Advances in Animal and Veterinary Sciences
Research Article
Formulation and Characterization of Plant, Animal, and Probiotic Based Fish Meals and Evaluating their Efficacy on Growth and Performance in zebrafish (Danio rerio)
Riya Ann Samuel, Suravi Sasmita Dash, Riyaz Ali.L, Kuppusamy Alagesan Paari*
Department of Life sciences, CHRIST (Deemed to be University), Bangalore, Karnataka, India 560029.
Abstract | A comparative analysis on the effects of plant based (PD), animal based (AD) and probiotic based (PrD) diets on growth performance in Danio rerio was investigated. Different diets were administered as either single or combination diet (CD) containing PD, AD and PrD exhibited varying effects on growth and development. The probiotic bacteria isolated from Indian prawn (Penaeus indicus) was identified as Bacillus sp using 16s rRNA sequencing and phylogenetic analysis. The isolate was characterized by evaluating its ability to survive at different pH, temperature and simulated artificial gastric environment and was further subjected to varying concentrations of salt and organic solvents. Antibiofilm activity of the isolate was evaluated against fish pathogens; Vibrio harveyi (96.1±2.7%), Escherichia coli (96.2±1.5 %,), Pseudomonas aeruginosa (95.3±3.0%) and Staphylococcus aureus (96.7±2.8%). After the end of trail period, growth parameters were evaluated. Weight gain percentage was significantly higher in PrD (15.7±0.08 %) compared to other treatments. (p<0.05). Feed conversion ratio was least in CD (0.35±0.09) and feed efficiency (2.7±0.08) in CD was numerically high compared to other treatments. (p>0.05).The study promotes sustainable aquaculture by the use of alternative aqua feeds derived from plant or animal based sources. The study also highlights the usage of probiotics in improving growth performance, disease resistance in aquatic animals.
Keywords | Alternative feed, Feed formulation, Growth performance, Probiotics, Bacillus sp.
Received | May 14, 2021; Accepted | June 12, 2021; Published | July 28, 2021
*Correspondence | Kuppusamy Alagesan Paari, Department of Life sciences, CHRIST (Deemed to be University), Bangalore, Karnataka, India 560029; Email: paari.ka@christuniversity.in
Citation | Samuel RA, Dash SS, Ali L Riyaz, Paari KA (2021). Formulation and characterization of plant, animal, and probiotic based fish meals and evaluating their efficacy on growth and performance in zebrafish (danio rerio). Adv. Anim. Vet. Sci. 9(9): 1489-1497.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1489.1497
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Paari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Aquaculture is a rapidly growing food sector in the world with global production reaching a peak of 179 million tonnes annually (FAO, 2020). Aquaculture sector is in constant demand and is facing the problems of feed shortage, pathogenic diseases and excessive antibiotic usage. Rapid advancements in the field of nutrition biology, aquaculture technology and the practice of sustainable aquaculture are key factors that contribute for better aquaculture practices (Santis and Jerry, 2007). Fish meal (FM) is an extensively used ingredient in aquaculture owing to its vitamin contents, balanced amino acids, growth factor, and other growth promoting properties (Tacon and Metian, 2008; Goddard et al., 2008). In terms of aquaculture sustainability, extensive use of FM as an aquatic feed has been unyielding due to the shortages in the resource for feed production which in turn increase the cost of FM. Alternative feed sources that have better nutrient profiles and available at a lesser price can be used to make ideal formulations for catering nutritional requirements (Gerile and Pirhonen, 2017; Aleström et al., 2006). Cheap protein sources derived from plant and animal by-products from agriculture, oilseed plants, fisheries, domestic animal sources can be utilized as ingredients in fish feed formulation (Tacon, 1990; Zhou et al., 2011). Among the plant based meal, soybean diet consisting of 44-49% protein content constitute for 69% of diet from plant source for feed formulation (Ghadge et al., 2009; Siddiqui and Khan, 2014; Cromwell, 2017; Blaufuss and Trushenski, 2012). Similarly, groundnut meal known for its rich amount of protein, vitamins, and minerals source is another traditionally