Journal of Animal Health and Production
Research Article
Quantitative Analysis of Fluoroquinolones Residues in Broiler Meat using High Performance Liquid Chromatography Occupied by Mass Detector (LC-MS/MS)
Aqeel Mahdi Muhammad Al-Ebrahim1, Waleed Rizk El-Ghareeb2*
1Ministry of Environment, Water and Agriculture in Riyadh, Saudi Arabia; 2Department of Public Health, College of Veterinary Medicine, King Faisal University, P.O. Box: 400, Al-Ahsa, 31982, Saudi Arabia.
Abstract | A total of 120 broiler meat samples (60 breast samples and 60 thigh samples) were examined using a high performance liquid chromatography occupied by mass detector (LC-MS/MS) for determination of fluoroquinolones residues (Sarafloxacin, Danofloxacin, Ciprofloxacin, Enrofloxacin, Ofloxacin, and Marbofloxacin) at Al-Ahsa province. The antibiotics were validated according to the guidelines laid down by the joint FAO/WHO Expert Committee on Food Additives. Incidence of fluoroquinolones residues in examined breast meat samples were 44 (73.33%) for Sarafloxacin, 45 (75%) for Danofloxacin, 49 (81.67%) for Ciprofloxacin, 48 (80%) for Enrofloxacin, 50 (83.33%) for Ofloxacin, 45 (75%) for Marbofloxacin while in thigh muscles were 49 (81.67%), 45 (75%), 49 (81.67%), 48 (80%), 55 (91.67%) and 45 (45%) for Sarafloxacin, Danofloxacin, Ciprofloxacin, Enrofloxacin, Ofloxacin and Marbofloxacin, respectively. Mean±SE of Sarafloxacin, Danofloxacin, Ciprofloxacin, Enrofloxacin, Ofloxacin, and Marbofloxacin were 7.10±0.57, 8.05±0.50 and 24.24±1.82, 23.99±1.80 and 21.88±1.35, 22.98±1.45 and 32.36±4.52, 23.06±2.14 and 13.30±1.72, 14.49±1.87 and 8.21±1.06, 9.93±1.28 μg/kg in breast and thigh, respectively. All examined samples were within permissible limit except for enrofloxacin (one breast sample) and Sarafloxacin (20 and 14 breast and thigh samples, respectively) were higher than permissible limits. The study suggested the judicial use of antibiotics in food producing animals to safeguard human health.
Keywords | Fluoroquinolones, Residues, Broiler, Meat, LC-MS/MS
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | November 02, 2020; Accepted | November 23, 2020; Published | December 27, 2020
*Correspondence | Waleed Rizk El-Ghareeb, Department of Veterinary Public Health, College of Veterinary Medicine, King Faisal University, P.O. Box: 400, Al-Ahsa, 31982, Saudi Arabia; Email: [email protected].
Citation | Al-Ebrahim AMM, El-Ghareeb WR (2020). Quantitative analysis of fluoroquinolones residues in broiler meat using high performance liquid chromatography occupied by mass detector (LC-MS/MS). J. Anim. Health Prod. 9(s1): 56-60.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.56.60
Copyright © 2020 Al-Ebrahim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Poultry meat is considered an important source of protein for human. It is easily digested and have palatable taste and is economically cheaper than red meat. It is of low calories, its fat contains essential fatty acids and its protein is good source of essential amino acids. A lot of food producing animals and birds receive antibiotic for part or most their lives. This lead to accumulation of what is known as antibiotic residues in poultry meat and their offal (Jayalakshmi et al., 2017).
Antimicrobials are substances produced by living organisms that are able to kill or inhibit the growth of other microorganisms but the definition of antibiotic has also been used to include chemically derived synthetic antibacterial drugs (Harrison and Svec, 1998). Antimicrobials are widely used in veterinary practices as prophylactic, treatment several diseases and as growth promoters for increasing growth weight. Antibiotics are still used in large scale in veterinary field and poultry farms with insufficient protocol for control.
The presence of antibiotics or their metabolites in food is potentially hazardous to health as it may cause allergic reaction in people, imbalance of intestinal microflora and antibiotic resistance in pathogenic microorganisms.
LC-MS/MS is widely used to quantify various antibiotic residues in food products with good sensitivity and specificity. Tolerance limits and maximum residual limits (MRL) have been established around the world and agencies should monitor the food supply to ensure that antibiotic residue concentrations do not exceed the MRL levels. Little information about the residual levels of antimicrobials in poultry meat in Saudi Arabia. El-Ghareeb (2019) screened the residue levels of nine antimicrobials with the most common use in Saudi Arabia veterinary medical field. These antimicrobials were enrofloxacin, ciprofloxacin, tylosin, erythromycin, tetracycline, oxytetracycline, chlortetracycline, sulfamethazine and sulfaquinoxaline. The tested samples included muscles, livers and kidneys of camel, cattle and sheep slaughtered at Al-Ahsa, Saudi Arabia. Antimicrobial residues in the tested samples was quantitatively estimated using liquid chromatography tandem mass spectrometry (LC–MS/MS).
