South Asian Journal of Life Sciences
Research Article
Natural History Observations on Mass Congregation and Diapause Behaviour of Pre-Migratory Roosting Danainae (Lepidoptera: Nymphalidae) From Sahyadri Mountains, India
Milind Digambar Patil
College of Forestry, Department of Natural Resource Management, Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth (Agricultural University), Dapoli, Dist. Ratnagiri, Maharashtra 415712, India.
Abstract | Butterfly migrations are one amongst the most fascinating phenomena in the animal kingdom. Tropical butterfly migrations are unique in them since they are comprised with more than one species and chiefly governed by sequential seasonal events of monsoon. To investigate the population structure and diapause behaviour of congregated Danainae populations, a study was conducted at the congregation site in Gothos village during the period 05/02/2014 to 09/02/2014. The roosting colony comprised of three Danainae (Nymphalidae) taxa viz. (i) Oriental Blue Tiger Tirumala limniace exoticus, (ii) Oriental Striped Tiger Danaus genutia genutia and (iii) Indian Common Crow Euploea core core in order of dominance. Congregation was lasted for around three months. Out of the total visual estimates of 50,000 individuals, 522 individuals were randomly captured and permanently tagged. An excellent wing condition of captured individuals depicted their ‘fresh’ eclosion. Abdominal dissections of 30 female specimens (10 of each species) showed existence of reproductive diapause. Recording three consecutive years’ congregations (2012-13, 2013-14 and 2014-15) confirm the Gothos valley as the first documented ‘annual pre-monsoonal congregation site’ of Danainae in the Indian peninsula. The observations highlight specific niche requirements of freshly ecloded migratory broods of Danainae to overcome reproductive diapause followed by theiradvancing voyages in the rain-shadow region.
Keywords | Annual mass congregation, Butterfly tagging, Reproductive diapause, Roosting behaviour, Seasonal migration
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | February 29, 2016; Accepted | May 22, 2016; Published | June 15, 2016
*Correspondence | Milind D Patil, College of Forestry, Department of Natural Resource Management, Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth (Agricultural University), Dapoli, Dist. Ratnagiri, Maharashtra 415712, India; Email: [email protected]
Citation | Patil MD (2016). Natural history observations on mass congregation and diapause behaviour of pre-migratory roosting Danainae (Lepidoptera: Nymphalidae) from Sahyadri Mountains, India. S. Asian J. Life Sci. 4(1): 25-31.
DOI | http://dx.doi.org/10.14737/journal.sajls/2016/4.1.25.31
ISSN | 2311–0589
Copyright © 2016 Patil. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Butterfly migration is an evolved response towards spatiotemporal heterogeneity of environment (Ramenofsky and Wingfield, 2007; Roff and Fairbairn, 2007; Gotthard, 2008). It is a mass movement of individuals in a same direction along with simultaneous occurrences of both the sexes, which falls with internal inhibitory responses (Shields, 1974; Dingle and Drake, 2007). Seasonal butterfly migrations are primarily an outcome of reproductive diapause, which is an evolutionary physiological answer to the uncomplimentary external environmental conditions (Herman, 1981; Ramenofsky and Wingfield, 2007). A thermal condition which caterpillar experiences normally determines diapause decisions in its adulthood (Gotthard, 2008; Fujita et al., 2009). On the parallel, ambient temperature and photoperiod (Arikawa, 2001) determines larval host plant quality, thus indirectly regulates the extent of diapause (Gotthard, 2008).
Monarch butterfly Danaus plexippus Linnaeus, 1758 in Northern America has two distinct generations viz. non-migratory summer population and the migratory winter population. The former cohort lives around two to
five weeks as adult whereas the latter live up to nine months exhibits reproductive diapause (Oberhauser, 2009). Migratory monarchs sometimes make transient roosting colonies. Here the intermission interval ranges from ten minutes to three days (Urquhart et al., 1965; Meitner, 2004). Such places are utterly distinct from the overwintering places where the stopover period is around four months (Sourakov, 2008; McCord and Davis, 2010).
