Advances in Animal and Veterinary Sciences
Research Article
Assuring the Safety of Local and Imported Beef Meat from Different Slaughterhouses in Egypt
Hanan Korany Sayed1, Khalid Shawky Tolba2, Hassan Mohamed Sobhy3, Sahar Hussein Abdalla Hekal3*
1General Organization of Vet. Services – Ministry of Agriculture; 2Chief Researcher of Food Hygiene and Safety in Reference lab for Food Safety, Animal Health Research Institute, Dokki – ARC; 3Department of Natural Resources, Faculty of African Postgraduate Studies - Cairo University, Giza, Egypt.
Abstract | The current study was applied to investigate E. coli and Staph. aureus counts as well as serodiagnosis of E. coli, Salmonellae isolates and determination of enterotoxin production by Staph. aureus in 100 random local and imported fresh beef samples, from different slaughterhouses at Cairo and Giza Governorates. Staph. aureus was isolated from 22(44%) and 20 (40%) of examined local and imported meat samples respectively. E. coli was found in 40 (80%) and 34(68%) in examined local and imported meat samples, respectively. Staph. aureus enterotoxin types (A & D) were detected in two strains out of 5 randomly selected isolates from local meat only (20% of each). E. coli, one strain (20%) was isolated from fresh local meat; and classified as Enteropathogenic (EPEC), while two strains (40%) were Enterohaemorrhagic (EHEC) “O111:H2”, one strain (20%) was Enterotoxigenic (ETEC) “O15:H4”. On the other hand, one strain of E. coli (20%) isolated from imported meat was classified as Enteropathogenic (EPEC) and its serodiagnosis was O128:H2, while one strains (20%) was Enterohaemorrhagic (EHEC) “O26:H11” which harbored both (stx1 and stx2) genes and finally, the 3rd strain (20%) was Enteroinvasive (EIEC) “O159”. Meanwhile, Salmonella spp. was isolated from two samples (4%) of examined local meat; one strain was identified as S. Montevideo of Group “C1” with antigenic structure (O6,7,14:H g,m,s:1,2,7) and the 2nd strain was S. enteritidis of group “D1” (O: 1,9,12:H g,m). While, imported meat recorded 1 strain (2%) of S. Typhimurium of group “B” (O:1,4,5,12 H: i:1,2). A concerted effort, Hazard Analysis and Critical Control Points (HACCP) should be made to improve sanitary condition to mitigate bacterial contamination in slaughterhouses.
Keywords | E. coli, Egypt, Salmonella, Staph. aureus, Local and imported beef meat
Received | May 14, 2021; Accepted | June 12, 2021; Published | July 28, 2021
*Correspondence | Sahar Hussein Abdalla Hekal, Department of Natural Resources, Faculty of African Postgraduate Studies, Cairo University, Giza, Egypt; Email: [email protected]
Citation | Sayed HK, Tolba KS, Sobhy HM, Hekal SHA (2021). Assuring the safety of local and imported beef meat from different slaughterhouses in egypt. Adv. Anim. Vet. Sci. 9(9): 1472-1482.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1472.1482
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hekal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Meat has high nutritive value for human consumption. On the other hand, meat is considered as an ideal growth media for many microorganisms because of the high moisture content, high percentage of nitrogenous compounds, good supply of minerals, glycogen and a favorable pH for most microorganisms (Al-Metairie, 2011). As a result of inappropriate environmental conditions, unhygienic slaughtering processes, bad storage and transportation of meat as well as bad handling and retailing may be resulted in its contamination with different food spoilage and food poisoning microorganisms. (Ercolini et al., 2006).
