South Asian Journal of Life Sciences
Review Article
Coronavirus Outbreak- A Pandemic
Seema Singh1*, Nitin Ranjan Gupta2, Syed Tahseen Raza1, Rahul Singh4, Sanchita Srivastava5, Pooja Singh6
1Department of Physiology, Era’s Lucknow Medical College and Hospital, Lucknow, India; 2Associate professor Dept. of internal medicine, Hind institute of medical sciences and research. Ataria, Mau,Sitapur; 3Deputy General Manager SPMU,NHM; 4Consultant Psychiatrist at District Hospital Barabanki (U.P); 5Research Assistant, Department of Biotechnology, Era’s Lucknow Medical College and Hospital, Lucknow, India; 6Assistant Professor, Department of Biotechnology, Uttaranchal institute of science and technology, Dehradoon, India.
Abstract | A global health emergency termed as Corona Virus Disease COVID-19 became an international concern since December 2019. Novel coronavirus (nCoV) is a spherical or pleomorphic enveloped beta virus containing single- (positive-sense) RNA strand with a nucleoprotein surrounded by a capsid comprised of matrix protein. The envelope is surrounded by club-shaped glycoprotein projections. The virus spreads primarily from one person to another, typically through close contact or breathing drops caused when the infected person coughs or sneezes, hence it is necessary to keep away from a sick person more than two meters. Many pharmaceutical companies and testing organizations are therefore rushing to develop more reliable and rapid test kits to detect 19-nCoV among people suspected of being contaminated in only a few minutes and without the need to send samples to central laboratories. Coronavirus (Covid-19) vaccine latest update: with the production of a secure, reliable and accessible Covid-19 vaccine as the cornerstone to controlling the pandemic, research and testing are rising at a rapid pace around the world to find a cure for the current coronavirus.
Keywords: Corona Virus Disease COVID-19, Novel coronavirus (nCoV), Severe acute respiratory syndrome (SARS), Middle East Respiratory Syndrome (MERS), Virus
Received | June 22, 2020 Accepted | July 15, 2020; Published | August 10, 2020
*Correspondence | Seema Singh, Department of Physiology, Era’s Lucknow Medical College and Hospital, Lucknow, India; Email: [email protected]
Citation | Singh S, Gupta NR, Raza ST, Singh R, Srivastava S, Singh P (2020). Coronavirus outbreak- a pandemic. S. Asian J. Life Sci. 8(2): 64-74.
DOI | http://dx.doi.org/10.17582/journal.sajls/2020/8.2.64.74
ISSN | 2311–0589
Copyright © 2020 Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A global health emergency termed as Corona Virus Disease COVID-19 became an international concern since December 2019 (World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report 46, 6 March 2020). This fatal disease is characterized by fever, cough, and fatigue along with mild to severe respiratory complications (Yan et al., 2020). On 11 March, this deadly outbreak of the virus was declared as a pandemic by the World Health Organization (World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report 68, 28 March 2020).In 1968 the term “corona virus” was derived from the word crown due to its morphological similarity with a crown as observed under electron microscope. In 1975, the International Committee on the Taxonomy of Viruses established coronaviridae family which was further divided into two subfamilies, the coronaviruses and the toroviruses at the 10th International nidovirus Symposium in Colorado Springs, Colo., in June 2005. The coronaviridae family, Arteviridae and Roniviridae families are altogether clustered to form the Nidovirales order (Susan et al., 2005). Based on phylogenetic and genomic studies this novel coronavirus belongs to genera betacoronavirus and has a diameter of 60–140 nm, which enveloped oval or round and polymorphous virions of strong infectivity and general susceptibility to all people irrespective of age across the entire globe (Addie et al., 2014; Almazan et al., 2000). Betacoronvirus has a close proximity to the sequences of severe acute respiratory syndrome-related coronaviruses (SARSr-CoV) and also uses ACE2 as the entry receptor-like SARS-CoV (7). The understanding of COVID-19’s genetic and phenotypic structure in pathogenesis is critical for drug and vaccine development (Bailey et al., 1949).
As on July, 22nd 1,48,45,978 cases have been confirmed worldwide out of which 6,13,879were death cases however 84,28,427 were the recovered cases. India witnessed a spike of 84, 28,427 confirmed cases among which 7,24,578 had successfully recovered whereas 28,084 deaths have been reported till date. The rate of occurrence was spotted as 849 cases per 1 million people. Maharashtra has emerged as the worst affected state with over 3, 18,695 cases.
