South Asian Journal of Life Sciences
Research Article
Influence of Dietary Lipid Levels on Growth Performances, Survival, Feed Utilization and Carcass Composition of African Snakehead Parachanna Obscura Fingerlings
Diane NS Kpogue1,4*, Arsène Fm D’almeida2, Narcisse Houankanlin3, Jacques Dougnon3, Et Emile D Fiogbe4
1Ecole d’Aquaculture de la vallée, Université Nationale d’Agriculture, B.P. 43 Kétou, République du Bénin; 2Ministère de l’Agriculture, de l’Elevage et de la Pêche, Direction de la Production Halieutique, 01 BP 383 Cotonou, République du Bénin; 3Laboratoire de Recherches en Biologie Appliquée, Ecole Polytechnique d’Abomey-Calavi, Université d’Abomey-Calavi, 01 B.P. 2009 Cotonou, République du Bénin; 4Unité de Recherches sur les Zones Humides, Département de Zoologie, Faculté des Sciences et Techniques, Université d’Abomey - Calavi, B.P. 526 Cotonou, République du Bénin.
Abstract | Five iso – energetic diets were formulated to evaluate the effects of dietary lipids levels on growth parameters, survival rate, feed utilization and body composition of African Snakehead Parachanna obscura fingerlings with initial body weight 7.69 ± 0.14 g. Experimental diets were formulated to contain graded levels of lipid (5, 7, 9, 11 and 14 g/100 g of diet). Each diet was tested in triplicate during 8 weeks. During the experiment, any mortality was registered. Growth performances and nutrient utilization parameters of fingerlings fed on different diets varied significantly (P <0.05). Highest growth performances and nutrient utilization were obtained with fish fed on a diet containing 7% of crude lipid. According to the broken line models used to analyze the relationships between the dietary crude lipid (DCL) and the specific growth rate (SGR), the maximum dietary crude lipid requirement is 7% of the diet.
Keywords | Parachanna obscura, Fingerlings, Diet, Lipid requirement, Growth performances
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | April 05, 2018 Accepted | May 10, 2018; Published | June 20, 2018
*Correspondence | Diane NS Kpogue, Ecole d’Aquaculture de la vallée, Université Nationale d’Agriculture, B.P. 43 Kétou, République du Bénin; Email: [email protected]
Citation | Kpogue DNS, D’Almeida AFM, Houankanlin N, Dougnon J, Fiogbe EED (2018). Influence of dietary lipid levels on growth performances, survival, feed utilization and carcass composition of african snakehead parachanna obscura fingerlings. S. Asian J. Life Sci. 6(2): 36-40.
DOI | http://dx.doi.org/10.17582/journal.sajls/2018/6.2.36.40
ISSN | 2311–0589
Copyright © 2018 Kpogue et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In order to diversify aquaculture production in the world, some neglected species like Parachanna obscura which reveal interesting aqua cultural potential have been identified (Kpoguè et al., 2013a). P. obscura has high economic value (Bolaji et al., 2011) and occupies a prominent place in the diet of people (O’Bryen and Lee, 2007). Few data are available farming on this fish. Information on nutritional requirements of major dietary components such as protein, lipid and carbohydrates is a prerequisite for the formulation of an inexpensive and balanced diet for the fish. Indeed, the chemical composition and growth performance in fish can be influenced by dietary composition (NRC, 1993). Insufficient dietary protein results in poor growth and feed conversion ratio whereas excess levels are catabolized to provide energy, being neither economically beneficial nor environmentally friendly (Sugiura and Hardy, 2000). Increasing dietary non-protein energy level, often by increasing dietary lipid, can spare protein to a certain degree by directing protein to growth rather than for energy production. Dietary lipid provides essential fatty acids, phospholipids, sterols and fat soluble vitamins necessary for proper functioning of physiological processes as well as maintenance of biological structure and cell membranes function (Ghanawi et al., 2011). Therefore, it is important to formulate diets with proper lipid levels to meet the energy and fatty acids requirements for fish (Lopez et al., 2009).
According to the results of Kpoguè et al. (2013b), optimum dietary crude protein requirements for P. obscura fingerlings is 42.5% in formulated diets. No study has yet been conducted in it lipid requirement to date. The present study aims to investigate the effects of dietary lipid levels on growth performances, survival, feed utilization and carcass composition of African snakehead P. obscura fingerlings.
MATERIAL AND METHODS
Composition and Preparation of the Diets
Five iso-nitrogenous and iso – energetic experimental diets were formulated to contain with graded lipid levels namely 5, 7, 9, 11 and 14 g/100 g of diet, respectively (Table 1). These lipid contents were chosen based on the results of the lipid requirements of other snakeheads species such as Channa striatus (Aliyu-Paiko et al., 2010). Cod, soybean and cotton seed meals were used as the main protein source. Sardine oil was used as primary lipid source and maize meal is the principal carbohydrate source. The various ingredients were ground with hammer mill, weighed and mixed. Feed was manufactured by mixing the dry ingredients with boiling water and oil until the achievement of a desirable paste-like consistency. The resulting paste was transformed into pellets of 2 mm diameter byfood blender (MFM- 302- Denwa). After sun-drying at a temperature varying from 28 to 35°C during 3 days almost, the pellets were manually broken in small pieces.
