Journal of Animal Health and Production
Research Article
Blood Metabolic Profile of Lambs Fed Complete Diet Supplemented with Exogenous Fibrolytic Enzymes Cocktail
Yasir Afzal Beigh1*, Abdul Majeed Ganai1, Haidar Ali Ahmad1, Danish Masood Mir1, Mudasir Ahmad Bhat1, Showkeen Muzamil2
1Division of Animal Nutrition, Faculty of Veterinary Sciences & Animal Husbandry, Shuhama, Alusteng, Srinagar-190 006, Sher-e-Kashmir University of Agricultural Sciences & Technology of Kashmir, Jammu & Kashmir, India. 2Division of Veterinary Biochemistry, , Faculty of Veterinary Sciences & Animal Husbandry, Shuhama, Alusteng, Srinagar-190 006, Sher-e-Kashmir University of Agricultural Sciences & Technology of Kashmir, Jammu & Kashmir, India.
Abstract | Blood metabolic profile (BMP) serve as clinical indicators for the impact of dietary manipulations on physiological health status. The present study was undertaken with the aim to assess the effect of in-feed supplementation of exogenous fibrolytic enzymes (EFE) cocktail as feed additive on BMP in lambs. Crossbred lambs (n=12) of 4-6 months age were distributed into two groups of 6 animals each and fed for a period of 90 days followed by 6 days metabolism trial. Animals in both the groups were offered complete feeds either supplemented with EFE cocktail @ 0.6% DM (Enz) or without any supplementation as control (Con). Haemato-biochemical parameters of animals in both the groups at start (0d) and subsequently at monthly intervals (30, 60 and 90d) were investigated. The EFE supplementation had positive significant (p<0.05) effect on dietary digestible energy content as well as total digestible nutrient intake (g/d) compared to control. However, there were non-significant (p>0.05) effects of EFE supplementation on overall means of all blood cell indices except mean platelet volume (p<0.05) and platelet percent (p<0.01), energy metabolic profile, serum lipid profiles except low-density lipoprotein cholesterol (p<0.05) and atherogenic index (p<0.01), and hepato-renal functioning test profiles. The BMP of EFE supplemented lambs revealed better physiological health status compared to un-supplemented animals. It is concluded that inclusion of EFE cocktail in complete diets for lambs have no adverse effects on the haemato-biochemical parameter suggesting in-feed supplementation of the EFE to improve animals’s health conditions for successfully raising lambs intensively.
Keywords | Blood metabolic profile, Fibrolytic enzymes, Sheep, Supplementation
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | July 13, 2018; Accepted | September 23, 2018; Published | October 17, 2018
*Correspondence | Yasir Afzal Beigh, Division of Animal Nutrition, Faculty of Veterinary Sciences & Animal Husbandry, Shuhama, Alusteng, Srinagar-190 006, Sher-e-Kashmir University of Agricultural Sciences & Technology of Kashmir, Jammu & Kashmir, India; Email: [email protected]
Citation | Beigh YA, Ganai AM, Ahmad HA, Mir DM, Bhat MA, Muzamil S (2018). Blood metabolic profile of lambs fed complete diet supplemented with exogenous fibrolytic enzymes cocktail. J. Anim. Health. Prod. 6(4): 96-102.
DOI | http://dx.doi.org/10.17582/journal.jahp/2018/6.4.96.102
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2018 Beigh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Blood metabolic profile (BMP) is considered to be one of the significant sources for judging the clinical status, nutritional balances, deficit condition, treatment monitoring and prognostics, making it a useful tool for the diagnosis of metabolic disorders in all livestock species. Also, it may act as a good indicator for assessing the effects of feeding diets and supplementations on normal physiological parameters. Various factors effect blood biochemical and haematological indices, and one of which is nutrition. Dietary content (Kurtoglu et al., 2005) and also processing of feeds affect the blood profile of healthy animals (Aya et al., 2013).
