South Asian Journal of Life Sciences
Research Article
Occurrence and Antimicrobial Susceptibility Patterns of Escherichia coli from Processed Water in Gwagwalada, Nigeria
Samuel Mailafia2, Emelogu Chukwudi3, Iliya Dauda Kwoji1, Jasini Athanda Musa1, Mustapha Bala Abubakar1
1Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria; 2Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Abuja, Nigeria; 3Department of Microbiology, University of Abuja, Nigeria.
Abstract |This study was carried out to determine the occurrence and In-vitro antibiotic susceptibility patterns of E. coli from processed water consumed by humans in Gwagwalada, Federal Capital Territory, Abuja. A total of 240 samples; consisting of 60 bottled water, 150 sachet water and 30 tap water were randomly collected bi-monthly for six months. The samples were analyzed for the presence of E. coli using culture, isolation and complete biochemical characterization to further confirm the isolates. The findings of this study showed an overall prevalence rate of 5.0%. However, E. coli were not isolated from bottled and sachet water samples. An In-vitro antibiotic sensitivity test conducted, indicated that all isolates had 100% susceptibility to gentamicin and 100% resistance to penicillin, flucloxacillin and ampicillin. The percentage susceptibilities to other antimicrobial agents were 83.3 for tetracycline, 50.0 for co-trimoxazole, 16.7 for cefuroxime and 8.3 for erythromycin. This study has clearly showed that E. coli isolates from tap water samples could contain pathogenic strains which may be relevant in the pathogenicity of colibacillosis. The varying degrees of susceptibility displayed by antimicrobial agents could be attributed to the use and misuse of antibiotics in the study area. This study has demonstrated the occurrence and antimicrobial susceptibility patterns of E. coli from processed water in Gwagwalada, Federal Capital Territory, Abuja. It is worth noting that the presence of E. coli as a food-borne pathogen in processed water in Gwagwalada area council serves as a public health threat to humans who may consume such water. It is therefore recommended that immediate screening of emerging E. coli strains be performed prior to therapy. Similarly, the presence of drug resistant E. coli in tap water samples in areas covered by this study suggests the need for public health intervention particularly from Federal Capital Development Authority (FCDA), Abuja.
Keywords: Antimicrobial resistance, Escherichia coli, Gwagwalada, Water
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | November 29, 2018 Accepted | February 22, 2019; Published | July 14, 2019
*Correspondence | Iliya Kwoji, Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria; Email: [email protected]
Citation | Mailafia S, Chukwudi E, Kwoji ID, Musa JA, Abubakar MB (2019). Occurrence and antimicrobial susceptibility patterns of escherichia coli from processed water in gwagwalada, nigeria. S. Asian J. Life Sci. 7(2): 40-45.
DOI | http://dx.doi.org/10.17582/journal.sajls/2019/7.2.40.45
ISSN | 2311–0589
Copyright © 2019 Kwoji et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Water for domestic uses comes from surface water (such as rivers, lakes and reservoirs) and ground water which is pumped from wells or boreholes (Thani et al., 2016). Groundwater has been recognised as the most abundant and most important source of portable water for human use around the globe (WHO, 2015). In Africa, many households in rural communities still depend on rivers and boreholes as water sources for general use and personal hygiene (Palamuleni et al., 2015). However, due to limited resources, poor sanitation and disposal of human and animal wastes into water bodies leads to pollution thereby making the availability of safe water almost unattainable in developing countries (Paulse et al., 2012). According to Mwanamoki et al. (2014), these pollutants could include chemicals such as pharmaceuticals and pathogenic microorganisms (bacteria, viruses and parasites) which have been found to cause numerous waterborne diseases in humans and animals that use such untreated water from polluted sources. Like other water sources, processed water such as bottled water, sachet water, tap water and dispensed water may also be contaminated if there is a break in hygiene during processing. Esherichia coli is one of the coliforms that are readily present in faecal contamination originating from man and other warm-blooded animals (Doughari et al., 2011; Musa et al., 2016). Contamination of water by E. coli could be due to poor handling, processing and packaging during production (Adzitey et al., 2015). Additionally, the presence of antibiotic resistant bacteria in drinking water is becoming an emerging problem of global concern (Akturk et al., 2012). This is because there is an increased in the prevalence of antimicrobial resistant pathogens over the years, thus, making the dissemination of these organisms and their resistance gene more frequently to humans and animals more especially if present in water (Parvez et al., 2016). This situation is common in areas where antimicrobials are frequently used without strict legislations especially in developing countries like Nigeria (Mailafia, 2009; Geidam et al., 2012b; Kwoji et al., 2018). Thus, this study was carried out to determine the occurrence and antimicrobial susceptibility patterns of E. coli from processed water in Gwagwalada area council. The findings of this study will provide a baseline data on E. coli from water in the study area which contribute to the information on the epidemiology of the pathogen and device control strategies to curtail this food borne pathogen in the events of disease outbreaks.