Tropical butterfly migrations, in contrast, accompanied by more than one species (Shields, 1974) and can be correlated with sequential seasonal events of monsoon (Orr, 1992; Kunte, 2005; Palot, 2012; Ramesh et al., 2012). In Indian peninsula, migratory butterfly swarms chiefly composed of Euploea core Cramer, 1780, Euploea sylvester Fabricius, 1793 and Tirumala septentrionis Butler, 1874 show a unique pre-reproductive longitudinal migration pattern, perfectly synchronized by the south-western and the mid north-eastern monsoons (Kunte, 2005). For instance, E. core possesses distinct migratory and non-migratory populations. Migratory cohorts show peculiar life history traits: emergence, congregation at some specific sites, sporadic feeding on nectar and other alkaloids to overcome reproductive diapause and emigration towards the rain shadow region (Kuussaari et al., 1996; Canzano et al., 2003; Kunte, 2005; Ramenofsky and Wingfield, 2007). Such pre-migratory butterfly congregations usually observed near to the up-hills or lower side of the gully, with the termination of diapause, may facilitate mating opportunities (Kitching and Zalucki, 1981; Orr, 1992).
Analogous to the above commentary, the present paper describes the natural history observations on mass congregation behaviour of Danainae butterflies (Lepidoptera: Nymphalidae) from northern Western Ghats (Sahyadri Mountains) of India (Patil, 2014; Patil et al., 2014). Here, I discuss the community structure, diapause and behavioural aspects of three Danainae taxa viz. Oriental Blue Tiger Tirumala limniace exoticus Gmélin, 1790, Oriental Striped Tiger Danaus genutia genutia Cramer, 1779 and Indian Common Crow Euploea core core Cramer, 1780 which were congregated on area of about 4 ha, and were lasted for three months.
MATERIALS AND METHODS
Study Area
The study was conducted in a small village Gothos (16°02’23.40”N, 73°49’59.82”E, elevation- 107.89m above msl and 36.6km radial distance from the West coast of India), Sindhudurg District of Maharashtra State. It is a westerly-faced amphitheater of hills in the Sahyadri Mountains occupied with two distinct forest types; the lower moist deciduous type and the upper semi-evergreen type (Figure 1). Major tree species includes Artocarpus gomezianus Wall. ex Trecul ssp. Zeylanicus (Moraceae), Artocarpus heterophyllus (Moraceae), Bombax ceiba (Bombacaceae), Butea monosperma (Fabaceae), Careya arborea (Lecythidaceae), Ficus glomerata (Moraceae), Gmelina arborea (Verbenaceae), Schleichera oleosa (Sapindaceae), Strychnos nux-vomica (Loganiaceae), Syzygium cumini (Myrtaceae), Tabernaemontana alternifolia (Apocynaceae) and Terminalia paniculata (Combretaceae). Other dominant plant species includes Carissa congesta (Apocynaceae), Helicteres isora (Sterculiaceae), Ixora coccinea (Rubiaceae), and Leea indica (Vitaceae). Meteorological records at the nearest weather station - College of Horticulture, Mulde (~20km from the study area) show an average annual rainfall of 3000mm; temperature minimum 13°C, maximum 38°C and humidity minimum 25% and maximum 97% in this region.
Lower part of the hill has private land ownership, sparsely planted with Managa bamboo Dendrocalamus stocksii (Poaceae) and Cashew trees Anacardium occidentale (Anacardiaceae). The above adjacent forest is under the jurisdiction of Kadawal Forest Range (Divisional Forest Office, Sawantwadi) having experimental plantations of Teak Tectona grandis (Verbenaceae). Rainfed paddy Oryza sativa (Poaceae) cultivations practiced at the base of valley. These agricultural fields are left fallow till next monsoon. One such fallow area shows renaissance of Tumba Leucas aspera (Lamiaceae) (16°02’24.82”N, 73°49’58.24”E, elevation- 100.88m above msl) from January persists till mid-March. Congregated butterfly community took shelter in the forests adjoining the fields of L. aspera during its blooming period.
Data Collection
I selected the edge between forests and L. aspera fields, an area of around 2 ha, as a sampling site. Butterflies started congregating the forest from 24/01/2014. The number was increasing steadily and on 04/02/2014, visual estimates of the congregated swarm showed around 50,000 individuals of Oriental Blue Tiger Tirumala limniace exoticus (BT), Oriental Striped Tiger Danaus genutia genutia (ST) and Indian Common Crow Euploea core core (CC). Field observations were recorded during 05/02/2014 to 08/02/2014.
A permanent zigzag transact line was marked and walked roughly at a pace of one meter per two seconds (Hamm, 2013). Butterflies were captured using hand-held insect net. ‘Net’ captured individuals were calm down and gently removed from the net by keeping their wings closed. Species and sex of each captured individual were confirmed by various morphological cues e.g. colour pattern on wings, presence of scent glands etc. (Kehimkar, 2008). Three point scale was adopted to categorize the wing conditions of captured individuals viz. “intact” (perfect wing-margins), “chipped” (<20 mm2 of wing missing), and “very chipped” (>20 mm2 of wing missing) (Kitching and Zalucki, 1981). Additionally, to get acquainted with local perspectives of the butterfly congregation, face-to-face interactions were conducted with six families residing nearby the sampling area.
