Advances in Animal and Veterinary Sciences
Short Communication
First Molecular Identification of Dirofilaria repens Found in a Wolf Heart in Kazakhstan
Rabiga S. Uakhit1,2, Ludmila A. Lider2, Ainura M. Smagulova1, Sergey V. Leontyev4, Sarsenbay K. Abdrakhmanov3, Vladimir S. Kiyan1*
1Research Platform of Agricultural Biotechnology, 2Department of Veterinary Medicine, 3Department of Veterinary Sanitation, Saken Seifullin Kazakh Agrotechnical University, Nur-Sultan, 010011, Kazakhstan; 4Facility of biology and technology, Department of biology, Bioresources and Aquaculture, Novosibirsk State Agrarian University.
Abstract | The clinical manifestations of dirofilariasis are usually different and depend on the species: D. immitis is located on the right side of the heart and pulmonary artery. On the other hand, D. repens is localized in the subcutaneous tissue. According to the literature, dirofilariasis is a filaroid nematode present in animals and humans, which is mainly transmitted by mosquitoes. This is a rare case of finding helminths in the heart cavity of a wolf and raises questions about its distribution area in Kazakhstan. To determine the genotype of Dirofilaria spp. found in the heart of a wild wolf, identification was carried out by PCR using the species-specific primer SSU rRNA, as a result of which amplicons of 875 bp were obtained. The results of the study showed that the nucleotide sequence of the studied species was identified as D. repens, and this sequence was deposited in the NCBI GenBank database (accession number MT877205.1). The research results revealed the imperfection of the differential identification of Dirofilaria spp parasites only by their morphological structure and confirm the need to use molecular identification methods.
Keywords | Dirofilaria repens, Wolf, Heart, Molecular identification, SSU gene
Received | February 08, 2021; Accepted | May 27, 2021; Published | July 28, 2021
*Correspondence | Vladimir Kiyan, Research Platform of Agricultural Biotechnology, Saken Seifullin Kazakh Agrotechnical University, 010011, ZhenisAvenue, 62, Nur-Sultan, Kazakhstan; Email: [email protected]
Citation | Uakhit RS, Lider LA, Smagulova AM, Leontyev SV, Abdrakhmanov SK, Kiyan VS (2021). First molecular identification of Dirofilaria repens found in a wolf heart in Kazakhstan. Adv. Anim. Vet. Sci. 9(9): 1342-1346.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1342.1346
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Uakhit et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Dirofilariasis is a zoonotic parasitosis that is caused by adult nematodes of the genus Dirofilaria (Simón et al., 2012). The most relevant are Dirofilaria immitis and Dirofilaria repens due to their high predominance, frequency, and severe pathological effects among all Dirofilaria species (Orihelet al., 1998). Parasites, as mentioned above, are the cause of dirofilariasis in dogs, cats, and wild carnivores (Simón et al., 2012). It is also assumed that the above helminths areetiological agents of zoonotic contamination in humans, which in turn are transmitted by carrier (Albonicoet al., 2014; Becker et al., 2010). The life cycle of Dirofilaria species consists of a vector and final host. Representatives of the diverse female mosquito species of the Culicidae family act as vectors (Becker et al., 2010). Domestic and wild dogs are the best reservoirs in association with mammalian hosts and are the most common human transmission source (Simón et al., 2012).
In animals, the clinical presentationsare usually different and are species-dependent: D. immitis localizes in the right part of the heart and lung artery, and D. repens localizes in the subcutaneous tissue (McCall et al., 2008; Simónet al., 2012). In humans, the clinical data are species-dependent: D. repens is essentially associated with subcutaneous nodules, and D. immitis is primarily accountable for lung nodules (Genchi et al., 2011).
Dirofilariasis is common on nearly all continents (Simón et al., 2012). Endemic foci have occurred across all of Europe, mainly in Italy and France, Greece, Spain and Russia (Avellis et al., 2011; Bargues et al., 2006; Ermakova et al., 2014). Several reports have identified the spread of dirofilariasis to the Republic of Kazakhstan, which date from the beginning of the last century (Ch’ung-Hsiung, 1959). There are no statistical data that document this spread into Kazakhstan, but in 2014, there were 2 cases of infection in Almaty (Seidulaeva et al., 2015). These reports do not allow us to understand the full picture of the epizootiology of Dirofilaria species. In addition, there are no molecular genetic studies.