In agricultural countries worldwide, crop residues and other fibrous agro-industrial by-products form the bulk of ration for ruminant livestock (Alsersy et al., 2014). However, these feedstuffs are characterized by low nutritive value, have complex network formed by structural carbohydrates and lignin that restricts their efficient utilization (Krause et al., 2003), thus necessitating to find ways for optimizing their utilization for improving ruminant production efficiency. One option is the supplementation of feed additives, among which exogenous fibrolytic enzymes (EFE) has received considerable attention as alternative feed additive with the demand for organic food and ban on the use of synthetic compounds (antibiotics/hormones/arsenicals etc.) in animal diets. Exogenous enzymes are reported to have positive effects for improving feed utilization and animal performance (Morsy et al., 2016; Kholif et al., 2017). Besides, additions of exogenous enzymes to animal diets are safe with no adverse effect on animal health (Hussain et al., 2008). To exploit the health promoting activity of EFE cocktail, the present study was undertaken to assess the in-feed supplementation effects on various hemato-biochemical parameters in growing lambs.
MATERIALS AND METHODS
Animal Management and Experimental Feeding
The experimental protocols involved in the study were approved by the Institutional Animal Research and Ethical Committee in compliance to the Guidelines on the Care and Use of Animals for Scientific Purposes, National Advisory Committee for Laboratory Animal Research (NACLAR), 2004. Twelve growing crossbred (Fec-B gene carrying×Hampshire) male lambs (4-6 months age and 11.58±0.02kg mean body weight) of uniform conformation procured from sheep unit of Mountain Research Centre for Sheep and Goat, Faculty of Veterinary Sciences and Animal husbandry, SKUAST-Kashmir, were randomly distributed to two groups in completely randomized design (CRD). The lambs were stall fed individually for a period of 90 days followed by 6 days metabolic trial. Animals in all the groups were offered complete feeds to meet their nutrient requirements (ICAR, 2013). The complete feeds were based on oats straw- 40 parts, mixed grass hay- 20 parts and concentrate mixture- 40 parts without as control (Con) or with EFE cocktail (Enz) @ 0.6% DM. The EFE cocktail preparation (ALLENZIMIX-EP, supplied by Mitushi Pharma, Ahmedabad, Gujarat, India) had constituent enzymes as cellulase (800000 IU/g), phytase (400000 IU/g), β glucanase (450000 IU/g), xylanase (400000 IU/g) and pectinase (350000 IU/g).
All the lambs were kept under uniform management conditions, housed in well ventilated, hygienic and protected sheds with batten floor, and appropriate facilities for individual feeding and watering. All the animals were vaccinated against prevalent contagious diseases before the start of experiment.
Haemato-Biochemical Analysis
Periodic monitoring of haemato-biochemical indices were carried out. The blood samples were taken in the morning before watering and feeding from all the animals at the start (day 0) and subsequently at monthly intervals (day 30, 60 and 90) of the experimental trial. The samples were collected by puncturing the jugular vein with all aseptic precautions. For haematological studies, ~2mL of whole blood was collected into ethylene diamine tetra acetic acid (EDTA) vials (Hi-media, Mumbai) agitated for 15-20 seconds to prevent blood clotting. Determination of hematological parameters viz. haemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), red cell distribution width (RDW), mean platelet volume (MPV), platelet distribution width (PDW), platelet percent (Pct), total erythrocyte count (TEC), total thrombocyte count (TTC), total leucocytes count (TLC) and differential leucocytes counts (DLC) was performed in the whole blood immediately after collection on automatic haematology analyser-MS4 (Melet Schloesing Laboratories, France).