MATERIALS AND METHODS
Study Area
This study was carried out in Gwagwalada, Abuja. Gwagwalada is one of the six area councils of the Federal Capital Territory (FCT) created on 15th October, 1984. It has an area of 1,043km2 and estimated population of 157,770 based on the 2006 census (FCT Baseline Data, 2010). Gwagwalada falls within the Guinean forest-region and is endowed with a mix agricultural produce such as tuber and root crops (like yam, cassava, potatoes) and cereal grains (such as maize, sorghum, millet, guinea corn and rice). Gwagwalada under koppen’s climate classification features a tropical wet and dry climate. It is characterized by two weather conditions annually which include warm humid-rainy and dry seasons. In between the two, there is brief interlude of harmattan caused by the northeast trade wind characterized by dusty haze, adverse coldness and dryness. The rainy season starts from April and ends in October when daytime temperature reaches 28oC (82.4oF) to 30oC (86.0oF) and night time decrease to above 22oC (71.6oF) to 23oC (73.4oF). Rainfall in Gwagwalada area reflects the location on the windward side of Jos Plateau and the zone of rising air masses. Due to the hilly and mountainous nature of Gwagwalada area, orographic activities bring heavy and frequent rainfall of about 1,500 mm (59.1in) during the rainy season. Sources of water in Gwagwalada include; processed water (dispensed, bottled, tap and sachet water), boreholes, hand pumps, rainfall, open wells and streams. 66% of water consumed in Gwagwalada is tap water supplied by FCT Water Board. However, most communities in Gwagwalada Area Council source 34% of their water supply from rainfall, open wells and streams (FCT Baseline Data, 2010).
Study Design
This was a cross-sectional study carried out in Gwagwalada area council, FCT Abuja. Based on the number of samples, locations and different types of potable water (bottled, sachet and tap water) subjected to bacteriological analysis, a random complete block design was adopted in this study. In this experimental plan, water samples were collected at random in triplicate determinations from each sample locations for a period of six months based on bi-monthly collections (Tagoe et al., 2011).
Samples Collection
Twenty different brands of bottled water and fifty different brands of sachet water were collected from stores on the streets of Gwagwalada. The tap water samples were collected at ten different locations in Gwagwalada area council. The samples were repeatedly collected bi-monthly to ensure that different batches were selected for a period of six months. Laboratory analysis was carried out at the Microbiology laboratory, Department of Microbiology, University of Abuja, Nigeria. The study was conducted from August, 2014 to April, 2015.
Isolation and Identification of E. coli
The bacteriological media used for this study were all prepared according to manufacturer’s instructions. Water samples were first filtered using membrane filter, the filter paper was then inoculated into MacConkey broth (Fluka Biochemical, Germany) and incubated at 37oC for 24 hours. A loopful of the culture from MacConkey broth was streaked onto freshly prepared Eosin methylene blue agar plates (EMB, Oxoid, SA) and further incubated at 37oC for 24 hours. Colonies with green metallic sheen colouration on EMB agar were further purified by picking discrete colonies and sub-cultured onto fresh plates of EMB agar and incubated at 37oC for 24 hours. Colonies that had green metallic sheen on EMB agar were characterized microscopically and biochemically using Indole test, Methyl red – Voges Proskeaur test, Citrate test and sugar fermentation (Ngwai et al., 2010; Doughari et al., 2011).