Tagging
Each captured individual was marked with a unique identification tag (Sheppard and Bishop, 1973). Permanent identification tags [dimensions- 9×9mm, mass- 0.007g (<3.5% of mean body weight of sampled specimens of corresponding species) with one side glue] were used (Figure 2). Single sheet consists of 500 tags, printed with black colored waterproof ink. Each tag contains the information: 5 digit code number e.g. A0034, e-mail id and telephone number of the researcher.
Tag was placed at central discal cell of the lower side of hind wing, so that it should be visible when the wings are closed. Before placing the tag, the cell portion was gently rubbed for gluing assurance. Handling period for each captured specimen was minimized up to 30 seconds. Recaptured specimens were released immediately after reading their tag code. Netting and tagging activities were performed during 0900 to 1300 and 1400 to 1800 hours (IST).
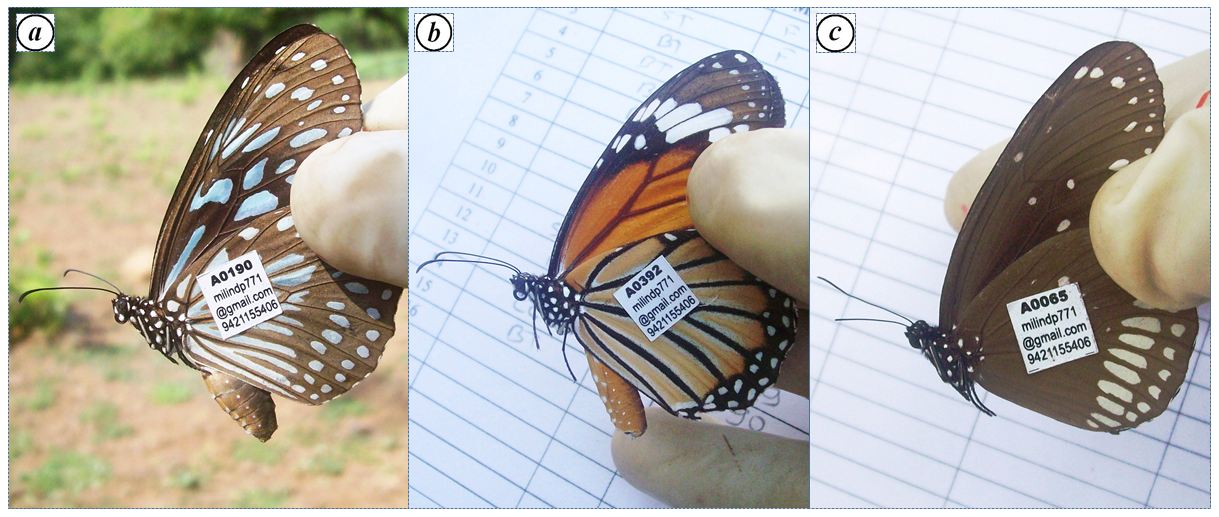
Figure 2: Tagged individuals of a) BT; b) ST; c) CC at the congregation site, Gothos village, Sindhudurg (2013-2014)
Table 1: Number of Danainae individuals captured and released at the congregation site (From 05/02/2014 to 08/02/2014), Gothos village, Sindhudurg
|
Species |
Day-1 |
T |
Day-2 |
T |
Day - 3 |
T |
Day - 4 |
T |
GT |
||||
|
M |
F |
M |
F |
M |
F |
M |
F |
||||||
|
BT |
50 |
34 |
84 |
42(1)* |
46 |
88 |
35(1) |
29(1) |
64 |
14(1) |
14 |
28 |
264(4) |
|
ST |
15 |
12 |
27 |
15 |
19 |
34 |
22 |
25 |
47 |
9 |
5 |
14 |
122 |
|
CC |
16 |
4 |
20 |
17 |
12 |
29 |
23 |
11 |
34 |
20 |
33 |
53 |
136 |
|
Total |
81 |
50 |
131 |
32(1) |
77 |
151 |
80(1) |
65(1) |
145 |
43(1) |
52 |
95 |
522(4) |
M- male, F- female, T- total, GT- grand total, *recaptured individuals)
Abdominal Dissections
In butterflies, reproductive diapause is more intense and lasts longer in females than the males (Herman, 1981). Thus to examine the reproductive track development, females were netted on 09/02/2014. Total 30 specimens (10 from each species) were randomly captured and kept in glassine envelops (Kunte, 2000). Butterfly morphometric (Dingle, 1996) and abdominal dissections were carried out at College of Forestry lab on 11/02/2014. Digital caliper and electronic weighing balance were used to measure the linear and mass measurements respectively. Labomed CZM4 Trino Stereo Microscope (40x) attached with digital camera (image resolution - 720×576 pixel) was used during dissections.