In the recent years, residues of veterinary drugs in food have received much attention due to increasing concerns of food safety by consumers (Jafari et al., 2017). Therefore, the present study was undertaken to investigate the following topics: Quantitative detection of selected fluoroquinolones residues (Sarafloxacin, Danofloxacin, Ciprofloxacin, Enrofloxacin, Ofloxacin, and Marbofloxacin) in random marketed poultry meat samples at Al-Ahsa using LC-MS/MS technique. A comparison was made among the detected antibiotic residues concentration in muscle and their international maximum residues limits (MRLs).
MATERIALS AND METHODS
All experiments were carried out in compliance with guidelines from King Faisal University, Saudi Arabia. The used chemicals were of HPLC standard, or the highest available quality.
Collection of samples
One hundred and twenty chicken meat samples (breast and thigh; 60 for each) were collected randomly during May 2019 until December 2019 from markets in Al-Ahsa province, Saudi Arabia. Each sample was transferred in separate sterile and labeled plastic bags to the laboratory in icebox until analysis for antibiotic residues.
Sample preparation
Freshly collected samples must be kept cold before and during shipping to laboratory. Once received at laboratory, samples must be frozen (˂-10ºC) prior to mincing/grinding if they cannot be prepared on the day of receipt. If sample is frozen, allow to thaw, but keep as cold as possible. Dissect away fat and connective tissue from samples. Mince finely or grind tissue in blender or vertical cutter –mixer. Store and frozen at (-10ºC) prior to analysis. Poultry meat samples were extracted and cleaned up according to Agilent Modified QUECHERS method kit.
Quantitative detection of fluoroquinolones residues by LC-MS/MS
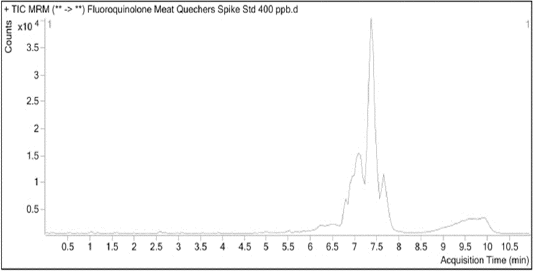
Fluoroquinolones residues detection and quantification were performed by using high performance liquid chromatography tandem mass spectrometry (An Agilent 6430 LC-MS/MS triple quadrupole system) according to Zheng et al. (2019) (Figure 1). The Multiple Reaction Monitoring (MRM) modes were used for quantification of antibiotics and their settings were shown in Table 1.
Table 1: MRM (Multiple Reaction Monitoring) Settings.
| Name | Fragment | Precursorion | Production | CE | Polarity | Rt.(min) |
| Ciprofloxacin | 150 | 332.1 | 314.1 | 20 | positive | 7.360 |
| Ciprofloxacin | 150 | 332.1 | 231.1 | 40 | positive | 7.360 |
| Danofloxacin | 151 | 358.2 | 340.1 | 20 | positive | 7.448 |
| Danofloxacin | 151 | 358.2 | 82.1 | 48 | positive | 7.448 |
| Enrofloxacin | 156 | 360.2 | 342.2 | 20 | positive | 7.394 |
| Enrofloxacin | 156 | 360.2 | 316.2 | 16 | positive | 7.394 |
| Ofloxacin | 150 | 362.2 | 318.2 | 20 | positive | 7.150 |
| Ofloxacin | 150 | 362.2 | 261.1 | 40 | positive | 7.150 |
| Marbofloxacin | 144 | 363.2 | 345.1 | 16 | positive | 6.949 |
| Marbofloxacin | 144 | 363.2 | 320.1 | 12 | positive | 6.949 |
| Marbofloxacin | 144 | 363.2 | 72.2 | 28 | positive | 6.949 |
| Sarafloxacin | 150 | 386.1 | 368.1 | 20 | positive | 7.652 |
| Sarafloxacin | 150 | 386.1 | 342.1 | 20 | positive | 7.652 |
Statistical analyses
Statistical analysis of the all the obtained data was performed as descriptive statistics by Excel program.
RESULTS AND DISCUSSION
Saudi Arabia ranked sixth in the world in consumption of poultry meat with an average of 39.7 kg / person per year during 2017 (OECD, 2018).
The extensive use of antimicrobials in livestock production might lead to several public health implications when contaminated meat is consumed. This study screened the residue levels of sex of the most commonly used fluoroquinolones in poultry production in Saudi Arabia.