Antimicrobial Susceptibility Test
The antimicrobial susceptibility test was carried out using Kirby-Bauer disc diffusion method (CLSI, 2016). Pure E. coli isolates were sub-cultured onto nutrient agar slant and incubated for 24 hours at 37oC. This fresh 24 hours cultures where then transferred to tubes containing Mueller-Hinton broth (Fluka Biochemical, Germany) and incubated at 37oC for 24 hours. The bacterial suspension was adjusted using sterile saline. The surfaces of Mueller Hinton agar (Fluka Biochemical, Germany) were streaked with the adjusted suspension and allowed to dry for five minutes. A panel of antibiotic discs (Biotech Lab, United Kingdom) containing eight different commonly used antibiotics namely: ampicillin (AMP 10 µg), tetracycline (TET 10 µg), gentamicin (GEN 10 µg), co-trimoxazole (COT 25 µg), cefuroxime (CRX 30 µg), penicillin (PEN 15 µg), flucloxacillin (FLX 5 µg) and erythromycin (ERY 5 µg) were placed on the inoculated agar surfaces, allowed for 15 minutes pre-diffusion and incubated in inverted position at 37oC for 24 hours. The zones of inhibition were measured using a transparent ruler and recorded. A control organism, E. coli ATCC 25922 was used to confirm the accuracy of the antibiotic sensitivity test. E. coli isolates were classified as resistant, intermediate or susceptible using the interpretation criteria of CLSI (CLSI, 2016).
Statistical Analyses
The data obtained from this study was summarized as percentages in the form of tables and chart. A chi-square analysis was carried out to determine the statistical difference in the occurrence of E. coli in water from the different sample sources using GraphPad Instat®. In line with the experimental plan used in this study, all the data were analyzed using one-way analysis of variance to determine the differences between mean values of the three types of water samples obtained from 10 different locations (Tagoe et al., 2011).
RESULTS
Table 1 shows the occurrence of E. coli from different water samples during the three sampling periods. An overall occurrence of 5.0% was observed with highest occurrence of 90.0% from well water samples (positive control), followed by tap water samples (40.0%). However, the occurrence of E. coli was 0.0% from bottled and sachet water samples collected during these sampling periods. Results of the biochemical tests conducted on the 12 E. coli isolates showed that the isolates were negative for Gram’s reaction, Voges Proskauer, citrate, oxidase, gelatin liquefaction, triple sugar iron and deoxyribonuclease tests but were positive to indole, methyl red, catalase and motility tests.
Table 2 shows the antimicrobial susceptibility of E. coli from water to different antimicrobial agents. All (100%) the E. coli isolates were susceptible to gentamicin but resistant to ampicillin, flucloxacillin and penicillin (0% susceptibility). The overall percentage susceptibilities of E. coli isolates to different antimicrobial agents tested were: gentamicin (100.0), tetracycline (83.3), co-trimoxazole (50.0), cefuroxime (16.7) erythromycin (8.3), penicillin (0), flucloxacillin (0) and ampicillin (0). Furthermore, the percentage resistances of the E. coli isolates were: ampicillin (100.0), flucloxacillin (100.0), penicillin (100.0), erythromycin (91.7), cefuroxime (83.3), co-trimoxazole (50.0), tetracycline (16.7), and gentamicin (0).