underutilized, inexpensive plant-based diet (Aguihe et al., 2012; Srivastava et al., 2018). Even though plant sources can be used as ideal formulations, several factors like anti-nutritional factors (ANFs) in soybean, failure of groundnut crops, susceptibility to fungal toxins limit their use (Ghadge et al., 2009; Yasothai, 2016). To overcome these complications from plant protein sources, alternative feeds from animal by-products are preferred (Henchion et al., 2017). Potential animal sources with favorable essential amino acid profiles include bone meal, shrimp meal, and earthworm meal (Sogbesan and Ugwamba, 2008). Long term economical and biological potential of bone and shrimp meal make it ideal additives in furnishing a balanced fish feed. More recently, earthworm meal is being used in the category of suitable feed ingredients (Ding et al., 2015; Tedesco et al., 2020). The amino acid profiles of animal based feeds are much similar to fishes and contain lipid contents with high proportions of omega-3 fatty acids (Beg et al., 2016). A proper ratio of all these blended protein sources can improve the quality of feed given to fishes and enhance their growth and development. A complementary feed material is formulated from plant, animal and bacterial source. Probiotics are live microbial feed supplements which benefit the host by improving its intestinal microflora (Vine et al., 2006; Wang, 2007; Yanbo and Zirong, 2006; Fuller, 1989). Probiotics supplemented along with the formulated diet can influence growth performance of fishes by secreting digestive enzymes which can facilitate better feed uptake and they can limit pathogenic growth by competition and production of extracellular secretions (Merrifield and Carnevali, 2014; Yanbo and Zirong, 2006). A wide variety of microorganisms can be used as probiotics in aquaculture including species of yeast, Bacilli, lactic acid bacteria, Pseudomonas sp. Enterococcus sp. (Wang et al., 2008; Ringo and Gatesoupe, 1998; Irianto and Austin, 2002; Satish Kumar et al., 2011). The use of probiotics can be highly beneficial in aquaculture since they can serve as an alternative to antibiotics, combat pathogenic diseases, many probiotic supplements have been proven to enhance growth, improve survival rate and even enhance reproduction in aquatic animals (Wang et al., 2008; Balcázar et al., 2006; Watts et al., 2017). Probiotics can improve the water quality of large aquaria by reducing ammonia content in the water and can also prevent pathogenic biofilm formation which makes them an essential component of sustainable aquaculture practices (Zhang et al., 2011; Sharma et al., 2018).
The present study was aimed at comparatively analyzing the effect of plant, animal, and probiotic based fish feeds on growth parameters in Danio rerio. Diets were formulated using plant-based (PD), animal-based (AD), and probiotic based (PrD) sources and were evaluated for their efficacy in improving growth performance in Danio rerio. The study promotes sustainable aquaculture by the use of alternative feeds derived from plant or animal based sources in order to mitigate the rising costs and shortage of fish feed.
Materials and Methods
Isolation, biochemical characterization and identification of potential probiotic bacteria
Indian prawn (Penaeus indicus) was used as a probiotic host for bacterial isolation. The gastrointestinal tract of the organism was homogenized and serially diluted with sterile saline solution, followed by plating onto De Man, Rogosa, and Sharpe (MRS) agar and incubated at 37°C for 48 hours. The morphological characteristics such as abundance of growth and size were studied on MRS agar plates. The isolates obtained were screened using the antimicrobial well diffusion assay and the safety of the isolated culture was assessed using hemolysis assay. A non-hemolytic strain showing effective antimicrobial activity was selected and further characterized using standard biochemical tests like Gram’s staining, motility ,catalase, indole, methyl red, Vogus Proskauer and Simmon citrate tests (Yadav et al., 2016).The isolated strain was identified using 16SrRNA-F and 16SrRNA-R primers using BDT v3.1 Cycle sequencing kit on ABI 3730xl Genetic Analyzer. A phylogenetic tree was constructed using the neighbor joining technique via MEGA v1.3.4 (Kimura, 1980; Kumar et al., 2016).
