Journal of Animal Health and Production
Libido Problem is Untraceable Through Testosterone and Luteinizing Hormone Rhythm in Zebu Breeding Bulls
Santu Mondal*, Mukesh Bhakat, Ajeet Singh, Tushar Kumar Mohanty, Muzamil Abdullah
National Dairy Research Institute, India.
Abstract | The libido problem is quite common in Zebu breeding bulls at tropical condition. When Breeding Soundness Evaluation (BSE) is normal, assessing the libido problem is complicated in adult breeding bulls.The present study was conducted to investigate the peripheral luteinizing hormone (LH) and testosterone levels in Zebu breeding bulls.The single dose of exogenous GnRH (10µg buserelin acetate, intramuscular) administrated in each animal of normal libido bulls (n=3) and poor libido bulls (n= 3). The jugular blood samplings were done at 1 h, 0 h (GnRH injection) followed by up to 6 h at 1 h interval. The average basal concentration, 1 to 6 h post-GnRH administration concentration (PGA) and Area under the curve (AUC) for testosterone, LH hormones were compared between the groups. The basal concentration of testosterone and LH hormones were found significantly higher (p<0.05) in poor libido bulls than normal libido bulls. The PGA and AUC were not significantly differed between the two groups. It was clear that the peripheral rhythm of testosterone and LH hormones were not useful to trace the libido problem in Zebu breeding bulls at the breeding centre.
Keywords | Zebu bulls, Libido, GnRH, Testosterone, LH
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | February 25, 2019; Accepted | April 12, 2019; Published | August 01, 2019
*Correspondence | Santu Mondal, National Dairy Research Institute, India; Email: [email protected]
Citation | Mondal S, Bhakat M, Singh A, Mohanty TK, Abdullah M (2019). Libido problem is untraceable through testosterone and luteinizing hormone rhythm in zebu breeding bulls. J. Anim. Health Prod. 7(3): 81-84
DOI | http://dx.doi.org/10.17582/journal.jahp/2019/7.3.81.84
ISSN | 2308-2801
Copyright © 2019 Mondal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Poor libido is a common complaint at any semen station in breeding bulls, especially in Zebu bulls. The huge numbers of breeding bulls are culled due to poor sexual willingness. The libido is a response to endogenous or exogenous stimuli mediated through a variety of physiological mechanisms, learned experience and motivation (Bryant, 1989). Although, expression of libido is primarily mediated by hormonal events, relationships among blood levels of luteinizing hormone (LH) or testosterone (Chenoweth et al., 1979, Henney et al., 1990). The sexual arousal processes involved in erection and ejaculation governed by parasympathetic and sympathetic pelvic nerves respectively (Hull et al., 1999). Sometimes sexual desire is called as a relief from psychological obstacles to sexual performance, where the result of previous bad experiences or native conditioning prevents them to express sexual willingness during a competitive situation (McDonnell et al., 1985). The culling rates of indigenous bulls are very much worrying leading to loss of good germ plasm under tropical climatic condition. Inadequate sex drive or poor libido is one of the main contributing factors for indigenous bull disposal, found 22.6% by Mukhopadhaya et al. (2010) at Artificial Breeding Research Centre, NDRI, India and 36.5% by Sudheer and Xavier (2000) at three AI centre in Kerala, India. Another finding reported that 8.36% of the crossbred bulls which have reached semen donation stage are culled due to non-donation of semen (Tyagi et al., 2006). There was 36.47 % Murrah bulls donated semen out of total reserved males (Shivahre et al., 2017), 58.97% of Holstein Friesian and Tharparkar crosses (KF) and 20% of Pure Tharparkar (TP) males produced semen of total reserved males (Panmei et al., 2016). The huge numbers of bulls were discarded and libido problem is one of the causes. There are several factors which affect libido like Hereditary, systemic diseases, psychogenic, endocrine problem and handling of bull as management problem. When physical examination of Breeding Soundness Evaluation (BSE) is normal, the poor libido in adult bulls is often more complicated at breeding centres. So, it was hypothesized that androgen insufficiency due to hypogonadotropic-hypogonadism may be one of the reasons. The testosterone and LH showed the diurnal pattern of secretion, so recommended blood sampling for the course of at least 8 h starting from morning is required, if an androgen insufficiency is to be confirmed (Nett, 1993). The present trials were undertaken to test the exogenous response of GnRH to peripheral testosterone and LH hormone in Tharparkar breeding bulls.