Table 1: Occurrence of E. coli Isolates from Water Samples
| S/No. | Water samples | No. of samples collected | No. (%) positive samples |
| 1 | Bottled | 60 | 0 (0) |
| 2 | Sachet | 150 | 0 (0) |
| 3 | Tap | 30 | 12 (40.0) |
| Total | 240 | 12 (5.0) |
P<0.0001
Table 2: Antimicrobial Susceptibility of E. coli Isolates to Antibiotics
| Antibiotics | Disc Content (µg) | Number (%) of E. coli susceptible to antibiotics (n=12) | Number (%) of E. coli resistant to antibiotics (n=12) |
| Gentamicin | 10 | 12 (100) | 0 (0) |
| Tetracycline | 10 | 10 (83.3) | 2 (16.7) |
| Co-trimoxazole | 25 | 6 (50.0) | 6 (50.0) |
| Cefuroxime | 30 | 2 (16.7) | 10 (83.3) |
| Erythromycin | 5 | 1 (8.3) | 11 (91.7) |
| Penicillin | 15 | 0 (0) | 12 (100) |
| Flucloxacillin | 5 | 0 (0) | 12 (100) |
| Ampicillin | 10 | 0 (0) | 12 (100) |
Table 3: Antimicrobial Susceptibility Patterns of E. coli Isolates.
| S/No. | Antimicrobial reactivity | Antimicrobial reactivity patterns |
| 1 | Susceptibility | GEN, TET, COT, CRX, ERY, PEN, FLX, AMP. |
| 2 | Resistance | AMP, FLX, PEN, ERY, CRX, COT, TET, GEN. |
The antimicrobial susceptibility and resistance patterns of E. coli from processed water in the study area are shown on Table 3. The isolates showed highest susceptibility to gentamicin, while the highest resistance was observed against ampicillin. The antimicrobial susceptibility and resistance patterns of E. coli isolates to antibiotics were arranged in descending order of the percentages of susceptibilities and resistance, respectively.
Figure 1 is a graph showing the relationship between percentage susceptibilities and percentage resistance. The percentage susceptibilities were inversely proportional to percentage resistance. The graph has equilibrium at 50% susceptibility and 50% resistance. At 50%, percentage susceptibility is equal to percentage resistance.
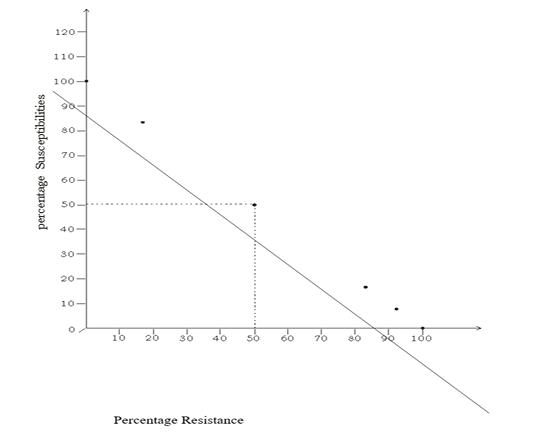
Figure 1: Graph Showing the Relationship between Susceptibilities and Resistance (Percentage Reactivity)
DISCUSSION
The growing demand for water for industrial, agricultural, environmental, municipal and domestic requirements has extended the requirements for an improvement in water treatment processes (Levantesi et al., 2010). In developing countries, due to deteriorating environments, the demand for clean drinking water supply is growing rapidly in recent times (Ibrahimagic et al., 2016). The presence of coliform bacteria and E. coli in water is an indicator of recent faecal contamination indicating the possible presence of disease-causing pathogens, such as bacteria, viruses, and parasites (WISC, 2013).