For biochemical analysis, ~8mL of whole blood samples were collected in well cleaned, dry test tubes allowed for clotting and then centrifuged (2000 × g for 10 min at 15°C) within 4 h after collection to obtain serum, which was immediately stored in eppendorf tubes in multiple aliquots at –20°C until analysis. After thawing, the serum samples were analysed for serum total protein (TP), serum albumin, serum urea nitrogen (SUN), serum creatinine, serum uric acid, total cholesterol (TC), total triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), non-esterified fatty acids (NEFA), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) in semi-auto biochem analyzer (Photometer-5010V5+, Robert Riele INC, Berlin, Germany) using commercial kits (DiaSys Diagnostics Pvt. Ltd., Navi Mumbai, India). Blood glucose was estimated in whole blood immediately after collection using SD-Codefree blood glucose meter (SD Biosensor Healthcare Pvt. Ltd., Gurgaon, Haryana, India). β-hydroxy butyrate (BHB) was analyzed with commercial kit (Transasia Bio-medicals Ltd.) using an enzymatic UV method. Serum globulin and serum albumin: globulin ration were obtained by calculations. Very low-density lipoprotein cholesterol (VLDL-C) was estimated using Friedewald et al. (1972) method. Low-density lipoprotein cholesterol (LDL-C) and Atherogenic index (AI) were measured by using the equations as below:
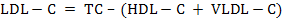
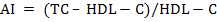
Statistical Analysis
The data were subjected to statistical analysis to draw the inference. Means of various parameters were estimated and test of significance (Independent-samples t-test) were performed using SPSS version 20.0 software for windows. Analysis included between-subjects main effect of diet, within subjects main effect of period of sampling and interactionbetween periods of sampling × diet. Data are reported as mean and standard error mean (SEM). Statistical differences was declared at p<0.05 or lower.
RESULTS AND DISCUSSION
Many feed products are fed to livestock usually without recourse to their health and physiological implications on the animals. The commonest parameter for measuring these implications is through the haemato-biochemistry of animals (Aro et al., 2013). BMP represent an integrated index of the adequacy of nutrient supply in relation to nutrient utilization, and give an immediate indication of nutritional status at that point in time (Cronje and Pambu-Gollah, 1996).
Content and Intake of Digestible Nutrients
Supplementation of EFE cocktail hadsignificant (p<0.05) positive effect on digestible energy (DE Kcal/g) while non-significant (p>0.05) effect on digestible crude protein (%DCP) and total digestible nutrients (%TDN) contents in comparison to control (Table 1). Likewise, intake of digestible nutrients were improved by EFE supplementation; however, statisticallysignificant (p<0.05) effect was observed only for TDN intake (g/d). Improvement in DCP, TDN (p>0.05) and DE (p˂0.05) in EFE supplemented dietary group might be due to higher dietary nutrients utilization which could have led to better BMP in the respective group. EFE supplementation enhance rumen microbial attachment and colonization to the plant cell wall and/or work in synergy with rumen microbial enzymes (Gado et al., 2011), thereby increases the hydrolytic potential within the rumen that enhance nutrient digestibility and availability. Likewise, significant (p˂0.05) intake of TDN (g/d) by EFE supplementation could have varied the blood metabolites under study, since blood parameters are sensitive to variations in nutrient intake (Pambu-Gollahet al., 2000). Radostits (1994) posited that nutritional intakes among other factors alter the blood values of sheep and goats.
Haematological Profile
There were non-significant (p>0.05) effects of the EFE supplementation on overall means for all the red blood cell, white blood cell and platelet indices except MPV and Pct (Table 2). Significantly higher MPV (p<0.05) and Pct (p<0.01) values were recorded in EFE supplemented group compared to control. However, all the haematological values were well within the normal physiological ranges for healthy sheep (Jain, 1986), suggesting general health of lambs in both the dietary treatment groups remained normal throughout the experiment. Higher values of RBC and platelet indices in EFE supplemented diet fed group compared to un-supplemented group might be due to improvement in nutriture of animals that enhanced the erythropoiesis or reduced the establishment of adult nematodes (Gilthiori et al., 2004). Improved host nutrition increases the rate of rejection of adult parasites without affecting the rate of establishment of infective larvae (Van Houtert and Sykes, 1996). The results of the present study are in accordance with the findings of Rivero and Salem (2015) who also reported that supplementation of exogenous enzymes to diets for growing lamb had no adverse effect on any measured haematological parameters. Also, Rivero et al. (2012) reported no significant differences for various blood cell counts by supplementation of exogenous enzymes to lambs.
