Journal of Animal Health and Production
Research Article
Endogenous Testosterone Hormone and Agonistic Behavior in Male Japanese Quail (Coturnix japonica)
Ahmed Mohamed Hanafy1*, Hassan Ahmed Khalil1, Ibrahim Magdy Hegab2,3
1 Department of Animal Production, Faculty of Agriculture, Suez Canal University, 41522 Ismailia, Egypt; 2College of Grassland Science, Gansu Agricultural University, Lanzhou 730070, Gansu Province, China; 3Department of Hygiene, Zoonoses and Animal Behaviour and Management, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Abstract | The objective of the current study was to estimate the relationship between endogenous serum testosterone hormones and agonistic behavior in male Japanese quail. Forty healthy adult Japanese quails (30 males and 10 females) were randomly distributed into three experimental groups. The 1st group (G1, n= 10 males) was housed individually, the 2nd group (G2, 10 males and 10 females) was simultaneously housed in one cage, and the 3rd group (G3; n= 10 males) birds were housed together in a single cage. Serum testosterone concentration has been estimated alongside different behavioral (head banging, eyelid injuries, head plumage deterioration) and sexual (cloacal gland area, foam production, absolute and relative testes weight) traits. The results revealed that there was a highly significant difference (p ≤0.001) among males housing status on testosterone levels. Serum concentrations of testosterone were significantly (p ≤0.001) increased in males in G2 and G3 by 91.35 and 98.41 %, respectively than that of males in G1. In addition, the results demonstrated that highly positive correlations (p=0.01) were found between serum testosterone concentration and each of head banging, eyelid injuries and head plumage deterioration, and moderate positive correlations (p=0.225) with foam production. In contrast, negative correlations were found between testosterone concentration and each of absolute testes weight (p=0.294), and cloacal gland area (p=0.074). These results demonstrate that changing levels of testosterone in the blood may be due to male’s status. The present results may provide a role of testosterone towards aggressive pecking behavior among males when kept lonely or with females.
Keywords | Testosterone, Pecking, Cloacal gland, Foam, Quail
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | March 30, 2018; Accepted | April 03, 2018; Published | June 25, 2018
*Correspondence | Ahmed Mohamed Hanfy, Department of Animal Production, Faculty of Agriculture, Suez Canal University, 41522, Ismailia, Egypt; Email: [email protected]
Citation | Hanafy AM, Khalil HA, Hegab IM (2018). Endogenous testosterone hormone and agonistic behavior in male japanese quail (Coturnix japonica). J. Anim. Health Prod. 6(2): 51-56.
DOI | http://dx.doi.org/10.17582/journal.jahp/2018/6.2.51.56
ISSN | 2308-2801
Copyright © 2018 Hanafy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Recently, Japanese quail (Coturnix japonica) has been used as a good laboratory model for poultry research due to their faster juvenile growth, higher egg production and shorter generation interval (Shit et al., 2010). The modern production systems and market demands mandate a reliance on the intensive farming system which guarantees high yield and minimizes the need for space and for labor. Nevertheless, this space restriction will inevitably lead to the appearance of abnormal behavioral patterns including pecking behavior which is one of the problems faced by poultry producers worldwide (Riber et al., 2007). Moreover, head injuries caused by aggressive pecking are an important welfare concern in quail farming when housed in the same place (Wechsler and Schmid, 1998). Aggressive pecking behavior in males, especially toward the head area, leads to several problems such as severe injuries (sometimes eye loss), cannibalism and even mortality which encompasses some economic concerns in quail farming (Mekawy, 2014). The previous findings have recognized testosterone hormone as the main regulator of inter-male aggression, particularly aggressive pecking behavior, among males of wild and domestic birds (Muller and Wrangham, 2004; Biswas et al., 2007). Suppressing testosterone levels through castration dramatically reduced or even diminished the inter-male aggressive pecking which is restarted by external administration of testosterone (Mills et al., 1997). Being maximally elevated during the breeding season, (Arboleda and Khan, 2016) higher testosterone levels may function to reduce pain sensitivity as a way of promoting aggressive behavior during competitive interactions (Hau et al., 2004). Besides, testosterone has multiple aspects of male reproductive physiology, such as the development of the reproductive organs, spermatogenesis, secondary sexual characteristics and in addition to enhance sexual motivation (Wada, 1986; Wingfield et al., 1999).