Data Resources
Characterization of complete coronavirus genome and structure
Novel coronavirus (nCoV) is a spherical or pleomorphic enveloped beta virus containing single- (positive-sense) RNA strand with a nucleoprotein surrounded by a capsid comprised of matrix protein. The envelope is surrounded by club-shaped glycoprotein projections. Some coronaviruses have also been reported to contain a hem agglutinin-esterase protein (HE) (Woo et al., 2010). It is illustrated through various literatures that among all known RNA viruses Coronaviruses have reported to possess the largest genomes of approx. (26.4–31.7 kb), with G + C contents varying from 32% to 43%. There are varied numbers of small ORFs present between the various set of conserved genes such as (ORF1ab, spike, envelope, membrane and nucleocapsid) as well as downstream to the nucleocapsid gene in diverse coronavirus lineages. The viral genome contains characteristic features, which include a unique N-terminal fragment within the spike protein. Genes encoding major structural proteins in all coronaviruses are found in the 5′–3′ order as S, E, M, and N (Boheemen et al., 2012).
At least six ORFs are present typically in a CoV. Except for Gamma coronavirus that lacks the first ORFs (ORF1a/b), approx. two-third of the whole genome length, encodes 16 nsps (nsp1-16). ORF1a and ORF1b contain a frameshift within which two polypeptides are produced: pp1a and pp1ab.These polypeptides are processed by virally encoded chymotrypsin-like protease (3CLpro) or main protease (Mpro) and one or two papain-like protease into 16 nsps. All the structural and accessory proteins are translated from the sgRNAs of CoVs. These polypeptides are processed into 16 nsps by virally encoded protease-like chymotrypsin (3CLpro) or main protease (Mpro) and one or two papain-like protease. Both the structural and accessory protein are derived from the CoV sgRNAs. In addition to these four main structural proteins, different CoVs encode unique structural and accessory proteins, such as HE protein, 3a / b protein and 4a / b protein (Fig. 2B, lower panel). These mature proteins are essential for several key aspects in maintaining genomes and replicating viruses (Cascella et al., 2020).
The coronavirus membrane is composed of three or four viral proteins. Membrane (M) glycoprotein is the most dominant structural protein; it crosses the membrane bilayer three times, leaving a short NH2-terminal domain outside the virus and a long lasting COOH (cytoplasmic domain) inside the virion (Woo et al., 2010).
The peplomers are constituted by the spike protein (S) as the type I membrane glycoprotein. In fact, S protein is the principal inducer of neutralizing antibodies. There is a molecular interaction between the envelope proteins with which the formation and composition of the coronaviral membrane is likely determined. M plays a major role in the intracellular formation of virus particles without needing S. In the presence of tunicamycin coronavirus grows and produces spikeless, noninfectious virions that contain M but devoid of S (Boheemen et al., 2012, Li et al., 2020).
Virus origin’s still a mystery
The virus spreads primarily from one person to another, typically through close contact or breathing drops caused when the infected person coughs or sneezes, which is why it is necessary to keep away from a sick person more than two meters (6 ft. 7 in) (Phan et al., 2020). But the cause of transmission of the coronavirus infection is not just sneezing or coughing. There are other ways of transmitting the virus that include spreading from one person to another through surfaces that the infected person has reached, particularly after studies have shown that the virus remains alive on surfaces for up to 9 days (Riou et al., 2019, Zhou et al., 2020). When people come into contact with these items or structures, they may then develop COVID-19 disease and then touch their eyes, nose or mouth. In addition, research to date suggests that 2019-nCoV is mainly transmitted by interaction with respiratory droplets rather than through air. An individual who suffers from mild cough and not feeling ill may have COVID-19 infections. It is yet another justification to practice good hygiene after using the toilet and before feeding, despite the risks involved. Several uncertainties related to various aspects of coronavirus origin are illustrated in Figure 1.