Table 1: Nutritional profile of lambs fed complete diet supplemented without and with EFE cocktail
| Attribute | Dietary groups | SEM | p value | ||
| Con | Enz | ||||
| %DCP | 9.98 | 10.70 | 0.347 | 0.332 | |
| %TDN | 59.02 | 65.30 | 1.724 | 0.059 | |
| DE (Kcal/g) |
2.42a |
2.71b |
0.075 | 0.046 | |
| DCPI | g/d | 87.38 | 101.08 | 4.848 | 0.173 |
|
g/kgW0.75 |
10.06 | 10.24 | 0.202 | 0.691 | |
| TDNI | g/d |
515.51a |
616.22b |
25.742 | 0.036 |
|
g/kgW0.75 |
59.60 | 62.52 | 1.033 |
0.173 |
|
The means across the rows with different superscripts differ significantly
DCP: digestible crude protein; DE: digestible energy; TDN: total digestible nutrients; DCPI: digestible crude protein intake; TDNI: total digestible nutrients intake
General Metabolic Profile
In energy metabolic profile, there were no significant (p>0.05) effects of EFE supplementation on overall means of blood glucose, serum NEFA as well as BHB levels (Table 3). The values of blood glucose concentration varied non-significantly between the groups, although were within the normal range (Kaneko et al.,1997). In support to the present findings, no change in the level of blood glucose due to enzyme supplementation has also been observed by Vahora and Pande (2006) in dairy cows. NEFA and BHB are released in the blood plasma when adipose tissue is mo-
Table 2: Haematological profile of lambs fed complete diet supplemented without and with EFE cocktail
| Attribute | Dietary groups |
SEM |
p value | |||
| Con | Enz | Diet | Period | Diet × Period | ||
| Red blood cell indices | ||||||
| Hb (g/dL) | 10.18 | 10.59 | 0.116 | 0.088 | 0.132 | 0.830 |
| PCV(%) | 30.89 | 31.71 | 0.451 | 0.370 | 0.173 | 0.897 |
| MCV (fL) | 26.85 | 27.00 | 0.250 | 0.759 | 0.673 | 0.985 |
| MCH (pg) | 8.88 | 8.93 | 0.095 | 0.779 | 0.377 | 0.944 |
| MCHC (g/dL) | 33.40 | 33.70 | 0.162 | 0.362 | 0.005 | 0.163 |
| RDW | 9.55 | 9.70 | 0.090 | 0.393 | 0.013 | 0.724 |
|
TEC ( ×106/µL) |
8.95 | 9.07 | 0.179 | 0.741 | 0.457 | 0.997 |
| Platelet indices | ||||||
| MPV(fL) |
6.08a |
6.70b |
0.132 | 0.027 | 0.052 | 0.965 |
| PDW | 7.40 | 7.49 | 0.087 | 0.584 | <0.001 | 0.469 |
| Pct (%) |
0.24a |
0.37b |
0.012 | <0.001 | <0.001 | 0.066 |
|
TTC ( ×103/µL) |
281.62 | 299.60 | 7.248 | 0.225 | 0.003 | 0.921 |
| White blood cell indices ( ×103/µL) | ||||||
| TLC | 9.81 | 9.22 | 0.175 | 0.104 | 0.015 | 0.026 |
| Lymphocyte count | 4.09 | 4.03 | 0.082 | 0.713 | 0.057 | 0.995 |
| Monocyte count | 0.35 | 0.36 | 0.008 | 0.534 | 0.020 | 0.978 |
| Neutrophil count | 3.28 | 3.26 | 0.090 | 0.889 | 0.437 | 0.999 |
| Eosinophil count | 0.55 | 0.53 | 0.036 | 0.813 | 0.007 | 0.793 |
| Basophil count | 0.09 | 0.10 | 0.004 | 0.091 | <0.001 |
0.100 |
The means across the rows with different superscripts differ significantly
Hb: haemoglobin; MCH:mean corpuscular haemoglobin; MCHC: mean corpuscular haemoglobin concentration; MCV: mean corpuscular volume;MPV:mean platelet volume; PCV: packed cell volume; Pct: platelet percent;PDW: platelet distribution width; RDW: red cell distribution width;TEC: total erythrocyte count; TTC: total thrombocyte count; TLC: total leucocytes count
Table 3: General metabolic profile of lambs fed complete diet supplemented without and with EFE cocktail