The present study was conducted to distinguish the genotype of Dirofilaria spp. that was circulating in Kazakhstan and was found in a wild wolf heart.
MATERIALS AND METHODS
Sample collection
D. repens was isolated from a wild wolf heart (Canis lupus) (Linnaeus) that was captured in the Kostanay region of Kazakhstan. Hunters conducted extraction of wild animals in compliance with all legislative norms. Work with the animals was carried out in a parasitological laboratory of a Veterinary Medicine Faculty and was approved by the Animal Ethics Committee. All procedures complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm).
DNA extraction
For DNA extraction, 1.5 ml of one piece of the mature form of helminthes D. repens was homogenized in Eppendorf polypropylene centrifuge tubes, and the phenol-chloroform
method was used. The volume and clarity of the obtained DNA were defined by measuring the absorption levels at 260 nm and 280 nm with a Nano Drop 2000 spectrophotometer (Thermo Scientific, USA). The obtained DNA was dissolved in ddH2O and stored at -70°C for long-term use.
PCR assays
Methods for species-specific Dirofilaria spp. DNA was analyzed by conducting a polymerase chain reaction (PCR) with small subunit ribosomal ribonucleic acid (SSU rRNA) (Albonico et al., 2014). The ribosomal SSU gene was amplified via the primer pair, namely, forward (5’-CCATGCATGTCTAAGTTCAA-3’) and reverse (5’- TCGCTACGGTCCAAGAATTT-3’). The primers were synthesized by DNA Synthesis LLC, Moscow, Russia.
Enlargement of the marker genes was conducted in a final reaction volume of 25 μl containing 10× Taq buffer with (NH4)2SO4, 2.5 mM MgCl2, 1 U Taq DNA polymerase, 200 μM dNTPs (Thermo Scientific, Carlsbad, California, United States), 10 pmol of each primer and 20 ng of essential nematode DNA from an adult piece as a template. PCR was conducted as follows: denaturation at 94°C for 30 sec, annealing at 50°C for 30 sec, extension at 72°C for 40 sec, and a final extension for 5 min at 72°C. The resulting restriction fragments were subjected to electrophoresis and were separated by ethidium bromide with 1.5% agarose gel and using a 1× TAE buffer solution.
Sequencing data and phylogenetic analysis
The PCR-amplified target gene particles were purified with a Quick PCR Purification Kit (Invitrogen, Lithuania) according to the manufacturer’s protocols. Sequencing was performed in the Molecular Biology Laboratory of the Research Platform of Agricultural Biotechnology Sequencing at Saken Seifullin Kazakh Agrotechnical University, according to the manual for Seq Studio Genetic Analyzer (Thermo Fisher Scientific Applied Biosystems). The nucleotide sequencesobtained were analyzed with Bio Capt software version 11.0. The nucleotide sequences of the studied species were compared with other sequences in the NCBI GenBank database using BLAST options. These sequences were correlated with different sequences in GenBank by BLAST analysis. Phylogenetic analysis was accomplished with MEGA X software.
RESULTS AND DISCUSSION
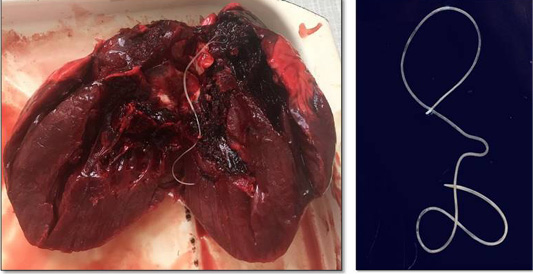
Hunters captured a wolf (female) near Torgay village, Dzhangeldinsky district, in the Kostanay region of the Republic of Kazakhstan. A complete parasitological study revealed an adult Dirofilaria spp. worm in the wolf’s heart (Figure 1). A total of 31 animals from various regions of the country were studied, and only one wolf was infected with the dirofilariasis pathogen (1 individual); the extent of invasion was 3.2±0.2%.
The Dirofilaria spp. individual detected had a length of 14.7 cm. Its cuticle had longitudinal ridges along the body and a filamentous body with a light-yellow color; it tapered toward both ends and was covered with a thin layer, striped cuticle.