Infectious disease transmission
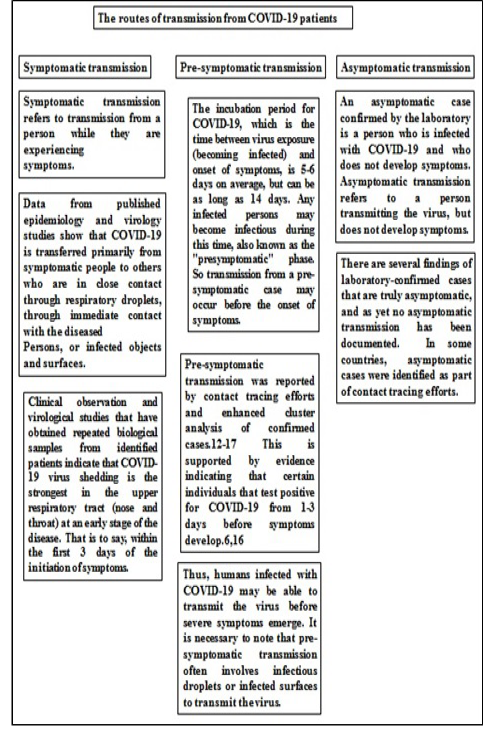
We provide a brief overview of available evidence of COVID 19-infected symptomatic, pre-symptomatic and asymptomatic transmission. Figure 2 illustrates three routes of transmission delineated by WHO in one of its situation reports (Maxwell et al., 2017).
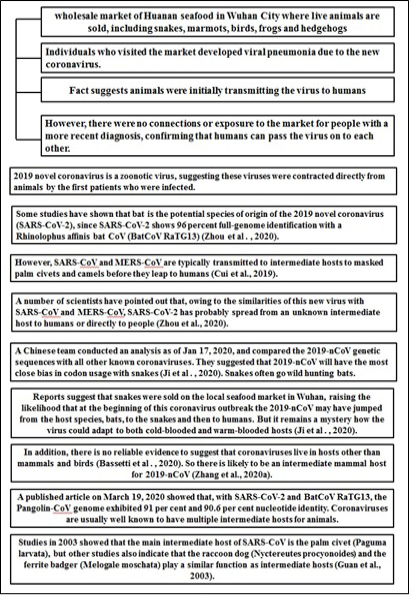
Figure 1: Uncertainty related to various aspects of coronavirus origin *(20)-Zhou et al 2020, (21)-Cui et al 2019, (22)-Ji et al 2020, (23)-Zhang et al 2020(a), (24)-Guan et al 2003
Potential effects of virus on pregnancy
At this period very little is known about the impact of 2019-nCoV on pregnant women and infants and there are currently no clear guidelines about the disease for pregnant women. The CDC has no evidence to suggest the incidence of adverse outcomes of pregnancy for pregnant women with COVID-19 while two of the other SARS-CoV and MERS-CoV coronaviruses were associated with more serious illnesses and higher mortality in pregnant women (Assiri et al., 2016, Chen et al., 2020). Figure 3 illustrate the impact of this deadly virus on pregnant women.
Potential effects of virus on children
Notwithstanding 2019-nCoV’s rapid diffusion, it appears that children usually tolerate the virus with few complications (COVID-19 in Children, 2020). This trend is substantially similar to previous CoV outbreaks, such as SARS-CoV and MERS-CoV, the symptoms of which did not occur widely in children. Figure 4 illustrates the effects of corona virus on children.
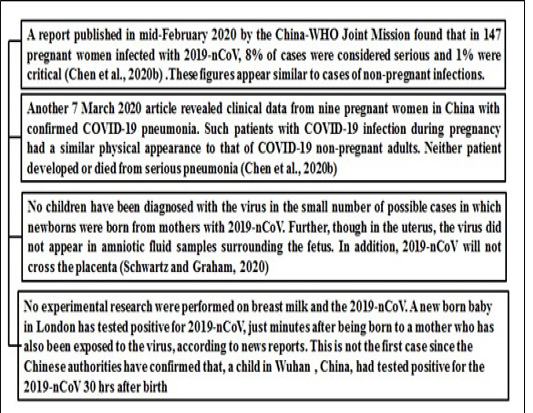
Figure 3: Reports which suggest impact of COVID19 on pregnancy *(28)-Chen et al 2020b, (29)-Schwartz and Graham 2020
Signs and symptoms of diabetes
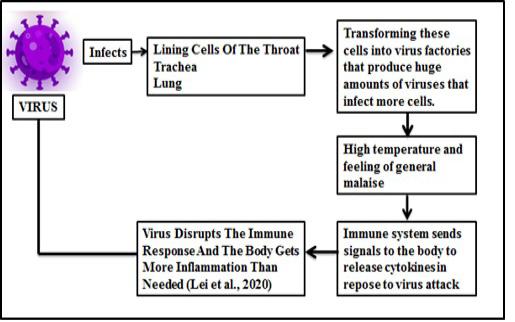
If someone has the nCoV19 infection, the first symptoms usually appear after five to six days, according to reports from the WHO (Phan et al., 2020). However, there are individual cases where the virus’ incubation period with a median incubation period of 14 days will last for up to 40 days (Zhang et al., 2020). The time of incubation for serious cases can vary from the mild cases and depend on the patient’s age and immune response. Among patients > 70 years (11.5 days) this period tended to be shorter than those aged < 70 years (20 days) (Zhang et al., 2020). COVID-19 symptoms are typically close to normal cold and