Since it was difficult to compute the total number of immature oocytes in each specimen, towards end of the ovarioles, it was not recorded. However, the stage of reproductive diapause was confirmed by lack of mature (chorionated) eggs, lack of ovarian activity and presence of huge mass of reserved fat in abdominal cavity (Orr, 1992; Canzano et al., 2003). Successful mattings were confirmed by the presence spermatophores in the bursa copulatrix (Kitching and Zalucki, 1981; Rindge, 1985; Orr, 1992; Watanabe and Sato, 1993; Oberhauser and Hampton, 1995; Cornish, 2001; Grill and deVos, 2004; Mikkola, 2007; Mikkola, 2008). Each dissected specimen was then labeled and kept in 70% alcohol (Kunte, 2000).
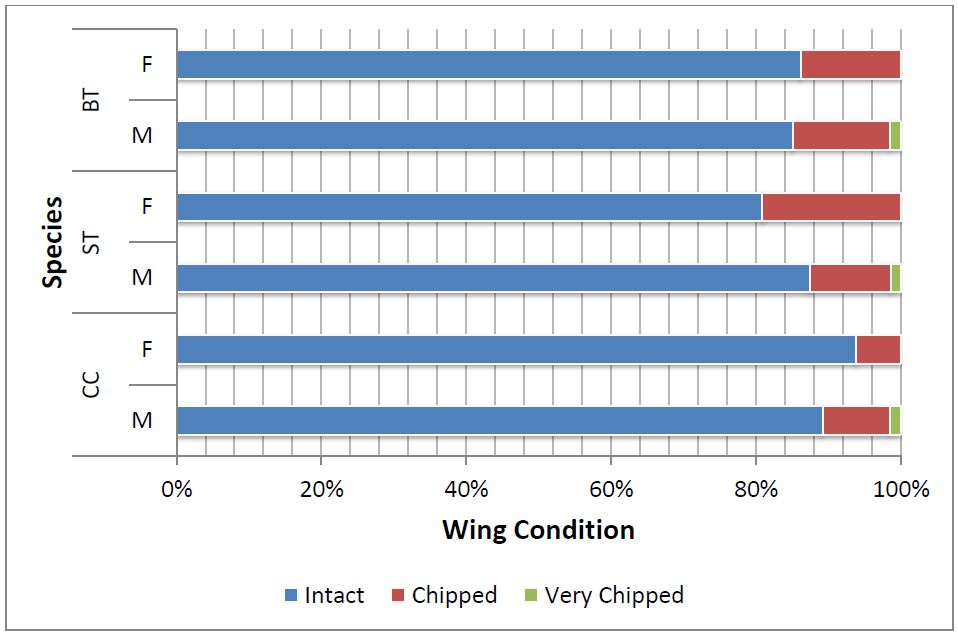
Figure 3: Female & male individuals of BT, ST and CC captured and released at the congregation site, Gothos village, Sindhudurg (2013-2014)
RESULTS
In all, 522 individuals (roughly 1% of the visual estimate) were randomly captured and permanently tagged. The order of dominance of captured species was BT (51%) followed by CC (26%) and ST (23%) (Table 1). Based on captured frequency, BT shows highest population amongst the congregated Danainae. The sex ratio of BT and CC was slightly male biased (Figure 3). A large number of total captured individuals had ‘perfect’ wing-margin (86%) and only 14% showed ‘chipped’ wing condition (Figure 4).