Egypt imports different types of meat to fill the gaps in the requirements of animal protein. (USDA, 2016). While the importation of beef meat in Egypt is crucial to close the gap in animal protein requirements, monitoring the frequency of antimicrobial resistance in imported meat must assure that quality and safety standards are met. The extent of microbial contamination and composition of microbial flora reflect the standard hygienic measures adopted during meat processing (Ko et al., 2013; Kim et al., 2016). Meat may be contaminated with various pathogenic microorganisms as E. coli, Salmonellae and Staph. aureus which renders meat constitute a hazard to human health (Nørrung et al., 2009). Moreover, some strains of E. coli could be able to causes several human foodborne illnesses ranging from gastrointestinal symptoms to sever bloody diarrhea and dysentery, also it considered the main cause of urinary tract complication represented by hemolytic uremic syndrome (HUS), pneumonia and meningitis (Johnson et al., 2006). Pathogenic E. coli strains have been broadly classified into two major categories; extraintestinal pathogenic and diarrheagenic E. coli which classified into six categories including Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC), Entero-invasive E. coli (EIEC), Enteroaggregative E. coli (EAEC), diffusively adherent E. coli (DAEC) and Entero-hemorrhagic E. coli (EHEC)/Shiga toxin-producing E. coli (STEC) (Monaghan et al., 2011; Clements et al., 2012).Salmonella has been considered as a significant foodborne disease and could be isolated from raw meat, poultry and their products. Cross contamination and unhygienic measures during food processing are considered a predisposing factor for contamination with Salmonella (Gorman et al., 2002). Staphylococcal foodborne intoxication occurs all over the world and caused by ingestion of already preformed Staph. aureus enterotoxins in food causing clinical signs as vomiting, diarrhea and even death in older people and children (Baumgartner et al., 2014).
The main sources of meat contamination occurred during slaughtering processes are hides and gastrointestinal contents of the slaughtered animals, the work environment. Also, carcasses can be contaminated during the slaughtering process through the contact with the animal’s skin, blood, hair, limbs, bile, equipment’s, water, air pollution and worker’s hands and clothes (Koffi-Nevry et al., 2011).
The circumstances at which the animal breed up, its health condition, slaughtered and carcass preparation are mostly affect the microbial load of the carcass in the abattoir. Firstly, carcass contamination takes place at the point of skinning and the following contamination occurs due to dust arises during removal of hide, workers hands or by contact between dirty hide and subcutaneous connective tissue (Zweifel et al., 2008). The degree of bacterial contamination of animal carcass differs according to plant sanitation. It is also affected by numerous factors as plant layout, rapidity of slaughtering processes and applying of good manufacturing and processing practices (GMP & GHP) as well as the skillfulness of the slaughtering plant workers and the presence of both un-skinned and skinned carcasses in the same area might be a source of meat contamination by many pathogenic agents (Alegre and Buncic, 2004; Hemmat et al., 2014).
Microbial examinations of slaughtered carcasses were not included through the routine of meat inspection in the slaughterhouses. (Muhammad et al., 2011; Bogere and Baluka, 2014).Therefore, the current study was applied to investigate E. coli and Staph. aureus counts as well as serodiagnosis of E. coli, Salmonellae isolates and determination of enterotoxin production by Staph. aureus in local and imported meat. Public health of isolated bacteria was discussed.
MATERIALS AND METHODS
Collection of samples
A total of 100 random fresh beef samples (locally produced and imported; 50 samples of each), were collected from different slaughterhouses in different Governorates; the local meat samples were from slaughter houses of El- Moneeb, El- Waraq and El- Basateen in Cairo and Giza governorates, while the imported meat samples were collected from El- Swiss slaughter house in Swiss canal governorate and Abu- Simble slaughter house in Aswan governorate. The collected samples were kept in its sailing bags and aseptically transferred without delay, in an insulated ice box to the laboratory without delay.
Preparation of sample homogenate
Twenty-five grams of the examined samples were aseptically transferred to a sterile stomacher bag and homogenized with 225 ml sterile buffered peptone water (0.1%) for 30-60 seconds to give an initial dilution of 1/10. Serial dilutions up to 106 was carried out according to APHA (2001). Prepared homogenate was used to carry out the following bacteriological examinations.
Bacteriological examination
Enumeration and Isolation of Staphylococcus aureus were done according to the technique recommended by FDA (2001).
Enumeration, Isolation and identification of B-glucuronidase-positive Escherichia coli according to (ISO 16649-2:2001).
Isolation of and identification of Salmonellae enterica according to (ISO 6579-1:2017).
Serological identification of Salmonellae according to Kauffman–White Scheme (Kauffman, 1974 for the determination of Somatic (O) and flagellar (H) antigens using Salmonella antiserum (DENKA SEIKEN Co., Japan).
Isolated Staphylococcus aureus strains were examined for their ability to produce different types of enterotoxins using Sac culture method and staphylococcal enterotoxin-Reversed Passive Latex Agglutination (SET-RPLA) test kit according to (Donnelly et al., 1967).