influenza, and do not get serious. For people suffering from underlying diseases such as diabetes, heart, lung and other diseases, however, it is different. In this situation, the disease may take severe forms that sometimes lead to death. Some individuals do not experience any symptoms (mild pneumonia). According to a recent WHO study on over 70,000 cases in China, the most frequent symptoms of COVID-19 are: fever (in 88% of cases), dry cough and sore throat (68%), weakness (38%) and diarrhea (4%), similar to SARS-CoV and MERS-CoV (33,34.). In addition, extreme shortness of breath occurred in about 20 percent of cases, and about 13 percent had severe headache or sore throat. 19-nCoV is commonly a member of the family of coronaviruses, which typically target the respiratory system. Many patients are prone to various complications including acute respiratory distress syndrome, acute heart injury and secondary bacterial infection (Schwartz et al., 2020). Figure 5 and 6 highlight the recent symptoms and mechanism of immune system in to response the viral entry.
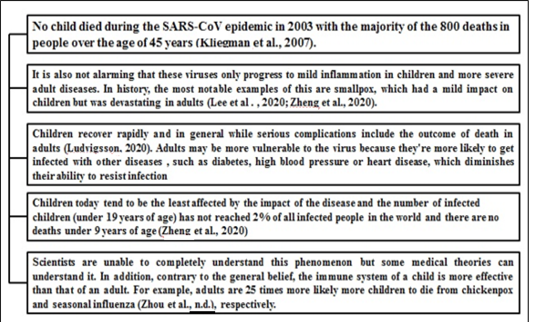
Figure 4: Reports which suggest impact of COVID19 on children *(30)-Kliengman et al 2007, (17)-Lee et al et al 2020, (31)-Zheng et al et al 2020 (32)-Ludvigsson et al et al 2020, (20)-Zhou et al et al 2020
Lung SARS pathology was connected with diffuse alveolar damage, proliferation of epithelial cells and a raise in macrophages. Multinucleate giant-cell infiltrates of macrophage or epithelial origin have been linked with putative syncytial development typical of many infections with coronavirus. Table 1 shows stepwise progression of the symptoms in human.
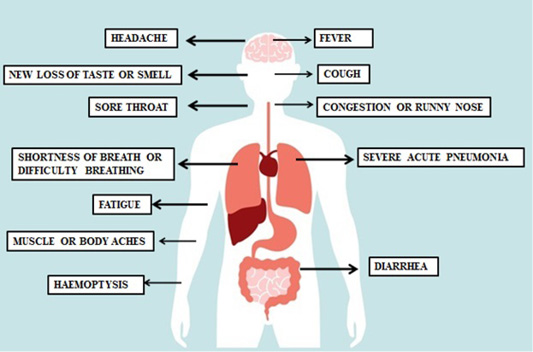
Figure 5: Symptoms of coronavirus-an update
Cases overview
The first two patients, who died in Wuhan, appeared to be in good health although they had been smoking for a long time and this resulted in their lungs weakening. On arrival at hospital, the first 61-year-old patient had acute pneumonia. Despite the use of a respirator, he had acute respiratory distress syndrome, his lungs failed and his heart just stopped.
He died after being hospitalized for 11 days. A 69-year-old man, the second patient, had acute respiratory distress syndrome. He died of a septicemia and acute pneumonia (Tarek et al., 2020). The causes of these symptoms need to be further researched.