A total of 120 broiler meat samples were analyzed for fluoroquinolones residues. The results revealed that the incidence of fluoroquinolones residues could be detected in local poultry meat samples at Al-Ahsa. Incidence of fluoroquinolones residues in examined breast meat samples were 44 (73.33 %) for Sarafloxacin, 45 (75 %) for Danofloxacin, 49 (81.67 %) for Ciprofloxacin, 48 (80 %) for Enrofloxacin, 50 (83.33 %) for Ofloxacin, 45 (75%) for Marbofloxacin while in thigh muscles were 49 (81.67%), 45 (75 %), 49 (81.67 %), 48 (80 %), 55 (91.67 %) and 45 (45 %) for Sarafloxacin, Danofloxacin, Ciprofloxacin, Enrofloxacin, Ofloxacin and Marbofloxacin, respectively (Table 2).
Table 2: Incidence of fluoroquinolones residues in examined poultry meat samples using LC MS/MS (N=120; 60 for each breast and thigh).
| Antibiotic | Positive | Range within positive samples(ppb) | ||
| Breast | Thigh | Breast | Thigh | |
| Sarafloxacin | 44 (73.33%) | 49 (81.67%) | 7.99 to 11.11 | 7.90 to 14.64 |
| Danofloxacin | 45 (75%) | 45 (75%) | 31.22 to 34.99 | 31.30 to 33.05 |
| Ciprofloxacin | 49 (81.67%) | 49 (81.67%) | 25.54 to 28.74 | 25.52 to 48.99 |
| Enrofloxacin | 48 (80%) | 48 (80%) | 12.52 to 238.09 | 12.52 to 74.36 |
| Ofloxacin | 50 (83.33%) | 55 (91.67%) | 15.32 to 17.23 | 15.34 to 17.21 |
| Marbofloxacin | 45 (75%) | 45 (45%) | 9.28 to 16.38 | 9.34 to 13.85 |
Chicken muscle, liver and egg samples were collected from 33 broilers and 5 layer farms in the eastern province of Saudi Arabia. Antibiotic residue-positive samples were identified in the product of 23 (69.7%) broiler and 3 (60%) layer poultry farm (Al-Ghamdi et al., 2000).
Mean±SE of Sarafloxacin, Danofloxacin, Ciprofloxacin, Enrofloxacin, Ofloxacin, and Marbofloxacin were 7.10±0.57, 8.05±0.50 and 24.24±1.82, 23.99±1.80 and 21.88±1.35, 22.98±1.45 and 32.36±4.52, 23.06±2.14 and 13.30±1.72, 14.49±1.87 and 8.21±1.06, 9.93±1.28 μg/kg in breast and thigh, respectively (Table 3). All samples contained Sarafloxacin residues were within the maximum residue limits (MRLs).
Percentages of positive broiler muscle samples were nearly equal in thigh and breast muscles. In some studies, presence of higher detectable antibiotics residues in the thigh as it considered as an injectable site, this was coincides with Biswas et al. (2007) who mentioned that out of the different routes of antibiotic administrations, the injectable were responsible for 46% of the violative residues in meat followed by oral.
All examined samples were within permissible limit except for enrofloxacin (one breast sample) and Sarafloxacin (20 and 14 breast and thigh samples, respectively) were higher than permissible limits.
Antibiotics residues have very dangerous health hazards. These hazards can be one off the following (transfer of antibiotic resistance bacteria to the human, immunopathological effects, autoimmunity, carcinogenicity, mutagenicity, nephropathy, hepatotoxicity, reproductive disorders, bone marrow toxicity or allergy (Nisha, 2008).
The result in Table 3 showed the incidence of the fluoroquinolones antibiotic residues in the highest positive broiler breast muscle samples analyzed by using LC-Mass/Mass which were 50 (83.33 %), 49 (81.67 %), 48 (80 %), 45 (75 %), 45 (75 %) and 44 (73.33 %) samples were positive for the presence of Ofloxacin, Ciprofloxacin, Enrofloxacin, Danofloxacin, Marbofloxacin and Sarafloxacin, respectively.
Table 3: Statistical analysis of fluoroquinolones residues (μg/kg) in examined poultry meat samples using LC- MS/MS (N=120; 60 for each breast and thigh).