Experimental Fish, Rearing Conditions and Feeding Trial
Fish were collected from the swamp “Dra” in Takon village (South-Est of Benin). After their collection, fish were transported to the experimental Station of the Laboratory of Research on Wetlands belonging to the Faculty of Sciences and Technics of Abomey Calavi University where they were then put in circular tank for 2 weeks. During this period, fry were trained to progressively accept the formulated diet. A mixture of the different experimental diets (20% of each) was used as feed during this phase. After this conditioning period, 40 fingerlings (7.69 ± 0.14 g) were stocked per a 225 liter tank for 8 weeks. Each diet was tested in triplicate. Water in all tanks was renewed continuously (1 L/min). To prevent fish from jumping out, tanks were covered at 50% with a perforated wooden plank. During the experiment, fishes were hand fed daily every 2 h from 08:00 am to 08:00 pm up to apparent satiation.
Water quality parameters such as temperature, pH, dissolved oxygen and nitrite were daily measured in each tank throughout the experimental period. These parameters mean value were 27.91 ± 0.32°C, 6.26 ± 0.23, 6.21 ± 0.07 mg/L, respectively.
At the beginning and the end of the experiment, all fish were counted and weighed per tank and 20 fingerlings were sampled forindividual weight and total length. All fish were counted and weighed every 7 days during the experimental period in order to adjust feed ration. No feed was given to the fish on the sampling day.
Table 1: Formulation and proximate composition of experimental diets
| Ingredients (%) | Dietarylipid (%) | ||||
|
5 |
7 |
9 |
11 |
14 |
|
|
Cod meala |
45 |
45 |
45 |
45 |
45 |
|
Soybeanmeal |
20 |
20 |
20 |
20 |
20 |
|
Cottonseedmeal |
20 |
20 |
20 |
20 |
20 |
|
Maizemeal |
10 |
8 |
6 |
4 |
2 |
|
Sardineoilb |
0 |
2 |
4 |
6 |
8 |
|
Premix (min-vit) c |
3.5 |
3.5 |
3.5 |
3.5 |
3.5 |
|
Ferroussulphate |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
CMCd |
1 |
1 |
1 |
1 |
1 |
|
Proximate analyses |
|
|
|
|
|
|
Dry matter (%) |
89.2 |
89.1 |
89.3 |
89.2 |
89.3 |
|
Ash (%) |
9.61 |
9.52 |
9.62 |
9.62 |
9.54 |
|
Crude lipide (%) |
4.98 |
6.90 |
8.83 |
10.75 |
13.78 |
|
Crudeprotein (%) |
43.71 |
43.52 |
43.33 |
43.15 |
42.96 |
| Gross Energy (MJ/100g) |
1.88 |
1.77 |
1.81 |
1.86 |
1.90 |
| Protein/Energy (g/MJ) |
23.25 |
24.59 |
23.94 |
23.20 |
22.61 |
aRieber and Son, N. 5002 Bergen, Norway; b Sigma-Aldrich products (Bornem, Belgium) ; c Drugstore , premix (vitamin – mineral) contains (‰):Vitamin A 4 000 000 U.I; Vitamin D 800 000 U.I;Vitamin E 40 000U.I; Vitamin K3 1600 mg; Vitamin B1 4 000 mg; Vitamin B2 3 000 mg; Vitamin B6 3 800 mg; Vitamin B12 3 mg; Vitamin C 60 000 mg; Biotine 100 mg; Inositol 10 000 mg Pantothenicacid 8 000 mg; Nicotinicacid 18 000 mg; Folicacid 800 mg; Cholin chlorure 120 000 mg; Colbat carbonate 150 mg; Ferroussulphate 8 000 mg; Potassium iodide 400 mg; Manganeseoxide 6 000 mg; Cuivre 800 mg; Sodium selenium 40 mcg; Lysine 10 000 mg ; Methionin 10 000 mg ; Zinc sulphate 8 000 mg; d SIGMA product Protibel (yeast Saccharomyces) Bel industries, 4 rue d’Anjou Paris 8ème.
Chemical and Calculations
Fishes samples were analyzed by standard methods for dry matter (oven drying) at 105°C for 24 h, crude protein (CP) (N- Kjeldahl ×6.25) and ash (oven incineration at 550°C for 12 h). Total lipids were extracted according to Bligh and Dyer (1959).