Testings done for covid19
Many pharmaceutical companies and testing organizations are therefore rushing to develop more reliable and rapid test kits to detect 19-nCoV among people suspected of being contaminated in only a few minutes and without the need to send samples to central laboratories. The ELISA -- enzyme-linked immunosorbent assay -- antibody test is a kind of blood test. These are similar to rapid antibody tests, which detect antibodies in the blood to identify the infected individual with in case of COVID-19. Through
Table 1: Step wise progression of the symptoms in human
| S. No. | First step | Second step | Third step |
| Oxygen flows to the blood within the lungs but in the case of serious pneumonia | The alveoli tend to fill with water which may cause shortness of breath. | Cough with sputum(a thick mucus that contains lung cells killed by the 19-nCoV) | |
| nCoV19 infecting digestive system | Appetite loss, diarrhea, vomiting and major disorders of the digestive system. | Destroys the gastrointestinal bacteria (Fan et al 2020). | |
| High expression of ACE2 found in the lungs membrane,, kidneys cells and seminiferous ductal cells of the testis. | Affect renal tubular cells and testicular cells | nCoV19 binds directly to such ACE2 bearing cells and deteriorates the kidney and testicular tissue of patients. (Huang et al, 2020). |
latest updates it has been known to us that “National Institute of Virology, Pune, has successfully developed the 1st indigenous anti-SARS-CoV-2 human IgG ELISA test kit for antibody detection of COVID-19. This reliable test will be critical in monitoring proportion of the population exposed to SARSCoV2 infection. The test kit is named as “COVID KAVACH ELISA” and its first batch released by Zydus-Cadila is approved by Indian Council of Medical Research (ICMR).This kit will be helpful in “SERO-SURVEY”, which means it could detect prevalence of antibodies that appear after the patient has recovered.
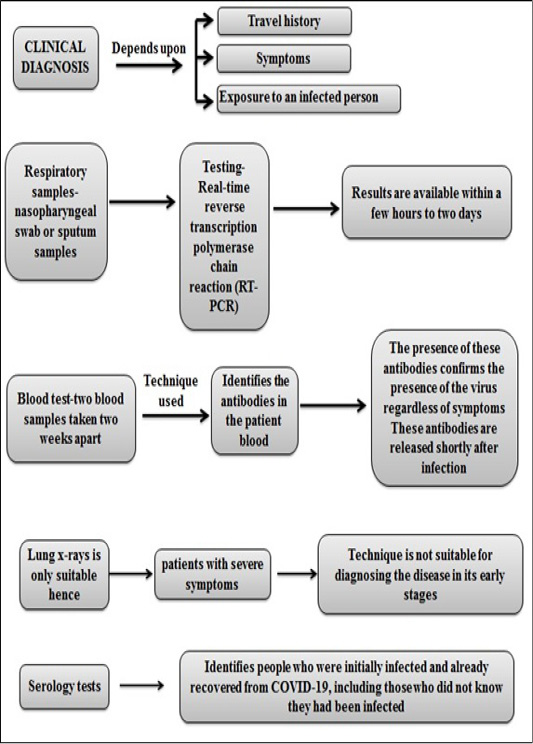
According to researchers the SARS-CoV-2 viral antigens produce antibodies via stimulating the body’s immune system which can be detected with IgM and IgG antibody tests. IgM is produced during early onset of the disease and is detectable. Later IgG is produced which is maintained for long-term immunity. ERBA Mannheim’s kit, called ErbaLisa® COVID-19 ELISA kits, can detect both antibodies (Mousavizadeh et al., 2020). Clinical diagnosis of COVID-19 is illustrated in Figure 7.
Mechanisms of viral pathogenicity
Lymphopenia and sustained inflammation have been found to be dominating in majority of cases of severe disease and death.
The biological mechanisms behind the epidemiological anomaly of severe acute respiratory syndrome (SARS) and COVID-19 is observed somewhat similar (Lei et al., 2020). Some comorbidities related to the disease are mentioned in Figure 8.
Overview of therapeutics and available treatment
Currently there are no different COVID-19 vaccines or drugs available at this time. Treatments are being explored, and will be examined in clinical trials.
Trials done through plasma treatment
The FDA approves the utilization of blood plasma from persons who have recovered from COVID-19 with a high neutralizing antibody titer, which could be a reliable donor source of convalescent plasma (CP) (Cheng et al., 2005). Clinical and vaccine trials have been summarized in Table 2 and Table 3.
CP is a classic adaptive immunotherapy, implemented for more than a century to the management and cure of many infectious diseases. This plasma therapy can be more successful if COVID-19 patients are administered early to remove the virus before it does significant damage to their lungs.