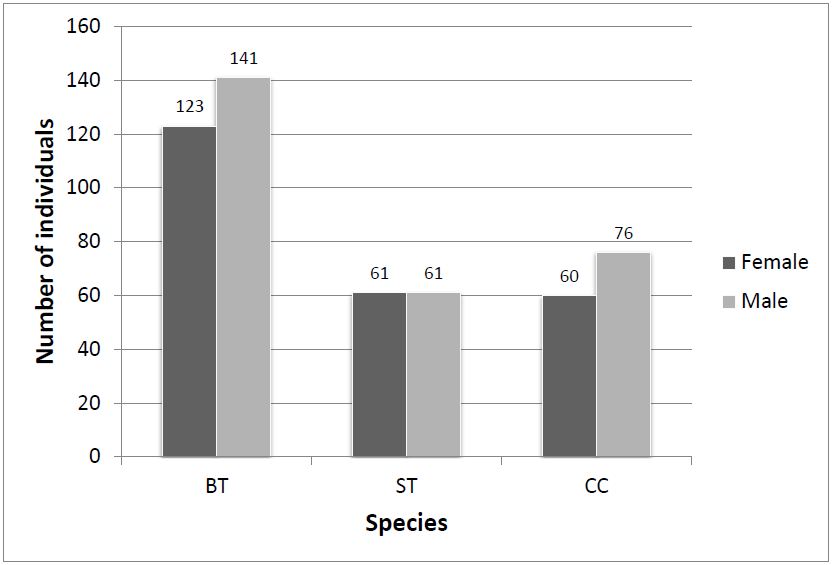
Figure 4: Wing condition of female (F) and male (M) individuals of BT, ST and CC butterflies captured at the congregation site, Gothos village, Sindhudurg (2013-2014)
The fresh and bright coloured wings depicted the recent eclosion of the congregated individuals. Abdominal dissections of all 30 female specimens exposed the sizable mass of reserved fat bodies. Out of 10, presence of spermatophores in the bursa copulatrix was recorded in nine BT specimens (Figure 5a). However, no mature oocytes were observed in any of the BT specimen. So also spermatophores were not recorded either in ST or in CC (Figure 5b and 5c). This shows that there exists reproductive diapause amongst the congregated Danainae. Some additional morphometric observations are given in Table 2.
Other Field Observations
Congregated individuals perched on low hanging vines, dried twigs, dried or fallen bamboo culms, branches and

Figure 5: Images of Bursa copulatrix of a) BT; b) ST; c) CC specimens captured at the congregation site, Gothos village, Sindhudurg (2013-2014) (Scale bar: 1mm)
Table 2: Morphometric observations of three Danainae taxa (females, 10 specimens each) captured at the congregation site, Gothos village, Sindhudurg
|
Species |
body (g) |
wing (g) |
head (g) |
thorax (g) |
abdomen (g) |
Forewings (cm) |
|
|
BT |
Mean |
0.3093 |
0.0418 |
0.0130 |
0.1265 |
0.1355 |
4.5220 |
|
SD |
0.0594 |
0.0060 |
0.0015 |
0.0232 |
0.0394 |
0.2716 |
|
|
SE |
0.0187 |
0.0019 |
0.0004 |
0.0073 |
0.0124 |
0.0859 |
|
|
ST |
Mean |
0.1927 |
0.0269 |
0.0098 |
0.0735 |
0.0815 |
4.1890 |
|
SD |
0.0215 |
0.0027 |
0.0008 |
0.0094 |
0.0122 |
0.1206 |
|
|
SE |
0.0068 |
0.0008 |
0.0002 |
0.0030 |
0.0038 |
0.0381 |
|
|
CC |
Mean |
0.1739 |
0.0273 |
0.0103 |
0.0737 |
0.0615 |
4.3220 |
|
SD |
0.0244 |
0.0045 |
0.0016 |
0.0113 |
0.0096 |
0.1630 |
|
|
SE |
0.0077 |
0.0014 |
0.0005 |
0.0035 |
0.0030 |
0.0515 |
|
tree trunks. CCs were roosting or resting chiefly on the forest floor matted with dried leaves. Nectaring was exclusively observed on L. aspera, although relatively far-off fields of L. aspera (>50m away from forests’ edge) remained unoccupied. In morning hours, many individuals were probing herbaceous vegetation as well as the earth near to the nectaring grounds. In the afternoon nectaring activity was ceasing gradually. Meanwhile they oriented themselves by facing the sun ventral wing surfaces. Moreover, three mating pairs of BT were observed near the L. aspera plots.