Probiotic characterization
The isolate was evaluated for its ability to survive in different pH (2-10), temperature (15 ̊C- 55 ̊C), sodium chloride concentration (2%-10%), phenol concentration (0.2%-0.6%) in MRS broth and was incubated for 24 hours. Later the isolate was plated onto MRS agar plates and the Log (CFU•mL-1) was calculated. The ability of the isolate to survive in simulated artificial gastric juice for durations (2, 4 and 6 hours) was assessed. After incubation, the isolate was plated onto MRS agar and Log (CFU•mL-1) was calculated. (Halder et al., 2017; Corcoran et al., 2005).
Antibiofilm activity of the isolate
The antibiofilm activity of the isolate was assessed against Escherichia coli, Vibrio harveyi, Pseudomonas aeruginosa and Staphylococcus aureus by performing the modified crystal violet assay. A 100µl aliquot of the bacterial isolate was added along with the control into the wells of the microtiter plate. After 45 min, plates were washed with sterile distilled water. 1% crystal violet was added and again incubated for 15 min. De-staining was carried out using 100µl of ethanol and the absorbance was measured at 450nm using a microplate reading equipment (Costa et al., 2018).The percentage inhibition was calculated using the equation:
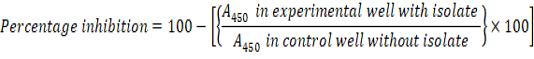
Determination of carbohydrate fermentation profiles
The carbohydrate fermentation profile was established using nine different carbohydrates, maltose, mannitol, xylose, galactose, fructose, lactose, sorbitol, inositol and sucrose. The assay was performed in a 96 well microtiter plate. 160 µl of active cell culture in modified MRS broth containing bromocresol purple was added in each well along with 40 µl of different sugar solutions. After incubation at 42 ˚C overnight the change of color and turbidity in the wells were noted with yellow color change indicating a positive result and no color change indicating a negative result (Erkus, 2007).
Feed preparation and formulation
Proximate analysis of the feed constituents in both plant based (PD) and animal based (AD) was performed based on the percentage of carbohydrates, protein, fat, monounsaturated fatty acids (MUFA), poly unsaturated fatty acids along with moisture and ash content (Table 2). Corn starch was added to act as a binder to prevent leaching of nutrients and disintegration of raw ingredients (Volpe et al., 2012).The probiotic based diet (PrD) involved administering the probiotic isolate at a concentration of 7 Log (CFU•mL-1) along with a commercially available diet. Combination diet (CD) was prepared by combining PD and AD based constituents in the ratio of 1:1 and supplemented along with 7 Log (CFU•mL-1) probiotic bacteria. Commercial fish diet served as control. The composition of different diets is shown in Table 1.
Experimental design and growth performance measurements.
Four experimental groups along with a control were designed. Uniform sized zebra fishes (Danio rerio) were purchased from a nearby fisheries store. The fishes were segregated into the following groups; group fed with plant-based diet (PD), group fed with animal-based diet (AD), group fed with probiotic-based diet (PrD), group fed with combination diet (CD). Each of the groups contained 10-12 replicates of uniform sized Danio rerio fishes. The fish were acclimated with the basal diet for 2 weeks before beginning the study.
Growth parameters-Weight gain percentage, feed conversion ratio and the feed efficiency were recorded from the beginning of the feeding period (Day 1) to the end of the feeding experiment (Day 30). The fishes were fed once a day with diets (4% of their body weight) and the growth parameters were measured at a five day interval. Additional parameters like pH, ammonia content, and temperature were monitored frequently to ensure a clean environment for the fishes to grow. Growth parameters were measured using the formulas below:
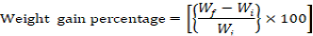
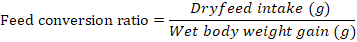
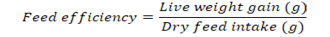
Note: Wf = Final weight in grams, Wi = Initial weight in grams
Table 1: Showing proportions of different ingredients used to prepare the different fish diets.