| Attribute | Dietary groups | SEM | p values | |||
| Con | Enz | Diet | Period | Diet × Period | ||
| Energy metabolic profile | ||||||
| Blood glucose (mg/dL) | 49.06 | 48.70 | 0.639 | 0.779 | <0.001 | 0.394 |
| NEFA (μM/L) | 186.81 | 182.75 | 1.534 | 0.196 | <0.001 | 0.010 |
| BHB (μM/L) | 54.66 | 53.97 | 0.692 | 0.627 | 0.089 | |
| Serum lipid profile (mg/dL) | ||||||
| TC | 85.43 | 87.74 | 1.083 | 0.295 | 0.082 | 0.774 |
| TG | 31.82 | 32.63 | 0.591 | 0.496 | 0.066 | 0.810 |
| HDL-C | 42.88 | 41.34 | 0.484 | 0.122 | 0.723 | 0.981 |
| LDL-C |
36.18a |
39.88b |
0.723 | 0.016 | 0.004 | 0.429 |
| VLDL-C | 6.36 | 6.53 | 0.118 | 0.496 | 0.066 | 0.810 |
| AI |
1.00a |
1.12b |
0.017 | 0.001 | <0.001 |
0.206 |
The means across the rows with different superscript differ significantly
AI:Atherogenic index; BHB:β-hydroxy butyrate; HDL-C: high-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; NEFA:non-esterified fatty acids; TC: total cholesterol; TG: total triglycerides; VLDL-C: Very low-density lipoprotein cholesterol
Table 4: Hepato-renal function test profile oflambs fed complete diet supplemented without and with EFE cocktail
| Attribute | Dietary groups | SEM | p values | |||
| Con | Enz | Diet | Period | Diet × Period | ||
| Liver function test profile | ||||||
| STP (g/dL) | 6.53 | 6.55 | 0.070 | 0.871 | 0.642 | 0.603 |
| Serum albumin (g/dL) | 3.60 | 3.57 | 0.048 | 0.795 | 0.177 | 0.818 |
| Serum globulin (g/dL) | 2.93 | 2.98 | 0.054 | 0.668 | 0.825 | 0.541 |
| Serum A:G ratio | 1.25 | 1.21 | 0.028 | 0.435 | 0.395 | 0.633 |
| ALT (IU/L) | 24.34 | 23.71 | 0.351 | 0.404 | 0.781 | 0.862 |
| AST (IU/L) | 71.61 | 70.12 | 0.712 | 0.307 | 0.162 | 0.994 |
| ALP (IU/L) | 236.87 | 231.01 | 3.331 | 0.293 | 0.001 | 0.604 |
| Kidney function test profile(mg/dL) | ||||||
| SUN | 36.46 | 34.97 | 0.414 | 0.081 | 0.085 | 0.841 |
| Serum creatinine | 1.45 | 1.48 | 0.013 | 0.314 | <0.001 | 0.692 |
| Serum uric acid | 1.24 | 1.25 | 0.012 | 0.608 | <0.001 |
0.748 |
ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; STP: serum total protein; SUN: serum urea nitrogen
bilized to supply for metabolic energy needs of the animal; although there levels in the blood of ruminants are small, but are important factors in determining caloric homeostasis of the body. In the present study, no significant effects were observed by EFE supplementation on overall serum NEFA and BHB levels in comparison to control which could be attributed to adequate levels of energy content of both the diets fulfilling the energy requirements of animals in the groups thereby restricting lipid mobilization; although, the values being numerically lower in the animals fed EFE supplemented diet presumably due to increased metabolism of glucose that might have depressed the fatty acid mobilization in peripheral adipose tissues in the respective group, so that less free fatty acid became available for hepatic uptake and oxidation.Use of EFE improves the energy status of ruminants by reducing plasma concentrations of NEFA and BHB, indicating reduction in mobilization of fat from adipose tissue (Dean et al., 2013). Also, Peters et al. (2015) observed no effects of EFE supplementation on serum BHB in early and mid-lactation Holstein cows.