After the feeding trial, fishes were collected, counted, weighed and the different parameters were calculated as follows:
Weight gain (g) = final body weight - initial body weight
Table 2: Growth performances, survival rate and nutrient utilization of P. obscura fingerlings fed diets containing different levels of lipid
| Parameters | 5% | 7% | 9% | 11% | 14% |
| Initial body weight (g) | 7.78±0.28a | 7.56±0.25a | 7.93±0.11a | 7.83±0.10a | 7.85±0.07a |
| Final body weight (g) | 16.23±0.13a | 18.36±0.18b | 16.74±0.20c | 15.24±0.28d | 14.75±0.24e |
| Weight gain (g) | 8.45±0.40a | 10.80±0.07b | 8.81±0.22a | 7.42±0.24c | 6.90±0.17d |
| SpecificGrowth Rate (%/day) | 1.53±0.09a | 1.85±0.05b | 1.56±0.04a | 1.39±0.03c | 1.31±0.02d |
| Feedefficiency | 0.50±0.02a | 0.52±0.07a | 0.47±0.02ab | 0.43±0.03ab | 0.38±0.07b |
| Survival rate (%) | 100±0.00 a | 100±0.00 a | 100±0.00 a | 100±0.00 a | 100±0.00 a |
| Coefficient of Variation (%) | 0.79±0.01a | 0.96±0.01ab | 1.20±0.01 c | 1.82±0.03d |
1.63±0.03e |
Means on the same line followed by different superscripts are significantly differents (P < 0.05).
Table 3: Body composition data for P. obscura fingerlings fed with diets containing different levels of lipid
| Parameters | Crudeprotein | Crudelipid | Ash | Moisture |
| Initial fish | 41.36 ± 1.11 | 11.21 ± 0.25 | 15.01 ± 0.51 | 74.28 ± 1.25 |
| 5% | 42.15 ± 3.12 a | 13.11 ± 0.14 a | 15.02 ± 0.33 a | 76.50 ± 0.51 a |
| 7% | 45.34 ± 0.27 b | 15.02 ± 1.01 b | 14.20 ± 0.50 a | 77.80 ± 0.25 a |
| 9% | 46.13 ± 1.12 b | 16.09 ± 0.06 b | 15.81 ± 0.37 a | 77.95 ± 1.12 a |
| 11% | 45.01 ± 0.31 b | 17.23 ± 1.23 c | 14.15 ± 0.12 a | 78.21 ± 0.12 a |
| 14% | 45.42 ± 1.17 b | 16.99 ± 1.13 bc | 14.95 ± 1.12 a |
79,21 ± 2.12 a |
Means on the same line followed by different superscripts are significantly differents (P < 0.05).
Specific growth rate (SGR; %/d) = 100 × [Ln (final body weight) - Ln (initial bodyweight)] / Duration of the experiment
Feed efficiency (FE) = (FB+DB-IB) / FD
Where IB (g) and FB (g) are the initial and final biomasses and DB (g) is the biomass of dead fish, and FD (g) is the total distributed feed quantity.
Survival rate (%) = 100 × FN/IN; (IN, FN = Initial and Final Number of fish respectively).
Coefficient of Variation (CV %) = Standard Deviation x 100/Mean.
Statistical Analysis
The mean values of final weight, SGR, FE, CV, survival rate and body composition were compared between treatments by one way analysis of variance (ANOVA 1) after verifying the homogeneity of variance using “Hartley’s test” for each treatment. Significant differences between treatments means (P<0.05) were determined using a Fisher’s test (Saville, 1990). Results are given as means ± standard deviation.
Mathematical model (dose – response) was used to assess the effect of dietary lipid level on specific growth rate of P. obscura fingerlings. The general equation of the broken line model (Robbins et al., 1979) is y = L+U(R-XLR) where L is the ordinate and R, the abscissa of the breakpoint. R is taken as the estimated requirement (dietary lipid that guarantees the maximum specific growth rate). XLR means X less than R and U is the slope of the line for XLR. By definition, R-XLR is zero when X > R.
RESULTS
Survival rates and growth performances parameters of P. obscura fingerlings during the feeding trial are shown in Table 2. Survival rates were 100% in all the treatments. They were not therefore significantly affected by the dietary lipid level (P˃ 0.05). In return, the dietary lipid level affected significantly fish growth (P<0.05) as indicated by growth parameters values (Table 2).The final body weight, weight gain, specific growth rate and feed efficiency improved as dietary lipid level increased from 5 to 7% in the diet. The specific growth rate varied from 1.31 ± 0.02 to 1.85±0.05%/day. Variation of feed efficiency among the experimental diets is shown in Figure 1. It varied from 0.38 ± 0.07 to 0.52 ± 0.07. Inclusion of dietary lipid above 7% of the diet did not produce any improvement in growth or feed efficiency (Table 2). The highest final body weight, weight gain and specific growth rate are obtained with fish fed on the diet with 7% of lipid (P<0.05).Therefore, the lowest growth performances and feed efficiency are obtained with fish fed on the diet with 14% of lipid (P<0.05).