The genomic DNA that was obtained fromthe helminth by applying the phenol-chloroform extraction method was analyzed by electrophoresis on 1% agarose gel. PCR was performed in a Simpli Amp thermo cycler (Applied Biosystems) under the conditions described above. The SSU area of the rRNA was amplified by species-specific primers, and the PCR product (with a size of 875 bp) was obtained (Figure 2).
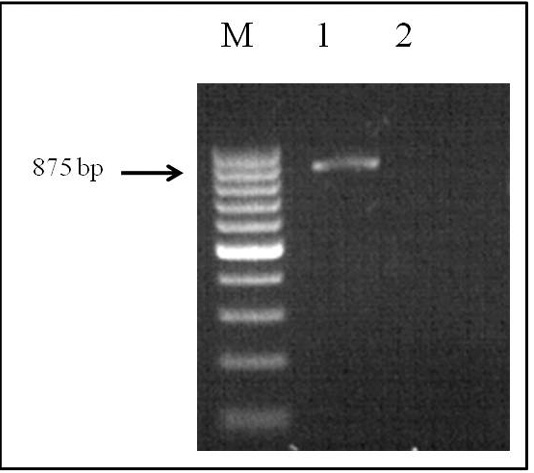
Figure 2: Electrophoretic analysis of PCR products: Lane M, DNA ladder (bp); lane 1, Dirofilaria spp. DNA; and lane 2, negative control.
Sequencing was performed by using a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). These sequences were compared with other sequences in GenBank by using BLAST analysis. The nucleotide sequence of the studied species was identified as D. repens and these data were deposited into the NCBI GenBank database (D. repens isolate 19-001-Kz, accession no. MT877205.1).
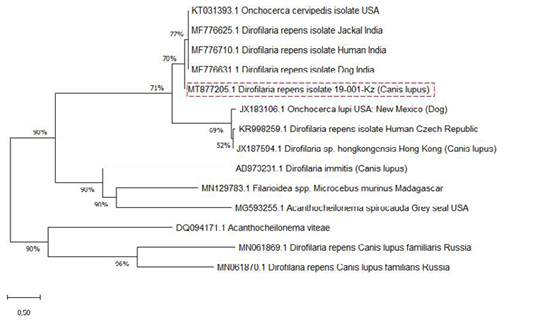
The SSU gene of Dirofilaria species in the current research paper was subjected to multiple sequence alignments with previously published studies of GenBank sequences using the MEGA X 10.2.0 program (Figure 3).
Analysis of the nucleotide sequence the SSU area of rRNA the Kazakhstan Dirofilaria nematode showed 77% uniqueness with those of D. repens strains from India (MF776625.1; MF776710.1; MF776631.1), 69% with D. immitis, and Onchocerca lupi from the USA and Czech Republic (JX183106.1; KR998259.1) but showed only 52% uniqueness with D. sp. hongkongensis from Hong Kong (JX187594.1).
The genetic ranges among the identified D. repens of the Kazakh wolf strain and reference strains were calculated based on the MEGA X software parameter model (Table 1). The genetic distances that were intermediate to those of the D. repens strains were deficient and ranged from 0.000 to 0.18 (Table 1, No. 1-5). There were relatively high taxonomic genetic distances, which ranged from 1.05 to 0.11 between Dirofilaria spp. and the nematode O. lupi (Table 1, No. 1-5 vs. No. 6); 1.06 to 0.12 between D. repens and D. sp. hongkongensis; 3.61 to 5.81 among D. repens and D. immitis; and 3.86 to 3.44 between Dirofilaria spp. and Filarioidea spp. which was used for an out-group (Table 1, No. 9).
Identification of immature worms of filariid species by examining their morphology is complicated. Often, misidentification of Dirofilaria species is due to limited technical expertise, especially under poor sampling conditions. Usually, the parasitesare rarely intact; the diagnosis is based on the histological characteristics of helminths which limit identification to onlythe genus level. DNA-based molecular methods have more specificity than serological methods (Rishniw et al., 2006).
Correspondingly, the localization of the investigated parasite in a wolf heart should be noted in this work. According to the literature data, D. immitis is known as a “heartworm” which indicates that this helminth is localized in host hearts. On the other hand, D. repens largely predominates over pulmonary dirofilariasis cases (Orihel et al., 1998).