| Sl.no | Name of the diet | Diet formulation | Percentage (%) |
| 1. | Plant based diet (PD) |
Soybean meal Groundnut meal Fruit peelA Corn starch |
56.25 25 6.25 12.5 |
|
2. |
Animal based diet(AD) |
Fish meal Bone meal Shrimp meal Earthworm meal B Corn starch |
48.65 15.13 24.33 1.08 10.81 |
|
3. |
Probiotic based diet(PrD) |
Crude protein C Probiotic suspension |
30 107 CFU mL-1 |
| 4. | Combination diet (CD) |
PD AD Probiotic suspension |
50 50 107 CFU mL-1 |
| 5. | Control |
Crude protein C |
30 |
NOTE:A- Major constituent of fruit peels-Orange peel, minor – pomegranate peel, banana peel; B- Dried in a hot air oven at 80ºC for 2 days, then ground into a fine powder and added as a raw ingredient; C- Commercial diet supplemented as 4% body weight ; CFU- Colony Forming Unit
Statistical analysis
All the data collected were analyzed using R v 3.6.3. One way Analysis of variance (ANOVA) test was used to evaluate the effects of each diet on the fish growth and performance with p <0.05 considered significant.
Table 2: Showing general isolate characteristics and the fermentation profile of nine carbohydrates
| Colony morphology | Off white, small, circular | Fermentation profile | |
| Shape | Bacilli | Maltose | + |
| Gram’s staining | + | Mannitol | + |
| IMViC tests |
-+++* |
Xylose | + |
| Motility | + | Galactose | + |
|
Hemolytic activity |
Non-hemolytic | Fructose | + |
| Anti-microbial activity | +++ | Lactose | + |
| Milk coagulation efficacy | + | Sorbitol | - |
| Tolerance to 0.6% trypsin | + | Inositol | - |
| Catalase test | + | Sucrose | - |
Note: *IMViC tests- Indole, methyl red, Vogus Proskauer and Simmon citrate test respectively.
Results and discussion
The potential isolates obtained on MRS plate were screened using the agar well diffusion assay. The isolates which showed antimicrobial activity were then safety assessed using the hemolysis assay. A non-hemolytic isolate was selected and was further characterized using standard biochemical and morphological assays (Table 2). Isolated culture was identified as Bacillus sp. using 16s rRNA sequencing and BLAST analysis. Bacillus sp. have been reported for a wide range of nutritional, physiological and metabolic diversity which has been widely used for its antimicrobial activity to tackle fish pathogens in commercial aquaculture (Sen et al., 2015; Kong et al., 2017).
The consensus sequence obtained was deposited in GENBANK (Accession number- MT355408). Phylogenetic tree was constructed using the neighbor joining method (Figure 1).
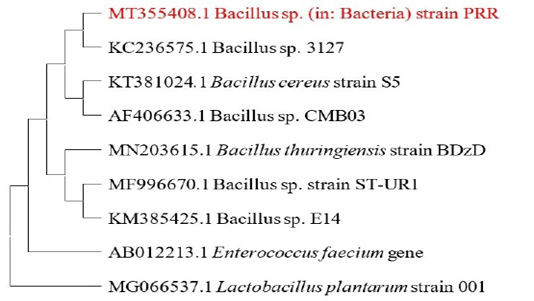
Figure 1: Shows the phylogenetic tree constructed using the maximum likelihood method. The tree with the highest log likelihood (-3972.48) is shown. Initial tree(s) for the heuristic search were made by using Neighbor-Join method coupled with BioNJ algorithm and then estimated using the Tamura-Nei model,at the end the topology with superior log likelihood value was selected. This analysis involved 9 nucleotide sequences. There were a total of 1559 positions in the final dataset. Enterococcus faecium and Lactobacillus plantarum were used as outgroups.
The isolate was able to grow at low pH ranging at pH 2 [5.9±0.04 Log (CFU•mL-1)] and pH 4 [6.56±0.07 Log (CFU•mL-1)].The optimum growth was between pH 6-8.The growth decreased at higher pH 10 [5.65±0.012 Log (CFU•mL-1)].T (Figure 2). Resistance to low pH is of great importance in assessing the survival of probiotic strains in the gastric environment (Musikasang et al., 2009; Kimura et al., 2006). Tolerance to gastric juice is considered as a key functional requirement for probiotics, which enables them to survive during passage through the gastrointestinal tract (Bevilacqua et al., 2010). The final growth observed after exposure of the isolate for a duration of 6h in simulated artificial gastric juice was found to be 6.46±0.01 Log (CFU•mL-1) (Figure 3). LAB are equipped with molecules like proton‐translocating ATPase that prevent internal cellular destruction or improve cellular strength to enable the tolerance of harmful exterior surroundings (Sanchez et al., 2007).