No significant (p>0.05) effects of EFE supplementation on overall means of serum lipids (TC and TG); however, significant differences were recorded for LDL-C (p<0.05) among serum lipoproteins and AI (p<0.01) between the treatment groups (Table 3), though the values in both the groups for the studied serum lipid parameters were within normal ranges for healthy sheep (Kaneko et al., 1997). This is in accordance with Morsy et al. (2016) who reported that addition of two commercial fibrolytic enzyme products to the total mixed rations of buffaloes did not affect (p>0.05) blood total lipids and cholesterol content. Numerical increase in serum TC in lamb fed EFE supplemented diet was primarily due to significant (p<0.05) increase in LDL-C suggesting hyperlipidemic activity of the EFE supplementation. Elevation (p<0.05) in serum LDL-C levels in animals fed EFE supplemented diet might be attributed to the improvement in lipid synthetic metabolic processes in response to the supplementation. However, no previous study reports are available in support of these findings regarding the effect of EFE as feed additive on blood lipoprotein profile.
Hepato-Renal Functioning Test Profiles
Among the indicators of liver and kidney functioning, no significant (p>0.05) effects of the EFE supplementation were recorded for overall mean of all the recorded parameters in comparison to the un-supplemented animals (Table 4), although the values in both the groups were within normal physiological ranges (Kaneko et al., 1997). Non-significant increase in serum TP and its fractions were also reported by Peters et al. (2015) in lactating dairy cows fed diets supplemented with exogenous enzymes. Rivero et al. (2016) reported non-significant differences in the levels of hepatic enzymes in sheep and goat fed diets supplemented with exogenous enzymes.
In kidney function attributes, numerically lower SUN levels in EFE supplemented group compared to controlmight probably be due to efficient utilisation of dietary proteins by addition of the feed additive. Lower N wastage by better utilization of generated ammonia in the rumen was represented in terms of lower SUN levels in EFE supplemented group. Similar levels of serum creatinine and uric acid between the groups indicated that supplementation had no adverse affects on glomerular filtration, thus safe for renal functioning. These findings of non-significant effects of exogenous enzymes on serum creatinine and uric acid levels in the present study are in close agreement to those reported by Rivero and Salem, (2015) for enzyme supplementation in sheep.
CONCLUSION
Inclusion of feed additives in diets of livestock ought to prove useful to small holder livestock producers with respect to enhanced animal health and productivity. Supplementing complete diets for growing lambs with EFE cocktail provided nutrients more than the recommended requirements with no adverse effects on blood cell indices, energy metabolic and serum lipid profiles and resulted in normal hepato-renal functioning suggesting in-feed supplementation of EFE for successfully raising lambs intensively.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Acknowledgements
The authors are grateful to the Directorate of Research for providing the funds and the Dean, FVSc & AH for facilitating the work to carry out the study.
Authors Contribution
All the authors contributed significantly to the paper. The principle/corresponding author along with D.M. Mir and M.A. Bhat carried the experimental trial and compiled the results. AM Ganai and H.A. Ahmed designed the protocol and S. Muzamil helped in laboratory analysis of the samples.
REFERENCES