Serological identification of E. coli according to (Kok et al., 1996) using rapid diagnostic E. coli antisera sets (DENKA SEIKEN Co., Japan) for diagnosis of the Enteropathogenic types.
Statistical analysis
Was carried out using Statistical Package for the Social Sciences (SPSS). One Way ANOVA, Ver. 20.
Ethical statement
Ethical approval was not needed in this study as the samples were collected from slaughtered cattle from the abattoir. However, consent from the abattoir authorities was taken prior collection of samples.
RESULTS AND DISCUSSION
Beef meat have been considered as highly desirable, nutritious and good quality protein rich food, meanwhile, meat is highly perishable because they provide a nutrient required for growth and multiplication of different types of microorganisms. The hygienic practices applied during slaughtering and processing of animals is controlling the quality attribute of meat and its shelf life as the increase in microbial load and its content of food poisoning and food intoxication organisms shall constitute a public health hazard (Kalalou et al., 2004)
Staph. aureus and E. coli counts (log10cfu/g) in examined local and imported meat samples
It is evident from the results recorded in Table (1) that Staph. aureus mean value was 2.31±0.1 log10cfu/g and 2.07±0.09 log10cfu/g in examined samples of local and imported beef meat with 22(44%) and 20(40%) positive samples, respectively. Results achieved in Table (1) also showed that E. coli mean count was 3.15±0.13 log10cfu/g with incidence rate (40/80%) in local beef meat and 2.98±0.11 (34/68%) in examined imported beef meat. There were no significance differences (P>0.05) between both local and imported meat regarding Staph. aureus and E. coli. Nearly similar results were recorded by (Abdalrahman et al., 2015) who could isolate staph. aureus by 50% (23/46) of examined retailed beef cuts, they added that Staph. aureus is one of the top five pathogens contributing to acquired foodborne illnesses causing an estimated quarter million cases every year in the US. Many investigators recorded incidence of Staph. aureus in fresh meat (Wu et al., 2018) 63 samples/43.3%), (Ge et al., 2017) (27.9%) in the USA, (Tang et al., 2017) (68%) in Denmark. In addition, (Bakr et al., 2004; Kitai et al., 2005; Van Loo et al., 2007; Buyukcangaz et al., 2013; Jackson et al., 2013; Bunnoeng et al., 2014; Dhup et al., 2015) found that 4 – 76.47% of raw meat samples were contaminated with Staph. aureus. Such differences in prevalence of Staph. aureus may be attributed to the sample sizes, sample types, hygienic status and geographic locations and collection period of investigation (Ge et al., 2017).
In this respect, (Elsharawy et al,. 2018) examined 40 fresh meat samples from Elkharja municipal slaughterhouse, New Valley Governorate, Egypt and it was found that mean E. coli count recorded (2.47x103±15.54x10) which is nearly identical to the results of the current study, while the mean Staph aureus count (9.94x102±15.7x10) was slightly higher, they concluded that applied hygienic measures in Elkharja slaughterhouse were low and more governmental efforts are still needed to control the microbial contamination and improve the environmental quality and infrastructure of Elkharga slaughterhouse in New Valley, Egypt. Also, similar results for Staph. aureus count was recorded by (Saad et al., 2019) (4.03 x 102 ± 0.75x102 cfu/g). The incidence of E. coli in imported fresh beef meat came in accordance with (Salman et al., 2015) (72%) from 150 samples collected from Alkadaro slaughterhouse, Khartoum-Sudan. Lower prevalence of Staph. aureus (8 isolates/16%) and E. coli (4/8%) obtained by (Abd El tawab et al. 2020). (Abdalla et al. (2009) found that Staph. aureus and E. coli were isolated by 10.54% and 8.86%, respectively from bovine carcasses in Khartoum – Sudan during 2008. Higher Staph. aureus count was recorded by Hemmat et al. (2014) (28×103±5×103 cfu/g) of shoulder beef meat collected from two traditional abattoirs of Elbehira province, while the incidence of Staph. aureus (12 isolates /40%) came in accordance with the present study. In this issue, Higher prevalence of Staph. aureus in examined raw buffalo meat samples in Nepal were recorded by (Kamana et al., 2018) (80%) respectively, while lower results were recorded by the same authors for isolation rate of E. coli (40%). They concluded that no significant difference between the type of meat and the presence of Staph. aureus and E. coli (P > 0.05). This agreed with statistical analysis of the present study.