The ability of an isolate to resist the gastric environment is a probiotic feature. Furthermore, the resistance can be enhanced by encapsulation, carbohydrate and protein substrate changes (Ding and Shah, 2007).
The isolate was able to tolerate different temperatures. Growth at optimum temperature 37 ˚C was maximum at 7.38±0.04 Log (CFU•mL-1). The growth diminished at temperature deviations of 10 ˚C in extremes of 37 ˚C. The growth at 25 ˚C was 7.02±0.06 Log (CFU•mL-1) and the growth at 45 ˚C was 6.83±0.09 Log (CFU•mL-1) (Figure 4). No growth was observed at low temperatures 15 ˚C and beyond 45 ˚C (data not shown). Probiotics have a variety of adaptation mechanisms to increase their thermostability which include increasing the production of heat shock proteins (HSPs), phosphotransferases and chaperonins (Chen et al., 2017; Hernández et al., 2018).
Probiotic growth at higher temperature is a good indication since a high fermentation temperature decreases contamination by other microorganisms (Chen et al., 2015;Terpou et al., 2019). Probiotics grown in a high salt concentration adapt by displaying loss of turgor pressure which can affect their overall physiology, enzyme activity, water activity and metabolism and thereby enabling the cells to proliferate. The growth observed at 2% concentration was 7.72±0.03 Log (CFU•mL-1) and the growth at 10% NaCl concentration was 7.15±0.05 Log (CFU•mL-1) (Figure 5). Therefore strains which can survive high osmotolerance are preferred in commercial applications (Menconi et al., 2014).
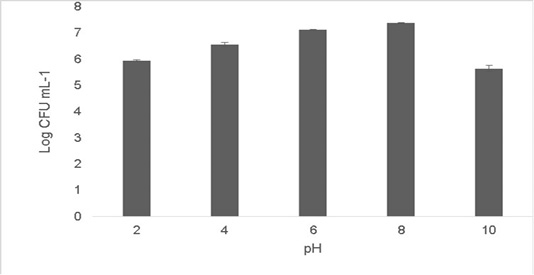
Figure 2: Shows cell viability of the isolate when subjected to pH ranges (2-10). The values shown are Mean±SD of triplicates.
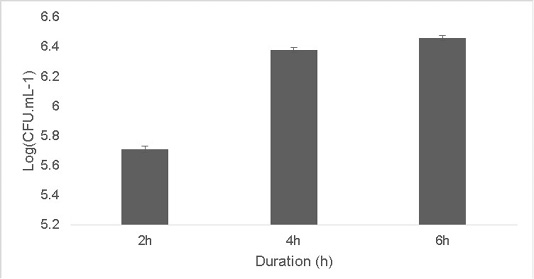
Figure 3: Effect of simulated artificial gastric juice on the growth of the isolate at duration of 2, 4 and 6h
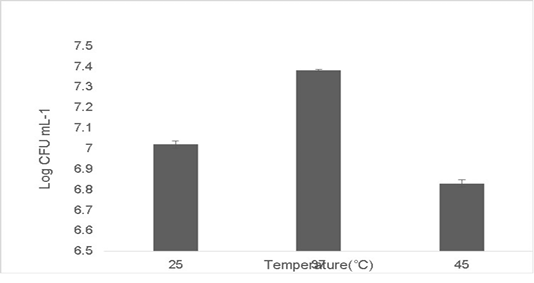
Figure 4: Shows heat tolerance of the isolate at ±10˚C deviations from 37 ˚C. Error bars indicate the standard deviation, the values are expressed as Mean±SD of triplicates.