| Statistical | Sarafloxacin | Danofloxacin | Ciprofloxacin | Enrofloxacin | Ofloxacin | Marbofloxacin | ||||||
| Breast | Thigh | Breast | Thigh | Breast | Thigh | Breast | Thigh | Breast | Thigh | Breast | Thigh | |
| Min | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Max | 11.11 | 14.64 | 34.99 | 33.05 | 28.74 | 48.99 | 238.09 | 74.36 | 17.23 | 17.21 | 16.38 | 13.85 |
| Mean±SE |
7.10± 0.57 |
8.05± 0.50 |
24.24± 1.82 |
23.99± 1.80 |
21.88± 1.35 |
22.98± 1.45 |
32.36± 4.52 |
23.06± 2.14 |
13.30± 1.72 |
14.49± 1.87 |
8.21± 1.06 |
9.93± 1.28 |
| Median | 9.13 | 9.21 | 31.86 | 31.86 | 26.56 | 26.51 | 33.31 | 24.56 | 15.87 | 15.67 | 9.99 | 10.80 |
| SD | 4.39 | 3.90 | 14.14 | 13.97 | 10.48 | 11.25 | 35.06 | 16.62 | 6.01 | 4.42 | 4.97 | 3.16 |
SD: Standard Deviation; SE: Standard Error; Min: Minimum; Max: Maximum.
Breast and thigh muscle samples appeared to have the same fluoroquinolone concentration (Table 3). This result not matched with Schnider and Donoghue (2004) who stated that breast muscles have high residue levels.
From Table 4, the Maximum residue limit for ciprofloxacin was 100 ppb for muscle and liver as stated by EAEM (1998). This indicate that the ciprofloxacin and doxycycline residues in the examined broiler muscle samples not exceed the maximum residual limits.
Table 4: Maximum Residue Limits (MRLs /poultry muscle) and Acceptable Daily Intake (ADI) for analyzed antibiotics (GCC, 2015).
| Antibiotic | MRL (µg/kg) | ADI (µg/kg BW) | Reference |
| Danofloxacin | 200 | 0-20 | Codex Alimentarius (2018) |
| Enrofloxacin | 100 | 2 | Commission Regulation (EU) No. 37/2010 |
| Marbofloxacin | 150 | 4.5 | Commission Regulation (EU) No. 37/2010 |
| Sarafloxacin | 10 | 0 – 0.3 | Commission Regulation (EU) No. 37/2010 |
* banned by US FDA; Canada and EC; BW: Body Weight.
This results were lower than Schreider and Donoghue (2004) who tested meat and liver samples for eight fluoroquinolones and found ciprofloxacin residues ranged from 10000-20000 ppb , on the other hand the results were higher than Nermeen (2013) who illustrated that out of 30 chicken muscles samples 11 were positive for the presence of ciprofloxacin residues with a mean value of 31.9 ppb, Shams et al. (2002) who detected ciprofloxacin residues in liver, kidney, breast and thigh muscles with mean values of 48 ±0.035, 56± 0.042, 14 ±0.009 and 23 ±0.021 ppb.
The main source of antibiotic residues is the improper use of antibiotic as (treatment, prevention and to enhance growth), extra label use and failure to observe withdrawal time for each antibiotic. The variation in antibiotic residues might be attributed to the difference in species and ages of poultry and dose of administration (Daoud, 1995).
Many factors are implemented in occurrence of antibiotic residues in poultry meat as: haphazard uses of antibiotic therapy in poultry farm, carless against withdrawal periods of drugs. Salehzadeh et al. (2007) collected 90 chicken samples represent 170 samples of muscle, liver and kidney. HPLC was used for separating, detecting and analyzing of enrofloxacin residues in samples. 8.8%, 13.3% and 24.4% of muscle, liver and kidney samples, respectively showed enrofloxacin residue above MRLs, respectively.
Fengxia and Hanwen (2010) mentioned that no residues for enrofloxacin or ciprofloxacin were detected in 20 chicken muscle samples randomly collected from local markets in china.
Conclusions and Recommendations
The obtained results in the current study revealed detection of the residues of fluoroquinolones antibiotic residues in broiler meat. All antibiotics were within permissible limit except for enrofloxacin and sarafloxacin. Control of the unscrupulous use of antibiotics in food animals and applying hygienic measures is very important to safeguard the public health and to protect the currently available antibiotics for future clinical uses.
Acknowledgments
The authors acknowledge the Deanship of Scientific Research at King Faisal University for the financial support (Grant No. 182012).
Author’s Contribution
All authors contributed to the study conception and design. Material preparation and sample collections were performed by Aqeel Mahdi M. Al-Ebrahim and Waleed Rizk El-Ghareeb. Chemical analysis and data managements were conducted by Aqeel Mahdi M. Al-Ebrahim. The first draft of the manuscript was written by Waleed Rizk El-Ghareeb. Authors commented on the previous versions of the manuscript and approved the final version before submission.
Ethical approval
This study was conducted according to the guidelines of King Faisal University, Saudi Arabia
Consent to participate
All authors approved to participate in this research work and in the manuscript.
Consent to publish
All authors approved this manuscript to be published.
Availability of data and materials
All data and materials will be available upon request.
Funding
This study was supported by the Deanship of Scientific Research at King Faisal University for the financial support (Grant No. 182012).
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES