers of hepatocytes in the liver section of co-treated HCC-rats with 5-FU and RES together (1.254%) (Figure 4).
Foci of weak positive immunostaining cells for CA19-9 were observed only in parts of the large bile ducts, not appeared in the small interlobular bile ducts, and negatively expressed in adjacent hepatic cells inside the liver parenchyma of all our experimental groups (Control 0.045%, HCC 1.107%, HCC+5-FU 1.049%, HCC+RES 0.727%, HCC+5-FU+RES 0.268%), CA19-9 was negatively expressed in HCC-hepatocytes and weakly positive immunoreactivity for large bile ducts (Figure 5).
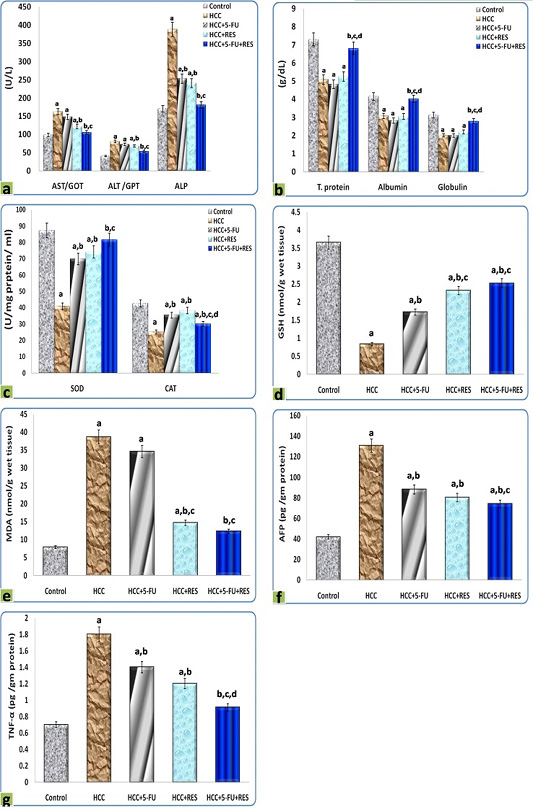
Figure 5: Immunohistochemical photomicrographs of rats liver sections showing CA19-9 expression in various groups (arrows); (a) untreated control, (b) HCC model, (c) HCC+5-FU, (d) HCC+RES, and (e) HCC+5-FU+RES, Scale bar = 50µm.
Biochemical analysis
The DEN administration resulted in a significant dramatic higher level of AST, ALT, ALP in serum, as well as MDA, AFP, and TNF-α concentrations in liver homogenates, on the other hand, decreased significantly the concentrations of total protein, albumin, and globulin in serum, as well as SOD, CAT, and GSH in liver homogenates, in comparison to the control group. Interestingly, the treatments of HCC-rats either with RES, 5-FU, or both together exerted a significant complete reversal of the increased liver function indices, oxidative stress biomarkers, inflammatory cytokine, and tumor marker concentrations, as well as, the decreased blood protein concentrations and the activities of antioxidant enzymes-induced by DEN. These results were statistically considered significant (p < 0.05) when compared with the control values and the different treatments (Figure 6).

Figure 6: Graphic diagrams showing sera levels of (a) AST, ALT, and ALP, (b) absolute T. protein, albumin, and globulin in rats treated with different treatments, (c) SOD and CAT, (d) GSH, (e) MDA, (f) AFP, and (g) TNF-α of rats treated with different treatments. The significance by using one-way ANOVA followed by Tukey-HSD test for j multiple comparisons. a: Refers to significant change when compared to control rats; b: Refers to significant change when compared to HCC rats; c: Refers to significant change when compared to HCC+5-FU rats; d: Refers to significant change when compared to HCC+RES rats.
Our results indicated that exposure animals to DMSO for 2 months did not show evidence of any abnormal changes in all morphological, macroscopic, and histological examinations compared to the normal control group. In agree with our results, Soliman et al. (2018) said that, rats who received DMSO for 2 months were showed normal colonic architecture.
According to the previous work by Ding et al. (2017), DEN-mediated hepatotoxicity is associated with elevated liver enzymes, hypertrophy, and hyperplasia. In the present study, we found that a significantly higher liver weight and enzymes in the HCC group, compared to the control group implying hypertrophy and hyperplasia of the liver. The morphological examinations of the liver in our work revealed a significant decrease in liver weight and volume of the hepatic nodules in the 5-FU plus RES group, compared to the HCC group and single interventions group, indicating a potent hepatocellular carcinoma inhibitory effect. The reduction in the steatotic HCC cells was notable in the RES and 5-FU+RES groups. Such findings are in line with a previous report by Hong et al. (2010).
A growing body of experimental evidence highlighted a beneficial and enhancing role of dual-treatments of natural agents with anti-cancer drugs in various types of malignancy. For example, it was reported that the combination of recombinant human lactoferrin with the 5-FU led to significant inhibition of growth of cancer cells through cell cycle arrest, as it induced G1/G0 growth arrest in it or through immunomodulation by inducing lymphocyte infiltration into tumor site (Wolf et al., 2007; Li et al., 2017). Likewise, dual-treatments of levamisole and taurine as a promising therapy for cancer in combination with cyclophosphamide modulated the immune response against the tumor cells, induced apoptosis in them, and potentiated the chemotherapy in Ehrlich ascites carcinoma-bearing mice (Ibrahim et al., 2018).
In this experiment, we planned to assess the synergistic effects of the combination RES with 5-FU in HCC models. The current study showed that co-treatments of RES with 5-FU could be a potent anti-tumor combination. In our research, pathological examinations of blood and liver tissues showed that the 5-FU recovered DEN-induced hepatic carcinogenesis, while RES reduced the cytotoxic consequences of 5-FU on hepatic cells, ameliorated the normal histological structure of the liver, enhanced the immune-modulatory efficacy, and induced cell apoptosis against HCC as they have less toxicity than pharmaceutical agents.
Over the past few years, our knowledge of the mechanistic approaches elucidating the anti-tumor effects of RES has notably expanded. RES is a potent anti-apoptotic agent and leads to impacts of the cell cycle through stimulation of several signaling pathways and inhibition of cell cycle regulatory proteins (Kosuru et al., 2016). RES was found to significantly inhibit the angiogenic properties of tumor cells (Savouret and Quesne, 2002). Additional proposed mechanisms for the anti-tumor activities of RES include the inhibition of potent regulators of cell proliferation (such as protein kinase C, and mitogen-activated protein kinases) (Xiao et al., 2019). Recently, it was found that exerted oxidative stress activities on cancer cells, while it has a well-established ameliorative effect on superoxide and reactive species production in normal cells. Thus, RES was proposed to induce targeted cell damage on malignant cells only, while it exerted a protective effect on normal cells (Heo et al., 2018; Cosín-Tomàs et al., 2019). The RES also exhibited an anti-metastatic property through the inhibition of the signaling pathway and epithelial-mesenchymal transition, as well as inhibition of cytokines-mediated cellular invasion (Yang et al., 2018; Wang et al., 2020). When RES combined with chemotherapy, it reverses the tumor cell’s resistance to chemotherapy (Xu et al., 2017).
In the setting of HCC, Bishayee and Dhir (2009) reported beneficial and chemo-preventive effects of orally administered RES on DEN-induced hepatic carcinogenesis in rats were arbitrated by reduction of cell growth and induction of apoptosis. Nonetheless, the synergistic effect of combining RES and 5-FU against liver cancer has not been well-studied yet. Our results displayed that 5-FU and RES combination produced a significant complete correction of the increased liver function parameters, oxidative stress biomarkers, inflammatory cytokines, and tumor markers concentration, as well as, the diminished blood protein concentrations and antioxidant enzymes activities, which were induced by DEN. These results indicating less toxicity than the pharmaceutical agent alone. These findings were in accordance with previous studies, Cosco et al. (2015) reported that RES and 5-FU co-loaded ultra-deformable liposomes and act as a new nanomedicine for squamous cell carcinoma treatment. Lee et al. (2015) indicated that co-treatment of RES with 5-FU reduced cell proliferation and angiogenesis in B16 murine melanoma tumors. Soliman et al. (2018) showed that RES with 5-FU has a synergistic effect as a chemotherapeutic combination in the treatment of colorectal cancer.
While it is not fully understood how the RES exerts a hepatoprotective effect, we hypothesized that this protective effect stems from the fact that RES contains high phenolic contents; the polyphenolics are known as hepatoprotective agents (Yasin et al., 2015). Remarkably, we found that the treatments either with RES, 5-FU, or a combination of both produced a significant and complete reversal of the liver function indices, serum proteins, activities of antioxidant, and oxidative stress biomarkers, compared with the HCC group. In general, it is important to draw attention to that, MDA level is a major indicator of oxidative stress in the tissue, and all these parameters are the best indicators of liver damage which reflected the pathological changes in the hepatocellular carcinoma in our work. These findings were in accordance with the results of Hong et al. (2010).