The antbiofilm activity of Bacillus sp. against fish pathogens; Vibrio harveyi (96.1±2.7%), Escherichia coli (96.2±1.5 %,), Pseudomonas aeruginosa (95.3±3.0%) and Staphylococcus aureus (96.7±2.8%) was recorded (p<0.05) (Figure 6). Probiotics can mitigate the problem of prevalence of pathogens in aquaria by disrupting formed biofilms. Probiotic antibiotic activity is due to secretion of bio surfactants, peptidoglycan binders, antimicrobial peptides and enolases (Spurbeck and Arvidson, 2010; Wu et al., 2018). Antimicrobial peptides are produced by genes like LCI other and gene clusters that help in production of secondary metabolites (Wu et al., 2018).
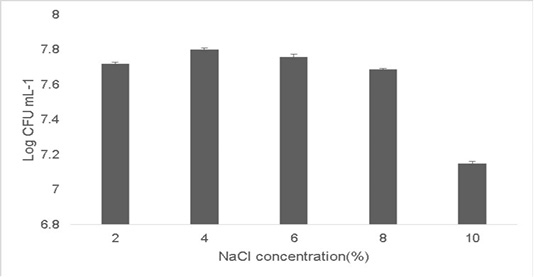
Figure 5: Osmotolerance of the isolate at varying concentration of NaCl (2%-10%). Error bars indicate the standard deviation, the values are expressed as Mean±SD of triplicates.
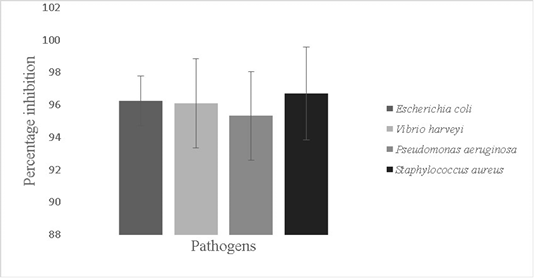
Figure 6: Antibiofilm activity of the isolate against pathogens; Escherichia coli, Vibrio harveyi, Pseudomonas aeruginosa and Staphylococcus aureus. The error bars indicate standard deviation and statistical significance indicated by * (P<0.05)
Proximate nutrient analysis PD and AD
The nutrient profiles of the plant and animal based diets were analyzed. The average energy was between 2500 kcal kg-1-3000kcal kg-1.The protein range was optimum between 45-50% of the feed composition. (Sogbesan and Ugwamba, 2008).The percentage of other components ;carbohydrates, fats (Mono unsaturated fatty acids, poly unsaturated fatty acids),moisture and ash have been described in Table 3.
Growth performance study
The results of the present study were hypothesized to show better performance in the fishes fed with a combination diet (CD) as it had the highest diversity of raw ingredients along with probiotic administration. However, this was not the case. PrD was found to exhibit greater weight gain percentage (15.7±0.08 %) in comparison with other treatments (p<0.05) (Figure 7). Feed conversion ratio and feed
Table 3: Showing proximate analysis of plant based diet (PD) and animal based diet (AD) per 100 g of the samples
| Diets | Energy (kcal)* |
% Carbohydrate |
% Protein |
% Fat |
% MUFA |
% PUFA |
% Moisture |
% Ash |
|
PD |
287.3 | 16.9 | 45.7 | 4.1 | 0.59 | 0.54 | 10 |
5.23 |
| AD | 263.5 | 9.0 | 48.1 | 3.9 | 0.28 | 0.06 | 8 |
4.6 |
Note: i. MUFA – Mono Unsaturated Fatty Acids, PUFA – Poly Unsaturated Fatty Acids ii. Energy = (Carbohydrate (g) x 4) + (Protein(g) x 4) + (Fat(g) x 9) *Energy in kcal per 100g of feed
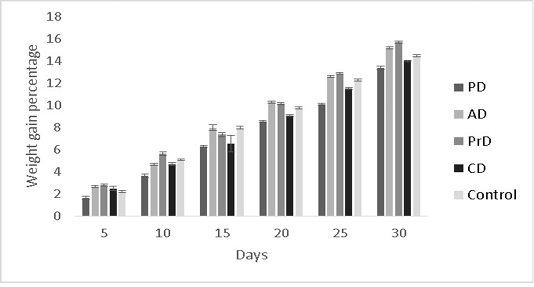
Figure 7: Showing the weight gain percentages of Danio rerio in the treatments PD, AD, PrD, CD and control. The trail was conducted for the duration of 30 days. The error bars indicate standard deviation.