The cloacal gland is considered as an external indicator for testicular function in Japanese quail male (Mohan et al., 2002). Area of cloacal gland and production of foam are dependent on the serum testosterone concentration (Biswas et al., 2007). Abood et al. (2011) reported that atrophy of testes and decreased concentration of serum testosterone caused reduction in cloacal gland size and foam production. Also, Mohan et al. (2002) reported that hemicastration and castration male quails reduced the size of foam gland and its foam production.
Therefore, the current investigation was conducted to estimate the relationship between the level of testosterone hormone, aggressive pecking behavior and some sexual traits for Japanese quail male.
Materials and Methods
Birds, Husbandry and Treatment
This experiment took place at Japanese quail Farm, Department of Animal Production, Faculty of Agriculture, Suez Canal University, Ismailia, Egypt. The study was conducted according to ethical guidelines approved by ethics of scientific research committee, Faculty of Agriculture, Suez Canal University, Ismailia, Egypt. After hatching all quail chicks were kept under normal brooding conditions in brooding floor pens until they were 10 weeks of age. Forty healthy adults [(30) males and (10) females] Japanese quails were individually weighed and randomly distributed into three experimental groups. The 1st group (G1); 10 males were housed in individual cages, the 2nd group (G2); 10 males were housed with 10 females in one cage (50x100 cm), and the 3rd group (G3); 10 males were housed together in one cage (50x50 cm). All groups were kept under uniform husbandry conditions and were provided with normal quail breeder ration 24% CP with 3000 Kcal ME)/Kg, and water ad libitum, with 16 h light/day.
Assessment of Intermale Aggressive Pecking
After 7 days from starting of the experiment some behavioral and physiological parameters had been measured. Aggressive pecking behavior and all related injuries (head banging and eyelid injuries) were visually examined for each male of all experimental groups. Headbanging was scored on a scale from 1 (normal head) to 5 (damage head). Eyelid injuries score, using a scale from 1 (normal eyelid) to 5 (all eyelid injuries), two eyelids injuries score (from 2 to 10) on the same scale as before. Head plumage deterioration scores of males of all experimental groups were visually investigated using a scale from one (completely feathered) to five (featherless) according to Khalil et al. (2011).
Reproductive and Hormonal Parameters
The cloacal gland areas (height and width per mm) were measured by using a caliper for all the males (Hanafy et al., 2015). The foam produced by each male was collected for three times with time interval 10 min. Quantitative measurements on cloacal gland foam production were conducted immediately using an electronic analytical balance. Behavioral and cloacal measurements were conducted by single observer to avoid inter-observer bias (Martin and Bateson 2007). All males of all experimental groups were slaughtered after 7 days from the start of the experiment by slitting the jugular vein. Immediately after blood collection, the abdominal cavity was opened, and the testes were removed and weighed using an electronic balance, and relative testes weights were calculated. The blood samples were centrifuged at 3000 rpm for 15 min and serum was stored at -20oC for further analysis. Serum testosterone was determined by ELISA kits (DiaMetra, Spello-Perugia, Italy) according to the manufacturer’s instructions. The sensitivity of the assay was 1.631 ng/mL, and the percentage of recovery was 95-103%. The intra- and inter-assay coefficients of variation were 4.08 and 5.54%, respectively.
Statistical Analysis
Data were analyzed using the General Linear Model (GLM) procedure of SAS (SAS Institute, 2011). Differences among means were detected using Duncan’s new multiple test. Correlation coefficients among traits were estimated. The level of significance at which the null hypothesis was rejected was α = 0.05.