Cytokines play a pivotal role in cancer initiation and progression (Grivennikov and Karin, 2011; Abdelaziz and Ali, 2014). TNF-α is one of the most widely recognized proinflammatory cytokines that have crucial roles in tumor development and progression; though induction of various signaling pathways, the TNF-α induces intracellular inflammatory status by activation nuclear factor-kappa β (NF-kβ) (Wang et al., 2016). The current body of evidence demonstrates a plethora of cytokines released by malignant cells and associated macrophages (Zhang et al., 2016). Administration of DEN elevated the serum levels of TNF-α and treatment with RES effectively attenuated TNF-α levels in our study. Studies have shown that flavonoids have antitumor and anti-inflammatory properties and can reduce the serum levels of cytokines (Torisu et al., 2000). Specifically, RES downregulates matrix metalloproteinase expression, leading to a subsequent decrease in the TNF-α levels (Ji et al., 2015).
Alpha-fetoprotein is widely used as a marker in the early detection of HCC. The increased level of AFP in HCC rats is an indicator of its production by malignant hepatocytes and was significantly reversed after 5-FU treatment (Abdel-Hami and Morsy, 2010). These findings were in accordance with our results and support them in the same way. The treatments of HCC-rats either with RES, 5-FU, or both together exerted a significant reversal of the increased AFP.
Cory and Adams (2002) reported that apoptosis is a vital cellular activity in the performance of hepatic cells. Bax is one of the apoptotic pathways, is a mitochondrial membrane protein that acts as a pro-apoptotic member, causes cytochrome C release, and activation of caspase-3 (Adachi et al., 2004). The level of Bax was increased in the hepatic cells revealing the possibility of the apoptosis occurrence after DEN administration in comparison to the control group. Similarly, other studies indicated that induction of apoptosis was always associated with elevated expression of Bax (Baiomy, 2016).
We observed that DEN administration led to an increase in Bax immuno-activity as a result of oxidative stress-induced Bax transmigration into the mitochondria. The detection of immuno-activated Bax is a very reliable way to identify cells destined to die by apoptosis, even before the presence of the morphologic or pathological characteristics such as DNA fragmentation (Csizmadia and Csizmadia, 2009). The combination of RES and 5-FU significantly permitted an extra effective reduction of cell proliferation and induction of apoptosis in cancer cells of mouse skin carcinogenesis than 5-FU alone (Dun et al., 2015).
In the current study, (-ve) immunostaining CA19-9, (+ve) immunostaining CK18 and Bax were demonstrated in liver sections of HCC-rats indicating the presence of hepatocellular carcinomas, while RES with 5-FU modulated the immune response against the tumor cells. CK18 is known to not be good in the distinguishment between primary hepatic carcinoma and metastatic cases from the gastrointestinal tract, while CA19-9 pattern is used for the diagnosis of hepatocellular carcinoma. Agree with our opinion of other scientific researches, CA19-9 pattern was positive immunostaining only in cholangiocarcinoma, metastatic carcinoma, and negative to HCC, so it was excluded a diagnosis of HCC, while CK-18 was of no role in the confirmation of the diagnosis of primary hepatic carcinoma as it has a positive immunoreactivity to HCC, cholangiocarcinoma, and metastatic carcinoma (Sawan, 2009). CA19-9 is a protein that exists on the surface of the cancer cells of various size interlobular bile ducts in areas of bile ducts epithelium proliferation. A number of large and middle-size interlobular bile ducts appeared as small foci of positive immunostaining cells for CA19-9 in the normal control liver section, while part of the large bile ducts and small biliary ducts positively immunostained for CA19-9 in liver carcinoma (Haglund et al., 1991).
Conclusions and Recommendations
Extensive evidence in the current study endorsed that the dual-treatment of the phytochemical substance with the antitumor drug had an observantly therapeutic and immune-modulatory efficacy against HCC. Our results suggest that chemotherapy using the anti-cancer drug and natural agent together represents a superior cancer treatment option.
ACKNOWLEDGEMENTS
Not applicable
Novelty Statement
It is the first time to explore the beneficial effects of combined use of resveratrol and 5-Fluorouracil in liver cancer.
AUTHOR’S CONTRIBUTIONS
AN designed and performed the experiments, supervised the study, analyzed the results and wrote the paper. RF assisted in paper writing, discussed the results,and contributed to the final manuscript arrangement. All authors read and approved the final manuscript.
Conflict of interests
The authors have declared no conflict of interest.
REFERENCES