Also, (Bogere and Baluka, 2014) recorded higher E. coli and Staph. aureus counts (8.4×104 and 2.7×103 cfu/g) respectively, in fresh meat samples collected from some abattoirs in Uganda Also, higher results of mean E. coli and Staph. aureus counts in meat were also recorded by (Hughes et al., 2015) in Ghana (5.97 and 5.5 log10cfu/g), respectively.
Table 1: Statistical analytical results of Staph. aureus and E. coli counts (log10cfu/g) in examined local and imported meat samples
| Types of meat | Statistical analysis | |||||
| Staph. aureus | E. coli | |||||
| Count | Positive samples | Count | Positive samples | |||
| No | % | No | % | |||
| Local |
2.31a±0.1 |
22 | 44 |
3.15b±0.13 |
40 | 80 |
| Imported |
2.07a±0.09 |
20 | 40 |
2.98b±0.11 |
34 | 68 |
Statistical analysis expressed for positive samples only.
No significance difference (P>0.05) between means having the same small superscripted litter in the same column.
(FANZA , 2016) recorded 95.24% of beef meat samples had quality unsatisfactory quality due the presence of E. coli. (Philips et al., 2007) in Austria found that 17.8% of retailed beef meat contaminated with E. coli with mean count (1.49 log10cfu/g), while Staph. aureus contaminated samples were recorded 28.1%.
Staph. aureus enterotoxin production in local and imported meat
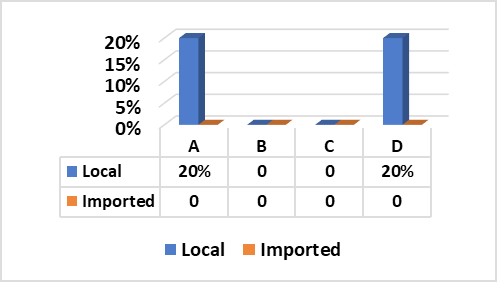
Fig. (1) Illustrated that Staph. aureus enterotoxins (SE) types “A” and “D” were successfully produced from two strains separately (20% for each) out of five tested strains obtained from local beef meat. In contrary, all random five Staph. aureus strains isolated from imported meat samples were negative for production of any type of enterotoxins.
These results go hand to hand with those recorded by (El-Shater-Nahla, 2010) (20% SEA and 26.8% SED) using SET-RPLA test kit, (Normanno et al., 2005) (26.5% SEA and 20.5% for SEA+SED) and (Normanno et al., 2007) (SEA, 18.4%). Furthermore, (Mathieu et al., 1991) found that SEA is the most common enterotoxin recovered from food-poisoning of all recorded outbreaks in the US (77.8%) followed by SED (37.5%) and SEB (10%). In addition to, Zagar et al. (2014) isolated 7 strains (14.8%) of Staph. aureus from examined beef meat samples, two samples (28.5%) found to contain enterotoxin type “A”. In Slovak republic, out of the 43 staphylococcal strains isolated from different foods, 15 strains (34.88%) were found to be enterotoxigenic and out of these 15 strains, seven (16.28%) contained enterotoxin “A” (Holeckova et al., 2002). Moreover, in 2011 in Marmara, Turkey, (Aydin et al., 2011) found that 13.8% of beef meat samples were contaminated with Staph. aureus out of which, 8.6% contained SEA. In Tehran, (Eshraghi, 2009) stated that Staph. aureus isolated from raw meat was able to produce SEA and SEA+SEC by 8% and 9%, respectively. These results, proved that the rates of presence of enterotoxin types A and D are the most common enterotoxins in meat which are identical to the results of the current research.
