Journal of Animal Health and Production
Research Article
Stability of Commercial Xylanases stored singly or Combined with Vitamins and/or Minerals Premix at different Temperatures
Mohamed Ahmed El-Sherbiny1, Mamdouh Abbas Abdel-Moneim1, Ahmed Mohamed El-Shinnawy1, Gamal Ali Abdel-Hafez Hamady2, Ghadir Aly El-Chaghaby1*
1Regional Centre for Food and Feed, Agricultural Research Centre, Giza, Egypt; 2Animal Production Department, Faculty of Agriculture, Al-Azhar University, Nasr City, Cairo, Egypt.
Abstract | Xylanase is an important feed enzyme that enhances the digestibility of animal feedstocks. Xylanase degrades arabinoxylan, thus improving the nutritive value of feed ingredients. In the present work, a 360-d study was performed to evaluate the effects of different storage forms and temperature on storage stability of exogenous xylanase. Two xylanase products Fibrozyme®M and Hostazym X 250 Microgranulated; were stored as pure forms, with-a vitamin premix, a mineral premix or a vitamin and mineral premix at 4 °C (RF) and 35 °C (R), respectively. Sampling was performed at 45 days intervals. Stability was estimated as the residual of xylanase activity (% of initial) at each sampling point. All interaction and main effects of temperature, time and treatment had a significant (P< 0.05) effect on xylanase enzyme stability except for the product (P> 0.05). When xylanase of different products were stored in different forms at 4 °C, the means of retained activity of Fibrozyme were 90, 98.5, 73 and 63.5% when it was stored singly, mixed with vitamins, minerals or vitamins plus minerals; respectively. For Hostazyme the retained activities were 81.7, 103, 85 and 88%, respectively. When both enzymes were stored at 35 °C; the retained activities of the first were 47%, 94.5%, 82.5% and 57% and those of the second were 61.25%, 106%, 95.5% and 86.5%, respectively for storing singly, mixed with vitamins, minerals or vitamins plus minerals. Results indicated that xylanases mixed with vitamins retained significantly higher (P<0.05) activity compared with those stored in a pure form, mixed with minerals or combined minerals and vitamins. It could be suggested to introduce both xylanases, examined herein, and vitamins premix in one product. More studies are needed to evaluate the effect of different enzyme sources, storage conditions and composition of the enzyme preparation on the stability of the product.
Keywords | Enzyme, Xylanase, Stability, Storage, Temperature
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | September 09, 2016; Accepted | October 11, 2016; Published | October 19, 2016
*Correspondence | Ghadir Aly El-Chaghaby, Regional Centre for Food and Feed, Agricultural Research Centre, Giza, Egypt; Email: [email protected]
Citation | El-Sherbiny MA, Abdel-Moneim MA, El-Shinnawy AM, Hamady GAA, El-Chaghaby GA (2016). Stability of commercial xylanases stored singly or combined with vitamins and/or minerals premix at different temperatures. J. Anim. Health Prod. 4(4): 111-117
DOI | http://dx.doi.org/10.14737/journal.jahp/2016/4.4.111.117
ISSN | 2308–2801
Copyright © 2016 El Sherbiny et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Enzymes are catalytic proteins with their active sites housed inside a 3-dimension structure, making them sensitive to some physico-chemical conditions (Pérez-Portabella, et al., 2001) such as pH, hydrothermal conditioning and frictional forces (Steen, 2001). The industrial enzyme business is steadily growing with an annual average increase of 4% in production of feed enzymes which represents around 17% of the global enzyme production (Iyer and Ananthanarayan, 2008). This is due to the improvement in production technologies, engineered enzyme properties and new application fields.
In many animal production systems, the limiting factor at formulating rations is the animal’s ability to digest different constituents of the feed raw materials, particularly fiber; monogastrics do not produce enzymes that digest fiber (Sheppy, 2001). A large proportion of this fiber is soluble beside insoluble arabinoxylan and β-glucan (Bedford and Classen, 1991). The soluble fiber (hemi-cellulose) can increase the viscosity of the contents of small intestine, impeding the digestion of nutrients and thereby reducing the growth of the animal. For breaking down the soluble fiber, the enzyme xylanase degrades arabinoxylan, thus improving the nutritive value of feed ingredients (Selvendran et al., 1987).