Whole body composition is presented in Table 3 for all treatments. Crude protein and lipid are significantly affected by dietary lipid level (P<0.05). Whole body protein content increased with increasing dietary lipid levels and reached the best value with diet contained 7% of lipid level. Therefore, whole body protein obtained with 7,9,11 and 14% of lipid level are not significantly different (P˃0.05).Whole body lipids increased significantly with dietary lipid levels and fish fed on the diet with 5% of lipid had the lowest (P<0.05). Diets with 11 and 14% induced the highest body lipid (P<0.05).
Relationships between dietary lipid in diet and SGR have been used to estimate the maximum dietary lipid requirements for P. obscura fingerlings. According to the broken line model (Figure 1), maximum lipid requirement for P. obscura fingerlings is 7% of the diet.
DISCUSSION
In any treatments, survival rates (100%) was affected by the dietary lipid level. These results confirmed that P. obscura is a hardy and rustic species which has important aqua-cultural qualities (Kpoguè et al., 2013 b). The SGR obtained in this study (1.31 – 1.85%/d) are closed to those founded by Aliyu- Paiko et al. (2010) for Channa striatus (1.55 – 2.56%/d) and Zehra and Khan (2011) for C. punctatus (1.21 – 1.82%/d). Inclusion of dietary lipid above 7% exhibited significant growth deceleration. Likewise, it did not produce any improvement in feed efficiency. The same trends have been shown by Lin and Shiau (2003) according to whom, the excessive dietary lipid may reduce feed consumption and thus depress the growth of fish.Similar observation was reported by several authors. Indeed, dietary lipid serves as an energy sources and enable for protein sparing (Mishra and Samantaray, 2004). Dietary lipids are known to affect the accumulation of lipid in fish body in a number of fish species (Arockiaraj et al., 2004; Segato et al., 2005; Wang et al., 2006). Within certain limits, increased dietary lipid levels can also improve diet utilization (Du et al., 2005). However, excessive dietary lipid supplement may causes growth deceleration (Gonzalez – Felix et al., 2015) and even confer external metabolic burden to the liver (Lu et al., 2015), resulting in excessive fat deposition (Richard, 2006, Song et al., 2009; Ghanawi et al., 2011). According to Ruyter et al. (2000), excessive dietary lipid may negatively influence the ability of fish to digest and assimilate fatty acids. An increasing lipid deposition in the fish due to the excessive consumption of dietary lipid (Martino et al., 2002) can produce fatty fish that may have an unpleasant flavor and become easily rancid at postharvest (Yong et al., 2015).
According to the broken line model (Figure 1), maximum lipid requirement for P. obscura fingerlings is 7% of the diet.
This result is similar to those recommended to other snakehead species fingerlings such as Channa striatus (6.5%) (Boonyaratpalin, 1981; Aliyu-Paiko et al., 2010) and C. punctatus (7%) (Zehra and Khan, 2011). P. obscura fingerlings dietary lipid requirement is close to those determined for other carnivorous species fingerlings like Carassius auratus (8%) (Bandyopdhyay et al., 2005); Mystus monastus (6.4% - 7.4%) (Raj et al., 2007); Clarias gariepinus and Silurus glanis (5 - 10%) (Bogut and Opacak, 1996); Epinephelus malabaricus (8.7%) (Lin and Shiau, 2003) and Pangasius hypophthalmus (8.7%) (Lin et al., 2011).
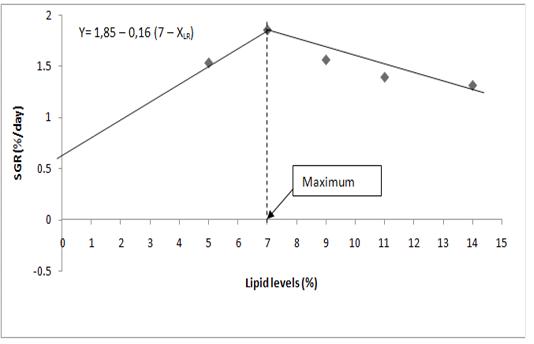
Figure 1: Determination of maximum dietary lipid requirement of P. obscura fingerlings according to the broken line model
CONCLUSION
The results of this study indicate that the maximum dietary lipid requirement for P. obscura fingerlings is estimated to be 7%. A further dietary lipid level damage the growth performances, feed efficiency and lead to an excessive body fat deposition.
ACKNOWLEDGEMENTS
This study was supported by the Ministry of Higher Education and Scientific Research of Republic of Benin.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
AUTHORS’ CONTRIBUTION
All authors contributed equally in this study and writing of the manuscript.
REFERENCES