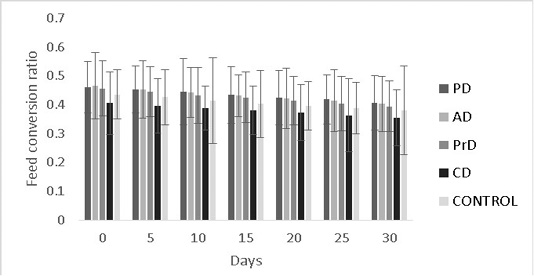
Figure 8: Shows the feed conversion ratios of Danio rerio treatment groups upon feed supplementation of PD, AD, PrD, CD and control. The parameter was monitored for a duration of 30 days. The error bars indicate standard deviation.
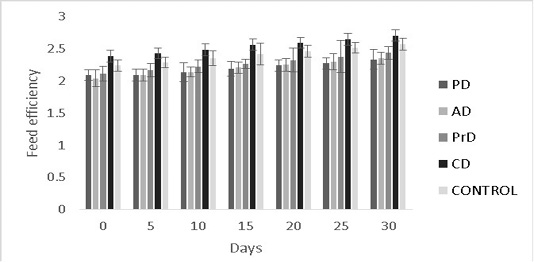
Figure 9: Feed efficiency of PD, AD, PrD, CD and control for a total duration of 30 days on treatment groups of Danio rerio. The error bars indicate standard deviation.
efficiency were numerically better in CD compared to other treatments (0.35±0.09 and 2.7±0.09) respectively (P>0.05) (Figure 8, 9 respectively).
Soybean meal has been found to contain anti-nutritional factors (ANFs) such as protease inhibitors, lectins, glycinin, and saponins which could possibly affect feed intake (Salem and Ghany, 2018). In another study, feed intake was highly affected when 35% of the feed was replaced with soybean meal, causing an 18% decrease in feed intake in comparison with the control group (Volpe et al., 2012). Similarly the replacement of fish meal with more than 20% of soybean meal can negatively affect growth. Moreover, soy protein, as well as soy saponins present in soybean meal, are known to trigger an immune response causing inflammation (Hedrera et al., 2013). Usage of soya concentrate can negate the detrimental effect of ANFs and other growth inhibitors (Ray et al., 2018). Soybean meal has a favorable essential amino acid profile (EAA) and is suitably used as an better digestibility component (Bandara, 2018). Probiotics can reduce inflammation caused due to plant proteins and improve growth and survival (Rendueles et al., 2012). This might be one of the possible reasons for better performance observed in fishes fed with CD compared to PD. Plant based diets if provided at early stages of the organism’s life can enhance acceptance and better utilization (Geurden et al., 2013). No mortality was observed in any of the treatments supplemented to the fishes throughout the experiment.
Conclusion
Alternate feeds formulated in this study had no negative effect on the growth and performance of Danio rerio. Bacillus sp supplement was effective in growth performance, contribute resistance against pathogens and improve water quality parameters in aquaria. Soybean meal refinement into concentrate can minimize the effects of ANFs and thereby improvise the feed intake. The use of alternative feeds along with probiotic supplements can reduce the load on fish feed industry and therefore making sustainable aquaculture practices more economical and to reap maximum benefits.
Acknowledgements
The authors acknowledge the support received from the Department of Life Sciences and Centre for Research, CHRIST (Deemed to be University) for the financial aid through MRP. (MRPDSC-1936).The authors also acknowledge the copyright assigned conditions required by Advances in Animal and Veterinary Sciences.
Conflict of Interest
The authors declare no conflict of interest
Author contribution
Riya Ann Samuel was involved in the experimental design, data collection, data analysis and revised this manuscript. Suravi Sasmita Dash was involved in the experimental design, data collection, data analysis and revised this manuscript. Riyaz Ali.L was involved in experimental design, data analysis and drafted this manuscript. Kuppusamy Alagesan Paari conceptualized the idea, involved in funding and revised this manuscript. All authors read and approved this manuscript
References