In feed production, enzymes are added to feed in a single (pure) form, yet there are many limitations using an individual component as feed additive. An individual component is used only in very small amount, it might be light or dense, tend to clump, and requires multiple feeders (Feed premix market: Global industry analysis and opportunity assessment 2015-2025).
There are several enzyme products in the market; however, few scientific studies have been carried out to examine the potency of exogenous feed enzymes added as enzyme preparation, vitamin premix, mineral premix or vitamin and mineral premix (Iyer and Ananthanarayan, 2008; Sulabo et al., 2011). It will be more beneficial to include enzymes in a premix with vitamins, minerals or both as it makes them easier to test and may decrease the price of the diet formulation.
Therefore, it is important to monitor the stability of exogenous feed enzyme activities, when such enzymes are included in commercial feed products (premixes or preparations). The aim of this study is to evaluate the commercial xylanase stability, following to single or combined storage with vitamin premix, mineral premix or vitamin plus mineral premix at two different storage temperatures.
Materials and Methods
This study was conducted at the Enzyme lab, Regional Center for Food and Feed, Agricultural Research Center, Giza, Egypt.
Characterization of Xylanases and Premixes
In this experiment, two commercially available xylanases with different activities obtained from the dried Trichoderma longibrachiatum fermentation extract, were used. Three types of premixes were also used; one vitamin premix (Bio-Vitasol produced by Bio pharmachemie, Vietnam, declared potency of vitamin A is 6 MU/kg and vitamin E is 4 g/kg), one mineral premix (Qilimix III produced by Tank, China), and one mineral plus vitamin premix (Vitamino Light, Multivitamins fortified with minerals produced by Anfotal Nutrition, China, declared potency of vitamin A is 12 MU/kg and vitamin E is 7 g/kg) (Table 1). The xylanase enzymes products used herein include: Fibrozyme®M (declared potency of 100XU/g) (P1); Hostazym X 250 Microgranulated (declared potency of 6000 EPU/g) (P2).
Table 1: Composition of the mineral, vitamin and mineral plus vitamin premixes used in the study
|
Items |
Mineral (M)a |
Vitamin (V) |
Mineral and vitamin premix (V+Ma) |
|
Vitamin A, IU |
- |
6000000 |
12000000 |
|
Vitamin D3, IU |
- |
1000000 |
2500000 |
|
Vitamin B1, mg |
- |
1330 |
2000 |
|
Vitamin B2, mg |
- |
2000 |
6000 |
|
Vitamin B6, mg |
- |
1000 |
3000 |
|
Vitamin B12, µg |
- |
3330 |
15000 |
|
Vitamin B3, mg |
- |
9000 |
30000 |
|
Vitamin B5, mg |
- |
4500 |
12000 |
|
Vitamin C, mg |
- |
3000 |
-------- |
|
Vitamin E, mg |
- |
4000 |
7000 |
|
Biotin, µg |
- |
30000 |
150000 |
|
Folic acid, mg |
- |
400 |
1500 |
|
Menadione, mg |
- |
1000 |
3000 |
|
Choline chloride, mg |
- |
-------- |
|
|
Ferrous sulphate, mg |
46670 |
- |
30000 |
|
Copper sulphate, mg |
6670 |
- |
5000 |
|
Manganese sulphate, mg |
66000 |
- |
80000 |
|
Zinc sulphate, mg |
46600 |
- |
60000 |
|
Potassium iodate*, mg |
660 |
- |
500 |
|
Cobalt chloride, mg |
130 |
- |
133 |
|
Sodium Selenate , mg |
200 |
- |
100 |
|
Chromium, mg |
0.130 |
- |
--------- |
aMineral (M): The stated values are for the elements expressed in mg/kg product; *: Value of Iodine expressed in mg/kg product
Raw Xylanase Products and Premixes
On day zero, one kg of each of the two xylanase products were divided into 8 open, plastic bags (2 as controls and 6 treatments). The control bags from each enzyme products were stored in a refrigerator (RF) at 4 °C and referred to as: P1RF and P2RF, or at controlled room temperature (R) at 35 °C and termed: P1R and P2R.