In this study, an overall occurrence of 5.0% of E. coli was observed in water samples analyzed from the study area. This finding is similar to previous reports in Bosnia, 5.5% (Ibrahimagic et al., 2015), Netherlands 7.4% (Hejinen et al., 2006), and Iran, 10% (Momtaz et al., 2013), but lower than the reports from Pakistan, 35.6% (Ahmad et al., 2009) and South Africa, 75% (Momba et al., 2006). The low prevalence of E. coli obtained from this study is in contrast with some previous reports that suggested E. coli as the most frequently isolated bacteria from processed water (Ezeugwunne et al., 2000; Doughari et al., 2011). E. coli was not isolated from sachet and bottled water samples in this study. However, an occurrence of 40% of E. coli from tap water was observed. The findings of this study are similar to the findings of Adzitey et al. (2015) who also reported 0% and 12% occurrences of E. coli in sachet and tap water, respectively. A similar occurrence of E. coli in drinking water was also reported by Garba et al. (2009) in a study conducted in Gusau, North-western Nigeria. The absence of E. coli from sachet and bottled water in this study could be due to adequate filtration, good hygiene, sterilization by UV light and addition of chlorine that is involved in water treatment plants during processing. Similarly, the presence of E. coli in tap water may be as a result of poor treatment, use of hose which is normally left on the ground before and after usage without prior cleaning, or the distribution of purified water through leaky pipes. The result of this study is in contrast to that of Ezeugwunne et al. (2000), who reported 36% occurrence of E. coli in sachet water from Nnewi, South-eastern Nigeria. The absence of E. coli in both bottled and sachet water samples was in contrast to previous studies reported by Ezeugwunne et al. (2009), Oyedeji et al. (2010) and Tagoe et al. (2011) in which E. coli was frequently isolated. The World Health Organization recommends the use of E. coli for the evaluation of microbial quality of water, and recommends that E. coli should not be found in any water meant for human consumption (WHO, 2008). Therefore, the tap water may not be said to have meet World Health Organization (WHO) standards. The international standard for drinking water stipulated that E. coli or total coliform bacteria must not be detected in any 100 ml samples (WHO, 2008). The presence of E. coli in processed water is a significant public health issue because E. coli can cause water borne infections.
The indiscriminate usage of antibiotics is recognized as a significant contributing factor to the development and spread of antibiotic resistance among bacteria (Kwoji et al., 2018; Ngwai et al., 2010). The 0% susceptibility of E. coli to ampicillin, penicillin and flucloxacillin in this study, conformed to the studies reported by Tagoe et al. (2011) in which the same result was obtained in bacterial isolates from drinking water sold in Cape Coast, Ghana. Previous studies by Ngwai et al. (2010), Shahriah et al. (2010) and Onyuka et al. (2011) reported low susceptibility of E. coli isolates to ampicillin. The high resistance observed in this study to ampicillin, penicillin and flucloxacillin, could be due to their free access, misuse and abuse. E. coli isolates displayed low susceptibility to some common antibiotics such as erythromycin and cefuroxime. Conversely, high E. coli susceptibility to gentamicin, tetracycline and co-trimoxazole encountered in this work agrees with the findings of Tagoe et al. (2011) who observed a high susceptibility of E. coli to gentamicin, tetracycline and co-trimoxazole while studying the antibiotic susceptibility pattern of bacterial isolates in sachet drinking water sold in the Cape Coast, Ghana. The general high resistance of E. coli to common antibiotics observed in this study might be attributed to possible abuse and misuse of antibiotics in Gwagwalada metropolis.
Conclusion
The study established the presence of E. coli in tap water and its absence from bottled and sachet water from Gwagwalada area council, Abuja. Additionally, antibiotic susceptibility studies of the isolates against commonly used antimicrobials revealed a varying degree of sensitivity and resistance of the isolates.
acknowledgements
The authors dully acknowledges all the laboratory staff of the Department of Microbiology, Faculty of Science, University of Abuja, Nigeria for their technical efforts towards the achievement of this work
Conflict of Interests
The authors declare that there is no conflict of interests whatsoever.
authors contribution
Mailafiya Samuel and Emelogu Chukwudi conceived the study and study design, Iliya Dauda Kwoji, Jasini Athanda Musa and Mustapha Bala Abubakar analyze data, wrote and edited the manuscript. All the authors were involved in revising and final approval of the manuscript.
REFERENCES