Materials and Methods
Experimental Animals
Total six Tharparkar bulls were selected for the experiment, reared at Artificial Breeding Research Centre, ICAR-NDRI, Karnal, body weight of animals varied from 380 to 450 Kg and age from 3.0 to 5.5 Years. The bulls were kept atindividual pen and given exercise on weekly basis for one hour in bull exerciser. All experiment bulls had the similar scrotal circumference (SC; 39 to 36 cm) and physiological BSE was optimum and selected animals were given group exercise from six months age before reaching puberty. The six bulls divided into normal libido bulls (n=3) and poor libido bulls (n= 3). The bull handler report along with our own semen collection records were taken into consideration for grouping of bulls. The bulls were handled by the same bull handler and the same platform was used for semen collection.
Blood Sampling and Hormonal Assay
4 ml vacutainers (vacuette® serum gel z tube) used for blood collection using vacuette needle (21 G & 1.5 inches) from the jugular vein. The blood sampling were done 1h to 6 h after given GnRH injection at 0 h of 10μg Receptals® (Buserelin acetate) Intra Muscularly (I/M) in each group targeted testosterone and LH levels. Blood samples were centrifuged immediately after collection in a high-speed centrifuge machine (HERMLE Z383K) at 3000g for 20 minutes. Then, 500 μl of separated serum samples were kept in 2ml micro-centrifuge tube separately for each hormone and stored at -200 C till analysis of hormones by ELISA kits (My Bio Source, USA). The intra-assay coefficients of testosterone and LH were 4.53% and 1.40%. The average basal concentration calculated by taking average of 1h and 0 h blood sampling with GnRH administration. After GnRH administration 1 to 6 hr. blood samples represent as post-GnRH administration concentration (PGA) and the area under curve (AUC) was estimated 1 h before GnRH upto 6 h after GnRH application.
Statistical Analysis
JMP 12.2.0 software (SAS) and GraphPad PRISM® (version 7.00) were used for statistical analysis. The standard curves were plotted to estimate the concentration of testosteroneand LH by using GraphPad PRISM® (version 7.00) as well as Area under curve estimated for each group of bulls. The numerical hormonal data were expressed as means± SEM and all parameters were compared between normal libido bulls and poor libido bulls by using Student’s t-test. The significant level was considered as p<0.05.
ResultS
Average basal concentration, 1 to 6 h post-GnRH administration (PGA) concentration and Area under curve (AUC) of LH and testosterone hormones (Means±SE) were compared between the Normal libido bulls and poor libido bulls group shown in Table 1. The average basal concentration of testosterone, LH hormones in normal libido bulls had significantly lower (p<0.05) against poor libido bulls (2.63 ± 0.14 vs. 3.27 ± 0.24 and 11.30 ± 0.56 vs. 14.10 ± 1.06). The PGA testosterone and LH concentration did not significantly varied (4.40 ± 0.24 vs. 4.28 ± 0.16 and 15.10 ± 1.24 vs. 15.90 ± 0.75) in between normal libido bulls and poor libido bulls. Similarly, the AUC of testosterone and LH also did not significantly change (28.40 ± 2.41 vs. 28.50 ± 1.25 and 98.80 ± 9.59 vs. 110.00± 9.43) within the two groups.
Table 1: Average basal concentration, 1 to 5 h post GnRH administration (PGA) and Area under Curve (AUC) of luteinizing hormone and testosterone hormone (Mean±SE) in normal and poor libido bulls.
|
Hormones
|
Testosterone (ng/ml) | LH (ng/ml) | ||
| Normal libido bulls | Poor libido bulls | Normal libido bulls | Poor libido bulls | |
| Average basal Concentration |
2.63a ± 0.14 |
3.27b ± 0.24 |
11.30a ± 0.56 |
14.10b ± 1.06 |
| 1 to 6h PGA Concentration |
4.40a ± 0.24 |
4.28a ± 0.16 |
15.10a ± 1.24 |
15.90a ± 0.75 |
| AUC (ng/ml x h) |
28.40a ± 2.41 |
28.50a ± 1.25 |
98.80a ± 9.59 |
110.00a ± 9.43 |
Means bearing different superscripts between the groups within a row (a and b; p<0.05) differ significantly for individual hormone.
The individual’s variations of LH and testosterone hormones are presented in Figure 1 and Figure 3. But, average concentrations (mean±SE) of both hormones in each group with GnRH administration are shown in Figure 2 and Figure 4. Each bull showed the different pattern of release for all the hormones, the peak values and time to attain peak were also varied whereas average concentrations of each group showed different pattern than individuals one.