Table 2: Clinical trials done for COVID19
| S.NO. | Drug | Efficacy | Clinical trials for COVID19 | Ref |
| 1. |
Favilavir (Developed by Toyama Chemical) |
An antiviral drug approved byJapan in 2014 to treat influenza. |
To treat nose and throat infections. (But not currently approved by the U.S. Food and Drug Administration (FDA) (Li and De Clercq, 2020) |
(Holshue et al 2020) |
| 2. | Remdesivir |
Developed by Gilead Sciences to target Ebola.
Showed efficacy by resisting two viruses similar to Covid-19, SARS-CoV and MERS-CoV, in animals (Wang et al., 2020b)
It terminates RNA transcription thereby inhibiting viral replication (Li et al., n.d.). |
On January 19, 2020, remdesiver was given to a 35-year-old man in Washington State who has recovered from COVID-19 (Holshue et al., 2020).
However, the efficacy and safety of this drug still needs validation in patients with 2019-through further clinical studies. |
(Dong et al,2020)
|
| 3. |
Chloroquine and Hydroxy- chloroquine |
It is used to treat malaria, chemoprophylaxis and certain inflammatory conditions which include rheumatoid arthritis, lupus and the blood disorder porphyria cutanea tarda |
Approved by the FDA to be tested against COVID-19
In vitro activity against SARS-CoV and SARS-CoV-2 is seen for both the drugs (hydroxychloroquine has relatively higher potency against SARS-CoV-2)
Hence both these drugs are recommended for treatment of COVID-19 patients in several countries, including in the U S. A
In a chinese study showed that when chloroquine was tested on more than 100 patients, it showed superior results to control drug inhibiting pneumonia, improving lung-imaging, promoting a virus negative conversion and shortening the disease course.
Side effects - worsening vision, nausea, digestive disorders and heart failure.
*A man in Arizona died and his wife was in critical condition after taking chloroquine prophylactically to prevent SARS-CoV-2 infection. |
(Gao et al, 2020)
(Cao et al 2020) |
| 4 |
Lopinavir/ritonavir (anti-retroviral drugs) |
Sold under the name Kaletra by AbbVie and are designed to treat HIV (AIDS). |
99 positive patients were treated
No benefit was observed compare to standard care
In South Korea, a 54-year-old man showed significant and substantial decrease in the levels of the β-coronavirus
According to the WHO, there may be of using it with other drugs such as interferon-β, oseltamivir or ribavirin
|
(Lim et al 2020) (Elfiky et al 2020)
(Caly et al, 2020)
|
| 5 | Tocilizumab | Marketed as Actemra, used to treat patients with moderate to severe rheumatoid arthritis to lower inflammation. |
An initial clinical trial was done in China on 20 acute COVID-19 patients. Nineteen patients (95%) were cured and discharged from hospital within two weeks.
The FDA has officially approved its phase 3 trial of in severe COVID-19 patients. |
|
| 6 | Darunavir (anti-retroviral HIV-1 protease inhibitor) | In February 2020, it significantly inhibited SARS-CoV-2 replication and its inhibition efficacy was 280-fold more than that in the untreated group | ||
| 7 | Ivermectin (anti-parasitic drug) |
Effective against the SARS-CoV-2 virus as studied by researchers in an in-vitro at Monash University in Melbourne, Australia. Although, clinical trials are still needed to confirm the effectiveness of the drug in humans with COVID-19 |
(Chen et al, 2005)
|
Table 3: Draft Landscape of Candidate Vaccines
| S.NO. | Company name | Production | Mechanism for COVID19 | Ref |
| 1. | Entos Pharmaceuticals |
DNA vaccine (Fusogenix naomedicine) proteo-lipid vehicle (PLV) |
It contains plasmid DNA encoding multiple antigens from key SARS-CoV-2 proteins to promote maximum protection. |
|
| 2. | Airway Therapeutics |
AT-100 human surfactant protein-D (rhSP-D) |
Effective in reducing inflammation and infection in the lungs in preclinical trials. Generating an immune response against various respiratory diseases |