(Shawish and Al-Humam, 2016) find a considerable diversity of Staph. aureus and its enterotoxin production where they isolated 19 Staph. aureus strains from minced meat (38%) out of which, 8(16%) were enterotoxin producers; 6(75%) were SEA producers, 4(50%) SEB, 5(62.5%) SEC and 4(50%) SED producer. For beef burger, out 11(22%) Staph. aureus isolates, 4(8%) were enterotoxigenic; 4(100%) were SEA producers, 2(50%) SEB and 2(50%) SEC producers. The aforementioned incidences of enterotoxin considered higher than that obtained in the present study. In addition, the majority of reported Staphylococcal Food Poisoning (SFP) outbreaks are associated with the classical enterotoxins, SEA-SEE and staphylococcal enterotoxin A (SEA) being considered the most common cause of SFP (Wieneke et al., 1993; Cha et al., 2006). Such variation in enterotoxin production may be due to the variation in the implemented traditional hygienic practices and food safety measures (Hussain and Dawson, 2013). Moreover, (Fisher et al., 2018) explained that Staph. aureus enterotoxins are a superfamily of secreted virulence factors that share structural and functional similarities and possess potent adverse effect on immunity. The classical (SEA-SEE) enterotoxin groups resulted in several serious human diseases, including toxic shock syndrome, pneumonia, and sepsis-related infections. Additionally, some of these enterotoxins are responsible for emetic activity and are frequently responsible for food poisoning outbreaks. Due to their robust tolerance to denaturing, the enterotoxins retain activity in food contaminated previously with Staph. aureus. The genes encoding the enterotoxins are found mostly on a variety of different mobile genetic elements. Therefore, the presence of enterotoxins can vary widely among different Staph. aureus isolates. Additionally, the enterotoxins are regulated by multiple, and often overlapping, regulatory pathways, which are influenced by environmental factors.
Isolation and identification of B-glucuronidase-positive Escherichia coli and Serological identification of E. coli
The results recorded in Fig (2,3 & Photo 1) indicated that among the five strains of E. coli that were randomly selected from 40 E. coli strains isolated from fresh local meat, one strain (20%) was classified as Enteropathogenic (EPEC) and its serodiagnosis was O146:H21 which harbored stx2 gene, while two strains (40%) were Enterohaemorrhagic (EHEC) “O111:H2” and both found to harbor (stx1, stx2 and eaeA) genes and finally, one strain (20%) was Enterotoxigenic (ETEC) “O15:H4” and found to contain stx1 only, while the 5th strain was non-pathogenic and subsequently not subjected to serodiagnosis.
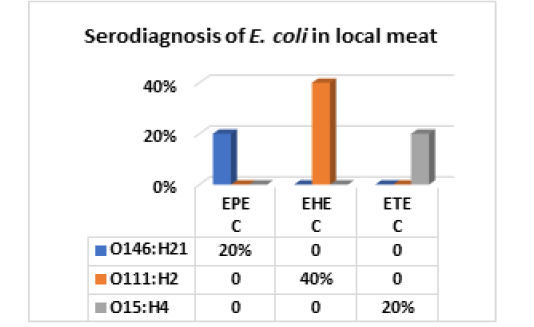
Figure 2: E. coli serovars in local meat
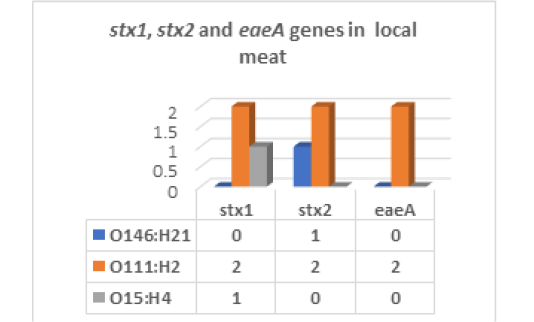
Figure 3: E. coli virulence genes in local meat
On the other hand, the results of serodiagnosis and virulence gens of the five randomly selected strains of E. coli out of 34 E. coli isolates from imported meat which registered in Fig (4,5 & Photo 1) showed that, one strain (20%) was classified as Enteropathogenic (EPEC) and its serodiagnosis was O128:H2 which harbored stx1 gene, while one strains (20%) was Enterohaemorrhagic (EHEC) “O26:H11” which harbored both (stx1 and stx2) genes and finally, the 3rd strain (20%) was Enteroinvasive (EIEC) “O159” and found to contain stx1 only, while the 4th and the 5th strains were non-pathogenic and subsequently not subjected to serodiagnosis. From the obtained results it could be concluded that local meat contained more pathogenic E. coli and virulence gens as compared with imported meat.