Two (100g) samples of each type of the three premixes were taken and put into two plastic bags; one was refrigerated at 4 °C and the other was stored in a controlled room temperature at 35 °C. At sampling, the bag was mixed. Samples from enzyme-containing bags were analysed for xylanase activity at zero day and at 45 days intervals up till throughout the course of the study (360 days), while vitamin mixture and vitamins plus mineral premix were analyzed for vitamin A (National Food Agency of Denmark, 1996) and vitamin E (Leth and Sondergaard, 1983) (available analysis) at zero day, 180 day and 360 day. Xylanase activity was assayed according to McCleary and Monaghan (1999).
Premixes
Samples from each xylanase product (3 treatments with each enzyme product) were added and mixed with vitamin premix (V), mineral premix (M) or vitamins plus minerals premix (VM). The added amount of xylanase enzyme from each product were estimated according to the recommendation of the registration document in Egypt, Fibrozyme®M (1000 g/ton), Hostazym X 250 Microgranulated 50 g/ton feed, vitamins premix (50 g/100L of drinking water), minerals premix (2000 g/ton) and minerals and vitamins premix (1000 g/ton). Each treatment was homogenized using a blender. Three bags from each enzyme product were stored in refrigerator (RF) at 4 °C and designed as: P1+VRF, P1+MRF, P1+VMRF, P2+VRF, P2+MRF, and P2+VMRF; while the other three bags for each enzyme product were stored at a controlled room temperature (R) of 35 °C and referred to as: P1+VR, P1+MR, P1+VMR, P2+VR, P2+MR, and P2+VMR. Samples from bags containing xylanase plus premixes were analyzed for xylanase activity (replicate analysis) at zero day and each 45 days throughout the course of the study (360 days). Potency of vitamin A and E in all treatments was also measured at zero day, day 180 and day 360.
Table 2: Probabilities of main effects and interaction of products, treatments temperature and time on enzyme stability (as defined by percentage of initial xylanase activity)
|
Item |
P- values |
|
Product |
0.31 |
|
Treatments |
0.001 |
|
Temperature |
0.001 |
|
Time |
0.001 |
|
Product x Treatment |
0.001 |
|
Product x Temperature |
0.001 |
|
Product x Time |
0.001 |
|
Treatment x Temperature |
0.001 |
|
Treatment x Time |
0.001 |
|
Temperature x Time |
0.001 |
|
Product x Treatment x Temperature |
0.001 |
|
Product x Treatment x Time |
0.001 |
|
Treatment x Temperature x Time |
0.001 |
|
Product x Treatment x Temperature x Time |
0.001 |
Statistical Analysis
Data were analyzed using a mixed model (MIXED procedure; SAS Institute (1991) to determine the interaction and main effects of product, storage form, storage temperature, and storage time on the activity of two commercially available xylanase sources. At the end of the storage period, means of xylanase activity were compared using Duncan’s new multiple range test (Duncan, 1955) at P≤ 0.05.
Results
All interaction and main effects of temperature, time and treatments were significant (P< 0.05; Table 2) on xylanase enzyme stability except for the product (P> 0.05).
Irrespective of treatments, activity of the two enzyme products stored singly (in the pure form) followed a similar trend (Figure 1 and 2), in which the retained activities had increased till day 135, then started to decrease significantly (P<0.05) till they reached the least activities at the end of the storage period (360 days). Yet, the retained xylanase activities were better in the pure form when stored at 4 °C (P1RF 90% and P2RF 81.7%) than when stored at 35 °C (P1R 47% and P2R 61.25%).