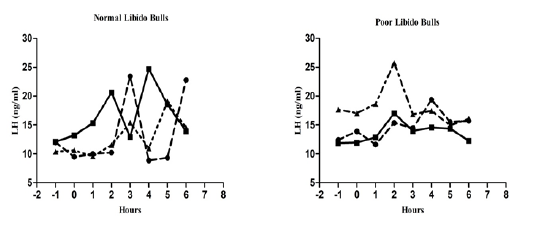
Figure 1: Individual bull luteinizing hormone concentration in both the groups before and after GnRH administration.
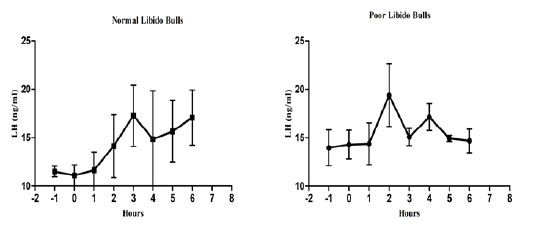
Figure 2: Average luteinizing hormone concentration in both the groups before and after GnRH administration.
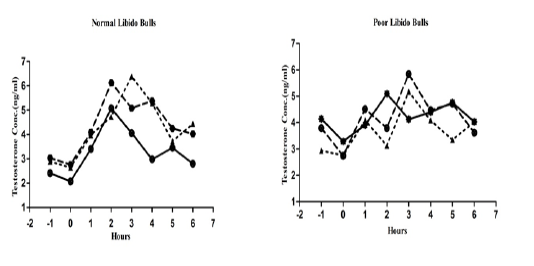
Figure 3: Individual bull testosterone concentration in both the groups before and after GnRH administration.
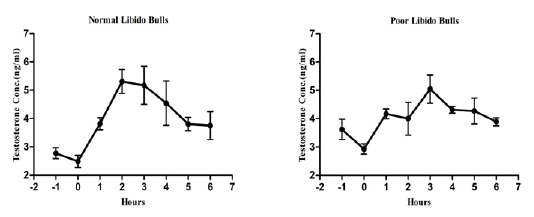
Figure 4: Average testosterone concentration in both the groups before and after GnRH administration.
Discussion
The basal concentration of testosterone and LH hormones were significantly (p<0.05) higher in poor libido bulls compared to normal libido bulls. Similarly, Boyd et al. (1988) reported that low testosterone concentration bulls showed higher libido. In normal bulls, the peripheral testosterone ranges from 2-20ng/ml (Katongole et al., 1971) and the threshold level of testosterone is required to maintain normal libido in bulls (Blockey and Galloway, 1978). In our study, both the group had more than 2 ng/ml testosterone concentration in peripheral blood level whereas poor libido bulls had more testosterone concentration. Likewise, low libido bulls had higher LH conc. during the first hour after GnRH treatment and also reported that after GnRH (10 ng/kg body weight, I/V) administration high and low libido group had similar testosterone level but initially high testosterone concentration in high libido group (Byerley et al., 1990). After GnRH treatment, the average level of testosterone and LH from 1 to 6 hand Area under Curve showed no significant variation between the two groups. It seems that the external application of a small dose of GnRH did not impact peripheral hormone levels. Due to the episodic nature of testosterone secretion (Katongole et al., 1971; Thibier, 1976a), the assessment of testosterone status requires frequent blood collections over a period of time (Post et al., 1987a). However, frequent blood sampling and restraint are reported to have depressant effects on the testosterone secretion (Sitarz et al., 1977; Post, 1978). Gonadotropin-releasing hormone (GnRH) treatment has been shown to overcome these depressant effects (Post, 1978), suggesting that GnRH response tests might provide a reliable appraisal of the testosterone status in bulls (Post et al., 1987b). The low libido bulls had a lack of sufficient testosterone concentrations but testosterone response to GnRH is not a viable indicator of libido (Byerley et al., 1990). So, the sexual willingness to dummy at AI centre not directly depends on peripheral hormone levels. Parlevliet et al. (2001) reported there was a psychological problem in equine for lack of libido and suggested for therapeutic rest, refraining from teasing; limit the mating frequency to restore the libido.
Conclusions
From the present study, it is concluded that peripheral hormonal rhythms of testosterone and LH did not resolve the libido problem of Zebu bulls although average basal testosterone and LH concentration were higher in poor libido bulls. Further investigation is needed with individual bull sexual behaviour and the psychogenic causes regarding libido problems.
Acknowledgments
I am thankful to the director of ICAR-NDRI, In charge of ABRC, all other researchers who helped me throughout this research project.
Conflict of interest
There is no conflict of interest.
Authors contribution
This comprehensive work was designed and materialised by all the authors.
References