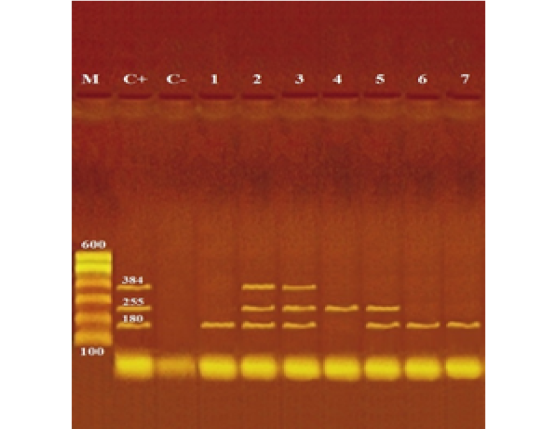
Photograph (1): Agarose gel electrophoresis of multiplex PCR of stx1 (180 bp), stx2 (255 bp) and eaeA (384 bp) virulence genes for characterization of pathogenic E. coli isolated from examined local and imported meat samples
Lane M: 100 bp ladder as molecular size DNA marker.
Lane C+: Control positive E. coli for stx1, stx2 and eaeA genes.
Lane C-: Control negative.
Lanes 2 & 3 (O111): Positive E. coli for stx1, stx2 and eaeA genes.
Lane 5 (O26): Positive E. coli for stx1 and stx2 genes.
Lane 1 (O15), 6 (O128) & 7 (O159): Positive E. coli for stx1 gene.
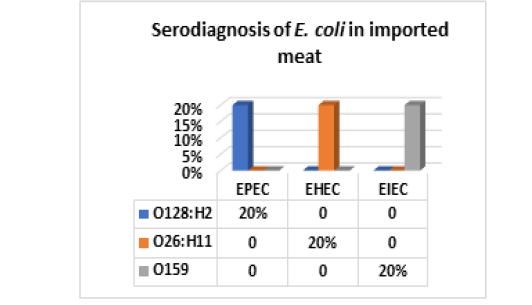
Figure 4: E. coli serovars in imported meat
Table 2: Incidence and serodiagnosis of Salmonella enterica in examined locally and imported meat samples (50 samples each)
| Type of meat / No. of positive isolates | Incidence of positive samples | Identified strains | Group | Antigenic structure | |
| O | H | ||||
|
Local meat: 1 |
4% | Salmonella Montevideo | C1 | 6,7,14 | g,m,s: 1,2,7 |
| 1 | Salmonella Enteritidis | D1 | 1,9,12 | g,m : - | |
|
Imported meat: 1 |
2% | Salmonella Typhimurium | B | 1,4,5,12 |
i: 1,2 |
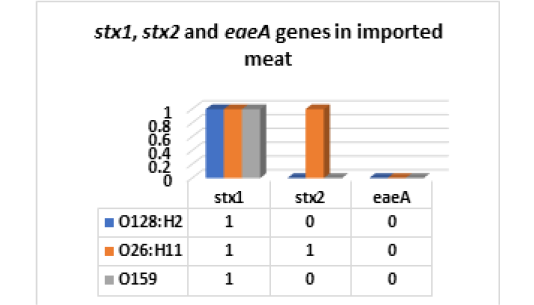
Figure 5: E. coli virulence genes in imported meat stx1: Shiga- toxin 1 gene; stx2: Shiga- toxin 2 gene eaeA: intimin gene
The obtained results are compatible with (Hussein, 2007) isolated members of O26 which successfully detected in the present study. Furthermore, the results are in line with that recorded by (Berger et al., 2010; Qardi et al., 2005; Steffen et al., 2005) mentioned that ETEC and EPEC are most common in developing world and appear to be the major causes of infantile diarrhea with potentially fatal consequences when untreated, while EHEC considered the main E. coli pathotypes associated with food poisoning outbreaks in the developed world. Nearly similar results were obtained by (Zweifel, 2003) detected stx1 and stx2 of non O157 E. coli isolated from sheep carcasses obtained from three Swiss abattoirs. (Monaghan et al., 2011); Datta et al., 2012; Hamed et al., 2015) stated that E. coli commonly considered non-virulent, but some strains have adopted pathogenic or toxigenic virulence factors that make them virulent to human and animals. Pathogenic E. coli strains have been broadly classified into two major categories; extraintestinal pathogenic and diarrheagenic E. coli which classified into six categories including Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC), Entero-invasive E. coli (EIEC), Enterohemorrhagic E. coli (EHEC)/Shiga toxin-producing E. coli (STEC), Enteroaggregative E. coli (EAEC) and diffusively adherent E. coli (DAEC).