Figure 1: Xylanase residual activity for Fibrozyme®M as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]

Figure 2: Xylanase residual activity for Hostazym X 250 as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]
For both xylanase products (Figure 3, 4, 5 and 6), when xylanase enzymes were mixed with vitamin premix, and /or vitamins and minerals a significant difference(P <0.001) was observed in the retained enzyme activities between those stored at 4 °C, being 98.5% for P1+VRF, 63.5% for P1+VMRF, 103% for P2+VRF and 88% for P2+VMRF and the corresponding values when stored at 35 ⁰C were 94.5% for P1+VR, 57% for P1+VMR, 106% for P2+VR and 86.5% for P2+VMR.
On the other hand, xylanase enzymes mixed with mineral premix (Figure 7 and 8) had better retained activities, when stored at 35 °C being 82.5% for P1+MR, 95.5% for P2+MR compared to those stored at 4 °C being 73% for P1+MRF and 85% for P2+MRF.

Figure 3: Xylanase residual activity for Fibrozyme®M mixed with vitamin premix as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]

Figure 4: Xylanase residual activity for Fibrozyme®M mixed with vitamins and minerals premix as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]

Figure 5: Xylanase residual activity for Hostazym X 250 Microgranulated mixed with vitamins premix as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]

Figure 6: Xylanase residual activity for Hostazym X 250 Microgranulated mixed with vitamins and minerals premix as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]
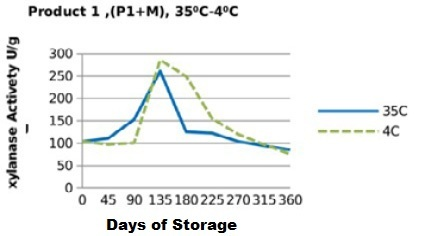
Figure 7: Xylanase residual activity for Fibrozyme®M mixed with minerals premix as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]
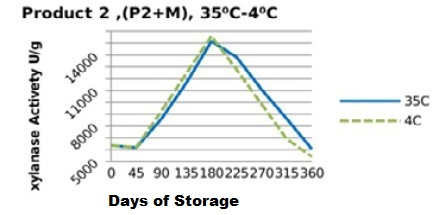
Figure 8: Xylanase residual activity for Hostazym X 250 Microgranulated mixed with minerals premix as affected by storage temperature [refrigerator (4 °C) and controlled room temperature (35 °C)]
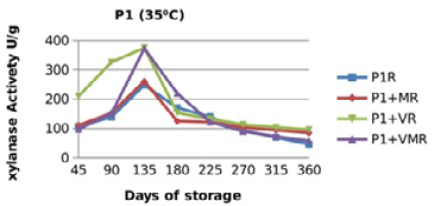
Figure 9: Xylanase residual activity for Fibrozyme®M whether in a pure form or mixed with vitamins, minerals or minerals and vitamins as affected by time at 35 °C
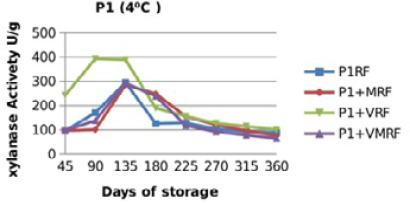
Figure 10: Xylanase residual activity for Fibrozyme®M whether in a pure form or mixed with vitamins, minerals or minerals and vitamins as affected by time at 4 °C
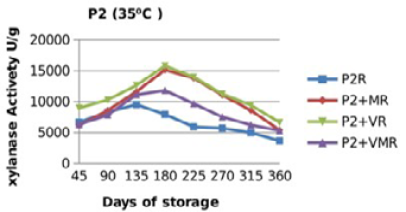
Figure 11: Xylanase residual activity for Hostazym X 250 Microgranulated either in a pure form or mixed with vitamins, minerals or minerals and vitamins as affected by time at 35 °C
There were significant (p≤0.05) differences in the retained activity of Xylanase stored combined with vitamin premix which retained higher activity compared with those stored singly in pure form, stored with mineral premix or combined with minerals and vitamin premix (Figure 9, 10, 11 and 12) and Table 3. It was observed that xylanase enzymes stored in pure form at 35 °C exhibited the minimum retained enzyme activity being, 47% for P1R and 61.25% for P2R.