Females D. repens reach 15-17 cm in length, males up to 7 cm, with widths of 0.4-0.5 mm. Additionally, the female tail is blunt and slightly curved ventrally. Males have no ornamentation on their heads, and only four protruding submediant head papillae are visible. The tail end is bluntly rounded (Lopez et al., 2006).
Table 1: Genetic distance of Dirofilaria strains.
| Dirofilaria repens | Onchocerca lupi |
Dirofilaria sp. hongkongensis |
Dirofilaria immitis |
Filarioidea spp |
||||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| MT877205.1 | ||||||||||
| MF776625.1 | 0,03 | |||||||||
| MF776631.1 | 0,03 | 0,01 | ||||||||
| KT031393.1 | 0,03 | 0,01 | 0,01 | |||||||
| MF776710.1 | 0,04 | 0,01 | 0,00 | 0,01 | ||||||
| KR998259.1 | 1,03 | 1,17 | 1,18 | 1,16 | 1,18 | |||||
| JX183106.1 | 1,05 | 1,18 | 1,20 | 1,18 | 1,20 | 0,11 | ||||
| JX187594.1 | 1,06 | 1,25 | 1,25 | 1,27 | 1,25 | 0,04 | 0,12 | |||
| AB973231.1 | 3,61 | 3,90 | 3,99 | 3,98 | 3,77 | 6,37 | 6,51 | 5,81 | ||
|
MN129783.1 |
3,86 | 3,79 | 4,05 | 3,90 | 3,99 | 4,60 | 5,85 | 4,41 | 3,44 | |
Adult D. immitis are 12-20 cm long (males) and 25-31 cm (females) and 0.7-0.9 mm wide (males) and 1.0-1.3 mm (females). In males, the posterior end of the body is tapered and bent in a spiral shape; in females, the posterior end of the body is blunt.
In contemporary research, molecular characterization of the isolate of wolf Dirofilaria found in Kazakhstan was achieved by conducting phylogenetic analyses of partial nucleotide sequences of the SSU area of the rRNA ribosomal gene. The SSU gene sequence used for genetic identification in the current work was previously used to differentiate filarial species (Albonico et al., 2014).
The nucleotide sequence was identified as belonging to D. repens. Phylogenetic analysis showed that the two groups, D. repens and D. immitis, are distinguished as separate groups in the presence of Dirofilaria sp. hongkongensis and the out-of-group species, O. lupi (Figure 3). It can be observed that D. repens species are closely related.
Considering the data that indicate that subcutaneous dirofilariasis by D. repens occurs only in the CIS and is common among the residents of Uzbekistan, Georgia, Armenia, Ukraine, Belarus, Russia, and Kazakhstan (Yushchuk, 2009; Tumolskaya et al., 2016), the case we are studying has not only biological significance but is also related to socioeconomic status. Very few dirofilariasis cases have been detected in Kazakhstan. Dirofilariasis has mainly been reported by observations of D. repens in dogs; in some cases, the rate of infection is 42.6% (Ch’ung-Hsiung, 1959). There are no data on heartworm infections of wolves in Kazakhstan.
In conclusion, we report a case of dirofilariasis in a wolf heart that was caused by D. repens. Molecular methods confirmed the identification of the causal agent by sequencing the ribosomal 18S rRNA SSU gene. These results indicate the imperfection of differential identifications of parasites only by morphological structure and confirm the need to use molecular identification methods. Above all, this is the first reported case of D. repens being found in the cavity of a wolf heart, which raises questions about the localization of this species in host bodies and its distribution area in Kazakhstan. The data obtained will serve as an example for practical parasitology and will expand the understanding of the localization of various types of dirofilariasis in carnivores.
ACKNOWLEDGMENTS
The research presented above was financially supported by the Ministry of Education and Science of the Republic of Kazakhstan to frame project AP08052252 for 2020–2022.
NOVELTY STATEMENT
The present study was show about the case of dirofilariasis caused by D. repens that found in a wolf heart circulating in Kazakhstan.
AUTHORS’ CONTRIBUTION
All authors shared equally in designing, conducting the study, and writing the manuscript.
Conflict of interest
The authors have declared no conflict of interests.
REFERENCES