E. coli of different serotypes and virulence factors was isolated from fresh meat and meat products with different diversities by many investigators; (Aidar-Ugrinovich et al., 2007) could isolate members of STEC represented by O7:H10, O22:H16, O111:H(-), O119:H(-) and O174:H21, whereas O26:H11, O123:H11 and O177:H11 were the most prevalent among EPEC strains in Brazil, The eaeA gene was detected in 25% of the STEC and 100% of EPEC strains. (Abd El-Tawab et al., 2015b) (O55:H7 (3/27.3%), O78 (2/18.2%), O111:H4 (1/9.1%), O26:H11 (2/18.2%), O119:H4 (1/9.1%) and O128:H18 (1/9.1%), (Binsy et al., 2016) (O3, O19, O22, O25, O29, O34, O36, O42, O50, O51, O53, O55, O65, O66, O73, O79, O105, O109, O115, O139, O140, O147, O152, O163, O164 and O173), (Shaltout et al., 2016) (O55:H7 EPEC (2/5.71), O125:H18 EPEC (1/2.86), O111:H4 EHEC (1(2.86) and O26:H11 EPEC (1/2.86%), (El-Sayed, 2019) (O55:H7 and O26:H11 and eaeA gene was detected in the tested strains. Furthermore, (Gyles, 2007) and (Aidar-Ugrinovich et al., 2007) mentioned that healthy dairy and beef cattle are a major reservoir of a diverse group of STEC that infects humans through contamination of food and water, as well as through direct contact. These results were compliant with the present research.
Incidence and serodiagnosis of Salmonella enterica in examined locally and imported meat : Results in Table (2) indicated that Salmonella spp. was isolated from two samples (4%) of examined local meat out of which one strain was identified as S. Montevideo of Group “C1” with antigenic structure (O6,7,14:H g,m,s:1,2,7) and the 2nd strain was S. Enteritidis of group “D1” (O: 1,9,12:H g,m). While, imported meat recorded 1 strain (2%) of S. Typhimurium of group “B” (O:1,4,5,12 H: i:1,2). In this regard, Food safety standards set by international organizations concerned with food safety parameters (EC No. 2073l2005; ICMSF, 2011; FDA, 2018 and FNANZ, 2018) that food should be free of salmonella spp. Therefore, the obtained results in the present study revealed that two samples (4%) of examined local meat and 2% of imported meat were rejected as they contained salmonella spp. and considered unfit for human consumption Nearly similar results were recorded by (Duffy et al., 2001) isolated Salmonella by (1.5%) from lamb carcasses. (Fegan et al., 2005) who could isolate Salmonella by 3% from both pre and post chilled beef carcasses. (Reid et al., 2002) found that the hides of the rump area of beef carcasses was less contaminated (2.2%) with Salmonella spp. as compared with brisket area (10%). This may be attributed to the contact of brisket with the slaughterhouse floor which is the main source of contamination or may be due to the live animal itself and the environment which serve as sources for pathogenic bacteria which contaminate the meat during slaughtering, dressing and evisceration processes. (Tassew et al., 2010) isolated two Salmonella strains (1.2%) from meat samples collected from hotels, butcher shops and abattoir (Hemmat et al., 2014) (2.2%) of rump beef meat. Furthermore, the obtained results coincide with Hendriksen et al. (2007) who concluded that S. enteritidis and S. typhimurium are the most dominated serovar isolated from meat and meat products and are considered the most common cause of human infection. In addition, (Sibhat et al., 2011) (2%) in Ethiopia with serotypes of S. Eastbourne and S. urbana. (Narvaez-Bravo et al., 2013) (6%) in Mexico, (Moaward et al., 2017) (3.3%) S. enteritidis from examined fresh beef meat in Egypt, while the incidence of S. typhimurium was 3 strains (10%) which considered higher than the results in the present study. Also, (Ead-Hoiam and Abdelgadir, 2019) could isolate Salmonella from 4 rump samples (3.3%), 2(1.7%) from shoulder, 9(7.5%) from flank and 1(0.8%) from neck beef meat in in karay slaughterhouse, Khartoum state, Sudan.