The lowest retained values of vitamin A and E were observed in vitamins and mineral premix mixed with enzymes and stored at 35 °C for 360 days, while the highest retained values for vitamin A and E were observed in vitamins mixed with enzymes or stored in a pure form at 4 °C for 360 days (Table 4).
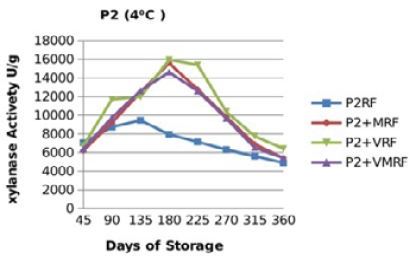
Figure 12: Xylanase residual activity for Hostazym X 250 Microgranulated either in a pure form or mixed with vitamins, minerals or minerals and vitamins as affected by time at 4 °C
Table 3: Effect of vitamins, minerals or minerals and vitamins premix on commercial xylanase activity stored at 4 °C or 35 °C for 360 days
|
Items |
Product 1* |
Product 2** |
||
|
4 °C |
35 °C |
4 °C |
35 °C |
|
|
Enzyme Pure (P) % |
90.00b |
47.00d |
4903b |
3675c |
|
Enzyme + Vitamin premix (P+V) % |
101.0a |
96.50a |
6426a |
6048ab |
|
Enzyme+ Minerals premix (P+M) % |
76.00c |
85.50b |
5411b |
6641a |
|
Enzymes + (Vitamins and Minerals premix (P+ (VM)) % |
65.00d |
58.50c |
5410b |
5343b |
a, b, c, d: means within columns with different letters are significantly different p<0.05; *Product 1: Fibrozyme M; **Product 2: Hostazym X 250
Table 4: Effect of different forms of mixtures on the retained potency values of vitamin A and vitamin E stored at 4 °C and 35 °C for 360 days
|
Items |
Days |
Vitamins (V) |
Vitamins and minerals (V+M) |
||||||
|
4 °C |
35 °C |
4 °C |
35 °C |
||||||
|
A (MU/kg) |
E (g/kg) |
A (MU/kg) |
E (g/kg) |
A (MU/kg) |
E (g/kg) |
A (MU/kg) |
E (g/kg) |
||
|
Pure (P) |
Zero |
6.59 |
3.69 |
6.58 |
3.65 |
16.25 |
7.61 |
16.20 |
7.40 |
|
180 |
6.29 |
3.23 |
5.82 |
3.23 |
15.45 |
7.50 |
15.31 |
7.35 |
|
|
360 |
6.26 |
3.21 |
5.65 |
3.20 |
14.30 |
6.50 |
14.37 |
6.40 |
|
|
Product 1 |
Zero |
6.50 |
3.60 |
6.49 |
3.60 |
16.20 |
7.55 |
16.15 |
7.30 |
|
180 |
6.20 |
3.18 |
5.74 |
3.18 |
15.40 |
7.44 |
15.26 |
7.25 |
|
|
360 |
6.18 |
3.07 |
5.58 |
2.95 |
13.85 |
6.44 |
13.52 |
6.00 |
|
|
Product 2 |
Zero |
6.55 |
3.62 |
6.51 |
3.65 |
16.22 |
7.58 |
16.18 |
7.35 |
|
180 |
6.23 |
3.20 |
5.78 |
3.20 |
15.42 |
7.46 |
15.28 |
7.27 |
|
|
360 |
6.10 |
3.10 |
5.62 |
3.08 |
14.08 |
6.45 |
13.44 |
5.78 |
|
Discussion
Xylanase is added to animal feed in order to improve the nutritional properties of agricultural silage and grain feed. According to Motta et al. (2013), the incorporation of xylanase into rye-based diet of broiler chickens resulted in a reduction of their intestinal viscosity which in turn led to an improvement in both the weight gain of the chicks and their feed conversion efficiency. As a catalytic protein, xylanase is sensitive to denaturation reactions. Denaturation is the unfolding of the enzyme tertiary structure to a disordered polypeptide, which may lead to an irreversible loss of activity or inactivation (Iyer and Ananthanarayan, 2008).