|
|
3. |
Canada's Medicago | Virus-Like Particle (VLP) | Stimulates cellular immunity with the aim of responding to the virus without the risk of infection | |
| 4 | Biopharma |
I-Mab (TJM2, a neutralizing antibody) |
Used in treatment for cytokine release syndrome common in patients suffering from a severe infection of SARS-CoV-2 TJM2 targets the human granulocyte-macrophage colony stimulating factor (GM-CSF), responsible for acute and chronic inflammation. |
|
| 5 | Tiziana Life Sciences |
TZLS-501 human anti-interleukin-6 receptor antibody |
Prevents lung damage and raised levels of IL-6 during the disease. Through various tests it is depicted that this treatment rapidly depletes circulating levels of IL-6 in the blood | |
| 6 | The University of Oxford's Jenner Institute and Italian pharmaceutical manufacturer Advent Srl |
ChAdOx1 (viral vector) |
Attenuated adenovirus which is capable of producing the spike (S) protein of SARS-CoV-2, which allows formation of endogenous antibodies against these proteins and, consequently, against SARS-CoV-2 (Alharbi et al., 2017). |
|
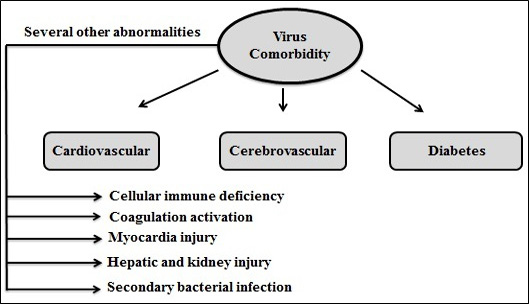
Figure 8: Comorbidities related to COVID19
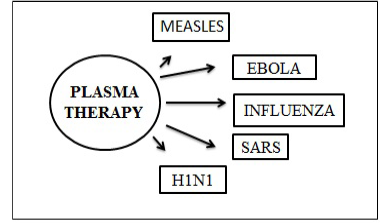
Figure 9: Plasma treatment used for earlier epidemics (Van Griensven et al., 2016; Cheng et al., 2005)
During the SARS epidemic in 2003, a research conducted on 80 patients in Hong Kong revealed that patients given CP therapy, recovered within two weeks of symptoms following as compared to other patients (Jawhara et al., 2020).
After studies conducted in China to measure the effectiveness of CP therapy New York hospitals also used the plasma blood as a potential antidote to the disease (MacLenam et al., 2006).
However, a small risk is associated with the use of CP therapy as it could also transmit blood-borne pathogen which includes human immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV) (51).
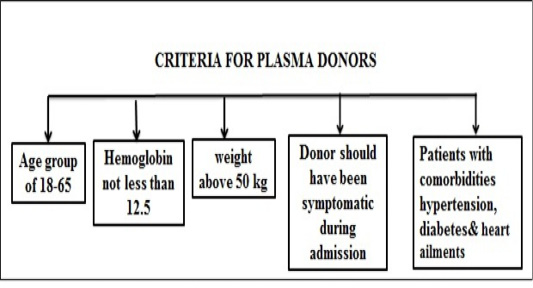
Figure 9 and 10 illustrate plasma treatment used earlier for epidemics and standardized guidelines for plasma donor.
Vaccine trials
Coronavirus (Covid-19) vaccine latest update: with the production of a secure, reliable and accessible Covid-19 vaccine as the cornerstone to controlling the pandemic, research and testing are rising at a rapid pace around the world to find a cure for the current coronavirus. In current events, US firm Moderna Inc, one of the front leaders in the vaccine race, has said it had begun dosing patients in a mid-stage study while Russian scientists intend to launch clinical trials within two weeks. There are about 120 vaccines at work worldwide, of which at least 10 are undergoing clinical trials, even as cases around the globe crossed the 61 lakh mark, including 372,566 deaths. Large pharmaceutical companies such as Gilead Sciences, Pfizer, Moderna, among others, are racing to find a successful vaccine. In India, Ramdev’s Patanjali has also joined the race to develop coronavirus vaccine.
China’s CanSino adenovirus vaccine, Oxford University’s adenovirus vaccine, Moderna’s mRNA vaccine and Novavax have surfaced as the most hopeful Covid-19 vaccine candidates to date (Crouch et al., 2000).
A Chinese vaccine, co - developed by Beijing Institute of Biological Products and China National Biotec Group Co, has finished Phase II evaluation and may be ready for market in late end of this year or slightly earlier next year, according to a report issued by the State-owned Assets Supervision and Administration Commission (Crouch et al., 2000).
Another Chinese biopharmaceutical company Sinovac Biotech, dubbed CoronaVac, has stuck hope on its inactivated vaccine and said it is 99 percent sure of its effectiveness. According to a report from Sky News, Sinovac researcher Luo Baishan said, “It has to be successful ... 99 per cent sure (Crouch et al., 2000).