Results
Serum Concentration of Testosterone, Pecking Behavior and Sexual Traits
Serum concentration of testosterone was markedly changed according to males housing status (Table 1). Males housed together (G3) or with females (G2) had increased (p <0.001) serum levels of testosterone compared with those recorded in males housed individually (G1). These increments were 91.35 and 98.41 %, respectively than that in G1. Moreover, there were significant (p ≤0.001) differences in aggressive
Table 1: Relationship of serum testosterone levels with aggressive pecking behavior and sexual traits of Japanese quail male
| Items | Groups | p-value | ||
|
G1 |
G2 |
G3 |
||
| Serum testosterone (ng/mL) |
5.67±0.29b |
10.85±0.75a |
11.25±0.65a |
0.001 |
| Aggressive pecking behavior traits | ||||
| Head banging (score) |
1.00±0.00c |
1.50±0.28b |
2.04±0.14a |
0.001 |
| Eyelid injuries (score) |
2.00±0.00c |
3.25±0.75b |
4.82±0.42a |
0.001 |
| Head plumage deterioration (score) |
1.00±0.00c |
2.37±0.12b |
3.13±0.09a |
0.001 |
| Body weight and sexual traits | ||||
| Body weight (g) |
245.80±6.12a |
233.00±7.34ab |
225.45±4.68b |
0.040 |
|
Cloacal gland area (mm2) |
597.60±19.15a |
558.50±32.47ab |
501.00±21.13b |
0.010 |
| Foam production (mg) |
49.75±3.16b |
76.51±4.17a |
46.72±3.91b |
0.005 |
| Absolute testes weight (g) | 7.42±0.59 | 6.59±0.41 | 6.63±0.79 | 0.612 |
| Relative testes weight (%) | 3.01±0. 23 | 2.84±0.23 | 2.86±0.31 |
0.801 |
Mean values bearing different letters within the row (a, b & c) differed significantly (P≤0.05) G1= Males housed individually, G2=Males housed with females, G3= Males housed together
pecking behavior traits among three experimental groups. The highest scores of headbanging, eyelid injuries and head plumage deterioration were estimated in G3, while the lowest scores were recorded in G1. Moreover, males housed together (G3) exhibited significantly more pecks than males housed with females (p ≤ 0.05).
As shown in Table 1, significant differences were found among groups in body weight and some sexual traits. The highest values of body weight and cloacal gland area were recorded in G1, while the lowest values were obtained in G3. Also, foam production was significantly increased (p ≤0.005) in G2 compared with those recorded in each G1 and G3. However, absolute and relative testes weights were higher in G1 than those recorded in each of G2 and G3, but without significant differences.
Correlation Coefficients Between Testosterone Hormone and Some Studied Traits
Results of correlation coefficients among serum concentration of testosterone hormone and aggressive pecking behavior and some sexual traits are presented in Figures 1 & 2. The results revealed that there were highly significant positive correlations (p=0.01) between testosterone concentration and each of headbanging, eyelid injuries and head plumage deterioration (r=0.588, 0.590 and 0.844, respectively; Figure 1-A, B and C). Regarding the sexual traits, there was a moderate positive correlation with foam production (r=0.301, p=0.225; Figure 2-A) and negative correlations were found between testosterone concentration and each of absolute testes weight (r=-0.259, p=0.294; Figure 2-B), and cloacal gland area (r= -0.425, p=0.074; Figure 2-C).
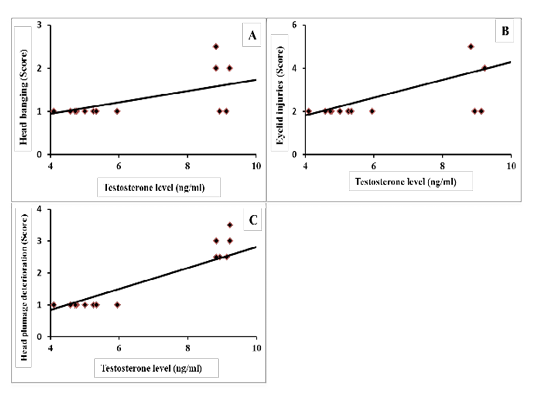
Figure 1: Coefficient of correlation between serum testosterone levels and aggressive pecking behaviors of male Japanese quail (A) Head banging (score), r = 0.588 (p = 0.010); (B) Eyelid injuries (score), r = 0.590 (p = 0.010); (C) Head plumage deterioration (score), r = 0.844 (p = 0.000).