It is a common practice that enzymes’ manufacturers provide averages in enzymes activity to account for the potential losses during storage and feed processing. There are limited data regarding the storage stability of commercial enzymes products except for those reported during product registrations (European Food Safety Authority, 2013).
The results of the present study demonstrated that, there were no significant differences (P> 0.05) in enzyme stability between the two types of xylanase products examined here. This might be due to that the xylanase in both products had resulted from the dried extract of the same source of fungi (Trichoderma longibrachiatum).In harmony with the present results, Sulabo et al. (2011) reported that when phytase is stored at room temperature (23 °C) or lower, the pure product retained most (approximately 85%) of its activity for up to 60 days of storage regardless of phytase source.
The present study results indicated that regardless of xylanase source pure Fibrozyme and Hostazym enzymes stored at 35 °C for 360 days retained 47% and 61.25% from their initial activities, respectively, while when stored at 4 °C retained 90 % and 81.7%, respectively. These retention rates nearly agree with those reported by the European Food Safety Authority (2013) for Hostazym products. In this report the product had retained 75 to 79% of its initial activity when stored for six months at 40 °C. There were no available data about Fibrozyme investigated in this study.
It was reported for other enzymes that when phytase was stored for 150 days at 5 °C the retained activities were between 80% and near 100% from the initial activities (Sulabo et al., 2011). However, the retained phytase activities were from 40% to 60 % when stored at 37 °C for the same period, they suggested that regardless of phytase source, the environmental conditions were sufficient to denature the enzyme and reduce its activity.
When each product of xylanase enzymes, investigated herein, were mixed with vitamins, and /or vitamins and minerals and stored at 4 °C for 360 days, the retained activities were 98% for P1+VRF, 63% for P1+VMRF, 103% for P2+VRF and 87% for P2+VMRF. While, when the same ingredients were stored at 35⁰Cthe retained activities were 94% for P1+VR, 56% for P1+VMR, 105% for P2+VR and 86% for P2+VMR.
The present results agree with the reported stability of Hostazym X 30000 which was evaluated previously when mixed with vitamin and mineral premixes and stored at 25 °C and 35 °C for up to six months (European Food Safety Authority, 2013); means of measured activities were 93% and 80%, respectively. Yet, the retained activity in our study was better at 35 °C (86%) compared with that reported by the manufacturers in product registration (80%).
It was suggested by Sulabo et al.(2011) that storing phytase enzyme mixed with vitamins and mineral at 25 °C or lower, may have advantages in retaining the original enzyme activity than when storing it at 37 °C.
Irrespective of enzyme source and temperature, xylanase products mixed with vitamins retained significantly (P<0.001) retained better activities than those mixed with mineral premix, mineral and vitamin premix and in pure forms. There are two hypotheses why this case occurred. The first hypothesis is that some vitamins had a fatty nature which could have acted as a coat and protected the enzymes from losing their activities. The second hypothesis is that most vitamins especially the B complex group function as precursors for enzyme cofactors that help enzymes in their work as catalysts. In this case, vitamins may be tightly bound to enzymes as a part of the prosthetic groups. Vitamins may also be less tightly bound to the enzyme, as they act as detachable molecules that function to carry chemical groups or electrons between molecules. These roles assist in enzyme –substrate reaction (Bolander, 2006).
According to Klibanov (1983), minerals had ion effects and with the presences of protein charged groups this leads to a salting out and compression of enzyme. Our findings agree with the results of Sulabo et al. (2011) who found that a loss of phytase activity was greater when phytase was mixed with vitamins and minerals premixes than when it was mixed with vitamin premixes. The objective of this study was not to identify specific vitamin or trace minerals that may have contributed to greater loss or improve in xylanase activity.
Conclusion
It could be suggested to introduce both types of tested commercial xylanase enzymes and vitamin premix in one product to reduce the cost of feed formulation for commercial animal production. Yet, more studies are needed to examine the effect of different enzyme sources, storage conditions and composition of enzyme preparation on the stability of the product.
conflict of interest
We declare no conflict of interrest.
Authors’ contribution
All authors have contributed to the work presented in this paper.
References