US Company Moderna Inc. reported recently that it had started dosing patients with its experimental coronavirus vaccine in a mid-stage study and ultimately plans to enroll 600 patients in the trial. The step suggests that the firm’s mRNA vaccine passed its initial safety tests. Reuters announced that Moderna plans to start late-stage trials in July (Crouch et al., 2000).
Russia, which has the third-highest Covid-19 toll in the world after the United States and Brazil, has stated that it plans to set up clinical trials on a vaccine within two weeks. In addition, authorities also accepted the country’s first anti-Covid-19 drug, according to a Reuters report (Crouch et al., 2000).
The US pharmaceutical giant Pfizer has said that a Covid-19 vaccine may be ready by the end of October 2020, that is being developed in partnership with the German firm Biontech (Crouch et al., 2000).
Discussion
Viral diseases continue to arise, and pose a significant public health problem, according to the World Health Organization (WHO). Several viral epidemics, such as the extreme acute respiratory coronavirus syndrome (SARS-CoV) in 2002 to 2003 and H1N1 influenza in 2009, have been reported in the last twenty years. Recently the Middle East coronavirus respiratory syndrome (MERS-CoV) was first reported in 2012 in Saudi Arabia. An outbreak of cases of unidentified low respiratory infections found in Wuhan, the largest metropolitan area in China’s Hubei province, was first identified to China’s WHO Country Office on December 31, 2019, in a timeline that reaches the present day. Available literature can date the emergence of symptomatic people back to early December 2019. These first cases were labeled as “pneumonia of unknown etiology” because they were unable to classify the causative agent. An expansive outbreak response plan was initiated by the Chinese Center for Disease Control and Prevention (CDC) and local CDCs. The etiology of this disease was ascribed to a novel coronavirus (CoV) family virus. The new virus tends to be very infectious, and has spread rapidly worldwide. The epidemic was declared a Public Health Emergency of International Concern (PHEIC) by the WHO at a meeting on 30 January 2020 according to the International Health Regulations (IHR, 2005), as it had spread to 18 countries with four countries reporting human-to - human transmission. An additional milestone occurred on 26 February 2020, when the first case of the disease was reported in the United States (US), not imported from China. The CoVs have been the main pathogens of new outbreaks of respiratory illness. These viruses can cross species boundaries for reasons yet to be established, and can cause disease ranging from common cold to more serious diseases such as MERS and SARS in humans. Interestingly, these latter viruses originally originated in bats and then spread to other mammalian hosts — the SARS-CoV Himalayan palm civet, and the MERS-CoV dromedary camel — before moving to humans. SARS-Cov-2 dynamics are currently unclear, although there is evidence that it is also of animal origin. World authorities are trying to put in place countermeasures to prevent their catastrophic consequences. Health organizations are organizing knowledge flows and issues guidelines and recommendations to help minimize the effects. Around the same time, scientists around the world are working relentlessly, and knowledge about the mechanisms of transmission, the clinical spectrum of illness, new treatments, and preventive and therapeutic approaches are evolving rapidly. There are still several uncertainties with regard to both the virus-host relationship and the evolution of the pandemic, with specific reference to the times when it would hit its peak. The therapeutic approaches for coping with the infection are currently only supportive, and prevention aimed at minimizing social transmission is our best tool. Aggressive efforts to isolate China have led to a gradual decline in incidents. In Italy, in northern regional areas, initially, and progressively around the peninsula, political and health authorities are making extraordinary efforts to control a shock wave that is seriously challenging the health care system.
Conclusion
There’s no particular treatment currently available for COVID-19. Given the high rate of human-to-pandemic transmission of this virus, it is important to identify the basis of its replication, structure, and pathogenicity for discovering a way to specific treatment or prevention. Because of the virus’ high similarity to its families, efforts have been made to provide COVID-19 drugs and vaccines. Differences in spike length as is longer in COVID-19 are likely to play a significant role in pathogenesis and treatment of this virus. Identifying the specific molecular details of the virus is however helpful in achieving treatment objectives.
Acknowledgements
We would like to acknowledge all the authors for their support and contributions in this study.
Conflict of interest
There is no conflict of interest.
authors contribution
SS designed and planned the research. NRG has written the manuscript. STR revised the manuscript and RS,SS and PS collected the data samples for the study.
References