It was observed that continuous human interference (sampling walks) increasing the level of disturbance at congregation site. Even a walk of researcher through sampling area lead the entire swarm winged up high in forest canopy and took 10-15 minutes to retreat. Here, the ‘handling effects’ may be the root cause of low recovery of tagged individuals (Table 1) which might enforcing previously captured individuals to avoid specific sites where they have been handled (Mallet et al., 1981). Intensive netting sometimes obtrudes usual behaviour of congregated Danainae (per. comm.). I also observed ‘community shifts’ of the entire congregated colony inside the forests of Gothos valley, twice e.g. there were no butterflies near the L. aspera fields after a week of sampling. Then an intense search revealed the swarm in the uphill side (16°02’20.42”N, 73°50’7.23”E, elevation - 151.79 MSL), some 50m from their initial roosting place.
Few subsidiary species were recorded intermingled with the congregated community viz. Oriental Dark Blue Tiger or Dakhan Dark Blue Tiger Tirumala septentrionis dravidarum Fruhstorfer, 1899 (300-400 individuals), Double-branded Black Crow Euploea sylvester coreta Godart, 1819 (70-100 individuals), Oriental Plain Tiger Danaus chrysippus chrysippus Linnaeus, 1758 (20-25 individuals) and Brown King Crow Euploea klugii kollari Felder and Felder, 1865 (20-25 individuals). Two specimens of Indian White Tiger Danaus melanippus indicus Fruhstorfer, 1899 were recorded near the L. aspera fields which was previously reported ‘uncommon’ to the peninsular India (Kehimkar, 2008).
People’s Perspective
Local people correlate the arrival and stopover period of Danainae to the blooming of L. aspera. In fact, the valley is locally called Pakhyacha ran (jungle of butterflies and moths). According to them, instability rains (rains during December - March) interrupt densely congregated swarms and make them disperse over larger area. Irregular interrupted gusts convey the message of arrival of S-W monsoon during early June. And with the onset of Mriga nakshatra (the 5th lunar mansion in Hindu astrology, indicating progress of S-W monsoon) butterflies started crossing the Sahyadri towards East. Advancing migrations benefited from earlier currents of air. Some of the respondents argue that these swarms might have been congregating the foot-hill to circumvent Indian summers. It was also highlighted that another larger species of butterflies annually congregate in the forest during pre-monsoon season but it never retreats from the forest.
DISCUSSION
In 2012-13, butterflies started congregating the Gothos valley in the last week of December, 2012. Populations reached at peak in the second week of January, 2013 and started decreasing gradually towards early-April (Patil, 2014; Patil et al., 2014). Again, in the year 2013-14, immigrants bloomed in the first week of February. This year, Gothos valley experienced the first showers of S-W monsoon on 02/06/2014, which triggered voyage of the roosting Danainae from the area. Thousands of butterflies (BT, CC and ST) were observed emerging from the forests, accompanied by thousand more from coastal and low land areas of Sindhudurg District. The direction of flight was against the prevailing monsoonal winds, more or less straight towards northeast. The ‘great migration’ was observed for three days (from 03/06/2014 to 05/06/2014). During the year 2014-15, again I witnessed similar butterfly gatherings at Gothos valley from last week of December. Unfortunately I was unable to conduct systematic survey of the congregated colonies.
Observations of three consecutive years’ congregations (2012-13, 2013-14 and 2014-15) confirm and solemnize the Gothos valley as an annual pre-monsoonal congregation site of Danainae butterflies in the northern Western Ghats. Moreover, this is the first report of annual pre-monsoonal congregation site of Danainae from Indian peninsula. I also affirm that there exist more than one such annual roosting sites of Danainae in the Sahyadri Mountains. Such places owing to their unique microclimate acting as secured refugee to the freshly ecloded broods of migratory Danainae to overcome reproductive diapause followed by their advancing voyages in the rain-shadow region.
Western Ghats, one of the world’s eight ‘hottest hotspots’ of biological diversity, gets inscribed in the UNESCO World Natural Heritage sites list in 2012. Certainly, these observations will help biologists for conducting future research on tropical migratory Lepidoptera, and conservationist / policy makers to preserve and maintain ecological integrity of the area from future threats.
ACKNOWLEDGEMENTS
I thank Shri Nagesh Daptardar, Honorary Wildlife Warden, Sindhudurg District, for his timely permission to conduct this study. I thank Professor Dr. Krushnamegh Kunte, National Centre for Biological Sciences, Bengaluru (India) and Mr. Peter Smetacek, Butterfly Research Center, Bhimtal (India) for their inspirations; Professor V. K. Patil, Professor Dr. A. D. Rane and Professor Dr. S. S. Narkhede, College of Forestry, Dapoli for providing lab and other facilities. I am also grateful to my family for encouragements.
CONFLICT OF INTEREST
The author declares that he has no conflict of interest related to the work.
References