Discussion
Testosterone is a major male steroid hormone from the androgen group in birds (Ottinger and Mahlke, 1984). Circulating testosterone concentrations could conceivably vary inter-specifically through changes in Gonadotropin-releasing hormone (GnRH) release from the hypothalamus, Luteinizing hormone (LH) release from the pituitary, testosterone synthesis in the testes or in other steroidogenic tissues (Adkins-Regan, 2011). It is primarily formed and secreted from Leydig cells in the testicles of males and the ovaries of females, although small amounts are also secreted by the adrenal glands (Burtis et al., 2007).
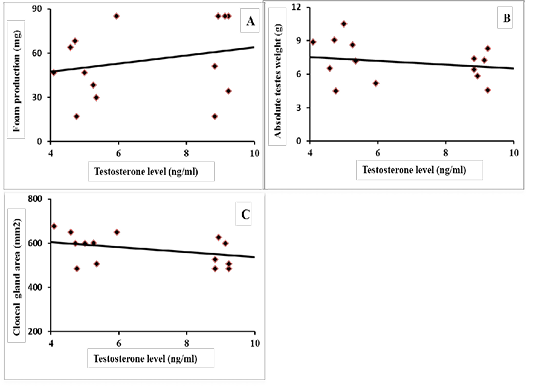
Figure 2: Coefficient of correlation between serum testosterone levels and some sexual traits of male Japanese quail (A) Foam production (mg), r = 0.301 (p = 0.225); (B) Absolute testes weight (g), r = -0.259 (p = 0.294); (C) Cloacal gland area (mm2), r = -0.425 (p = 0.074).
Testosterone influences multiple aspects of male reproductive physiology, such as development of the reproductive anatomy, spermatogenesis, secondary sexual characteristics, crowing, motivation, courtship and aggression behavior (Muller and Wrangham, 2004).
The results indicated that the serum concentration of testosterone was markedly changed according to males housing status. Males housed together or with females had significantly (p <0.001) increased serum levels of testosterone by 91.35 and 98.41 %, respectively than that estimated in males housed in individual cages. These increments may be returned to males housed together in the same cage, which may stimulate hypothalamus-pituitary-testicular axis to release excessive testosterone hormone. Also, results revealed that highly significant positive correlations (p=0.01) were found between testosterone concentration and aggressive pecking behavior which include head banging, eyelid injuries and head plumage deterioration (r=0.588, 0.590 and 0.844, respectively). These results are nearly consistent with Arboleda and Khan, (2016) who reported that during the breeding season, birds are aggressively defending themselves against intruders. The previous study also found a positive correlation between the concentration of testosterone and aggressive behavior in birds. In addition, they mentioned that males had a higher testosterone concentration and tended to be more aggressive behavior than females. Similarly, some species show testosterone peaks only during period of courtship. These variations in circulating testosterone levels during the breeding season are more closely associated with male aggression in reproductive contexts than with changes in reproductive physiology (Mutzel et al., 2011). Another interesting finding is the significant higher levels of aggressive behaviors in gender-mixed (G3) compared to male group G2. Aggressive pecking may serve primarily to establish social ranking and dominance hierarchy amongst individuals (Schlinger et al., 1987; Clutton-Brock and Huchard, 2013).
In the same vein, Mehta and Beer, (2009) reported that aggressive behavior may result from integrated approaches between behavioral endocrinology and cognitive neuroscience. Also, testosterone reduced the activity of the medial orbitofrontal cortex (OFC) in the frontal lobes in the brain while increased activity of OFC led to low levels of reactive aggression. Medial OFC activity is strongly associated with impulse control and self-regulation systems that integrates emotion, motivation, and cognition to guide context-appropriate behavior (Blair, 2004). Whereas OFC lesions lead to impulsive behavior and hyper-aggression (Strüber et al., 2008). Furthermore, receptors for androgens such as testosterone are found in the OFC (Finley and Kritzer, 1999). Testosterone influences aggression through reduced activity in the medial OFC by increase serotonin deficits in OFC (New et al., 2004; Siever, 2008), downregulate serotonin receptor mRNA expression and serotonin turnover in OFC (Ambar and Chiavegatto, 2009). Lower serotonin may lead to hypometabolism and reduced activity of the medial OFC. Moreover, Hau et al. (2004a) suggested that control of aggressive behavior by testosterone not only via efferent neural pathways but also by regulating the processing of sensory inputs probably at various levels of the peripheral and central nervous system. And they added that one function of testosterone during aggressive interactions is to reduce nociception, perhaps as a way of promoting aggressive behavior between birds (Hau et al., 2004b).
The cloacal gland of the adult male Japanese quail produces a meringue-like white foam which is always deposited into the female cloaca along with semen during copulation (Abood, 2011). The cloacal gland development, size change, and foam production depend on the physiological status of the testis and testosterone concentration in blood serum. The gonadotrophin hormones, food elements and daylight period have an effect on the size of sexual glands and its function (Biswas et al., 2007). In the current study, the cloacal gland area was significantly decreased in G3 while the largest area was found in G1. This could be convincingly linked with the level of aggression in the corresponding groups where G3 groups should the highest aggressive pecking behavior traits (Table 1). This inverse relationship between the cloacal gland area and the level of aggression may clarify the negative consequences of high fear and distress levels, due to the abnormal pecking behavior, on the development of cloacal gland in quail males. These results agree with Satterlee et al. (2002) where low-stress selected Japanese quail showed larger cloacal gland areas than males selected for exaggerated stress response.
The physiological role of foam on quail spermatozoa was described by Singh et al. (2011). They reported that the lactate present in foam increased sperm metabolism and motility by providing energy to quail sperm. Furthermore, low molecular weight (3–10 KDa) compounds in the foam were responsible for sperm disaggregating. The present results showed that a positive correlation between testosterone concentration and foam production (r=0.301, p=0.225). In contrast, negative correlations were found between testosterone concentration and each of absolute testes weight (r=-0.259, p=0.294), and cloacal gland area (r= -0.425, p=0.074). These results revealed that males housed individually showed the normal or physiological level of testosterone. This level may be sufficient to allow individuals to adjust their physiological process. Also, this group showed hypertrophy of cloacal gland area with low foam production, because these birds may not mate nor renew their foam. On the other hand, other groups showed high levels of testosterone which lead to increased copulation rate and foam output that causes decreased cloacal gland area and lowers foam output when measured. In fact, the present observation is in close agreement with Biswas et al. (2007) who reported that, a high degree of positive correlation between the concentration of testosterone and cloacal gland size. Mohan et al. (2002) determined that significant regression in the area of cloacal gland was associated with the reduction in serum testosterone in hemicastration and castration of male Japanese quail. Similarly, atrophy of testes and decreased concentration of serum testosterone motivated the reduction in cloacal gland size and foam production (Abood, 2011).
CONCLUSION
The present results suggest that changing levels of testosterone in the blood may be due to male’s status. These results may also provide a role of testosterone towards aggressive pecking behaviour among males when kept lonely or with females.
Acknowledgements
The authors would like to thank Prof. Akram Hamdy, Faculty of Agriculture, Minya University, Minya, Egypt for his valuable assistance in revising the manuscript.
Conflict of interest
The authors declare that they have no competing interest.
Authors Contribution
Ahmed Mohamed Hanafy, Hassan Ahmed Khalil and Ibrahim Magdy Hegab designed and performed the experiment, participated in manuscript writing and revision.
References