Lowe incidence of salmonella in fresh beef meat were recorded by (Vugia et al., 2004) (0.09 cases annual incidence among 100000 population and 7.8 cases among infants), the authors could isolate Typhimurium, Typhi, Enteritidis, Heidelberg, Dublin, Para typhi A, Choleraesuis, and Schwarzengrund, (Greig et al., 2001) (0.07%) of human salmonellosis in Australia, (Crim et al., 2015) (0.015%) in USA. While, many investigators; (Venkateswaran et al., 1988; Kalyapure et al., 1994, Binsy et al., 2016; Elsharawy and Mahran, 2018) failed to isolate Salmonella spp. from examined beef meat samples (0%).
In this issue, Higher results were reported by (Mc Evoy et al., 2003) 6 isolates (7.6%) from bovine carcasses in Ireland. (Aftab et al., 2012) concluded that 100% of fresh beef meat was contaminated with Salmonella in Peshawar, Pakistan. (Ahmad et al., 2013) 4(20%) and 7(35%) of examined beef meat samples from abattoir and R. outlets, (Abd El- Tawab et al., 2015 b) who isolated 3 strains of salmonella spp. (12%) out of which; 2 strains of S. typhimurium (66.7%) and one strain S. enteritidis (33.3%) from beef meat, (Muluneh and Kibret, 2015) could isolate Salmonella from hind limb, abdomen and Neck of beef meat samples with 6%, 10% and 7%, respectively in slaughterhouse of Bahir Dar town, Ethiopia. The serovars identified as 11(47.8) as Salmonella group A., 9(39.1%) as S. Arizona and 3 isolates (13.1) identified as S. typhi. (Birhanu and Menda, 2017) (4/19%), (Saad et al., 2019) (28%), (Kamana et al., 2018) (20% and 33.3%) from buffalo and goat meat respectively. (Abou El Nour-Mona and Sakr-Ebtehag In Nepal, 2020) (38%).
The presence of Salmonella on the cattle hides indicated that contamination could be potentially transferred to the carcass during slaughtering and dressing process (Bacon et al., 2002; Ruby et al., 2007). or during unhygienic slaughtering process (Li et al., 2004; Rhoades et al., 2009). In addition to, variation in analytical methods, environmental condition, samples and sampling procedure would be able to assess the levels of contamination (Fegan et al., 2005). Also, the variation which observed between different research data related to the prevalence rate of Salmonella and its detected serovars around the world may be attributed to several factors including cleaning and sanitation within the slaughterhouse, possible contamination of meat during processing and difference among the applied reference methods for detection of Salmonella (Rahimi, 2012). Moreover, workers hands and clothes, and the work environment act as additional sources for carcasses contamination through the contact with the blood, hair, limbs, bile, facilities, equipment, water supplies and air pollution (Koffi-Nevry et al., 2011; Muhammad et al., 2012).
COnCLUSION AND RECOMMENDATIONS
The present study concluded that local and imported beef meat are contaminated with E. coli of different serovars and some of them was able to possess different virulence genes. (stx1, stx2 and eaeA). Also, Staph. aureus was present in both local and imported meat with some strains were able to produce enterotoxins A and D. Moreover, Salmonella was present with different percentages. This may be due to neglected sanitary measures adopted during slaughtering, dressing, evisceration, storage, transportation and distribution of cattle carcasses. Therefore, a concerted effort should be made to improve sanitary condition in all carcasses processes to mitigate bacterial contamination in slaughterhouses. In addition, Hazard Analysis and Critical Control Points (HACCP) should be applied to improve carcass hygiene as well as to eliminate or reduce the presence of pathogenic bacteria of beef carcasses well as application of control measures for pathogenic bacteria through the food chain including slaughterhouses and retail outlets.
Acknowledgments
The authors are thankful to General Organization for Veterinary Services, Egypt for providing necessary facilities for this study. The authors did not receive any funds for this study.
CONFLICT OF INTEREST
None of the author has any conflict of interest to declare.
Authors’ Contributions
HKS collected the samples, and performed the laboratory work and data analysis. KST, HMS and planned the research and experimental design, supervised the work, checked the data analysis. SHAH prepare the manuscript
REFERENCES