South Asian Journal of Life Sciences
Research Article
A Model System for Conversion of Metmyoglobin to Bright Red Myoglobin Derivatives in Organic Sausages using Potential Probiotic Lactic Acid Bacteria
Sahar Farouk Deraz*, Ashraf Abd-Elsalam Khalil
Department of Protein Research, Genetic Engineering and Biotechnology Research Institute, City of Scientific Research and Technological Applications, Bourg Elarab, Alexandria, Egypt.
Abstract | Myoglobin is a globular sarcoplasmic protein with a non-protein iron protoporphyrin IX (known as heme), and is counted as the major responsible for the meat and meat products color. In this study, lactic acid bacteria (LAB) were isolated from dairy and meat fermented products, tested for their abilities to convert metmyoglobin (MetMb) to nitrosomyoglobin (NO-Mb). Out of 280 isolates, 17 isolates were able to convert MetMb to NO-Mb. The reddening capacity was further evaluated in sausage matrices, chemically (total and cured red pigment content) and instrumentally by measuring color parameters (CIE L* a* b*). The cured pigment concentrations (curing efficiency %) were increased over processing time in all sausage samples. Converting of total heme pigment to NO-Mb was substantially increased by 52.37, 49.22, 63.53, 30.87 and 32.5%, in treated sausages with S4, S7, K2, K3 and K9 isolates, respectively compared to nitrite-sausage control (31.1%).Correspondingly, most CIE color measurements showed the aimed changes during sausage curing. Sausages supplemented with S4, S7 and K2 LAB isolates showed darkened samples related to a slight decrease in color attributes (‘ L*’). a*’ values were increased, denoting positive movement toward redness while ‘ b*’ values were decreased, indicating a decrease in yellowness. Based on their abilities to convert MetMb to red Mb derivatives, S7 and K2 isolates were elected and then identified as Pediococcus acidilactici S7 and Lactobacillus plantarum K2, respectively. Both strains showed remarkable antimicrobial activities against various positive G+ and G̶ food-borne pathogens. They also presented survival rates of 93.75 and 88.53% in simulated gastric juice at pH 2.5 for 2 h, respectively. Pediococcus acidilactici S7 and Lactobacillus plantarum K2 greatly tolerated bile salts concentrations (0.1 to 0.3%) with survival rates of 85.62 and 100% for 3 h in the presence of 0.3% bile salts, respectively. These results suggested that these strains would be healthier alternatives to nitrate and nitrite application in organic oriental sausage.
Keywords | Cured pigment, Lactic acid bacteria, Metmyoglobin, Probiotic, Sausage.
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 10, 2018 Accepted | June 06, 2018; Published | June 08, 2018
*Correspondence | Sahar Farouk Deraz, Department of Protein Research, Genetic Engineering and Biotechnology Research Institute, City of Scientific Research and Technological Applications, Bourg Elarab, Alexandria, Egypt; Email: [email protected]
Citation | Deraz SF, Khalil AA (2018). A model system for conversion of metmyoglobin to bright red myoglobin derivatives in organic sausages using potential probiotic lactic acid bacteria. S. Asian J. Life Sci. 6(1): 22-35.
DOI | http://dx.doi.org/10.17582/journal.sajls/2018/6.1.22.35
ISSN | 2311–0589
Copyright © 2018 Deraz and Khalil. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Growth of indigence request for convenient, nutritious and healthy meat products with functional value is growing. The color of meat product is one of the important attributes which leaves a powerful visual impression with the consumer and foremost substantial factor of the decision-making process to buy meat products (Hunt et al., 1991; Aberle et al., 2001). Nitrite is known as responsible agent for the development of cured color and stability of cured meats products by generating nitric oxide (NO) from added nitrite by microorganisms found in the natural flora of meat or by purposed addition of microorganisms with nitrite-reducing characteristic (Shahidi and Pegg, 1992; Sanz et al., 1997). The pigment responsible for the pink color characteristic of cured meat is a ferrous complex of myoglobin containing nitric oxide (NO), namely, nitrosyl myoglobin or NO myoglobin (NO-Mb). The red myoglobin derivative formed by the reaction of myoglobin with NO generated from nitrite (Arihara et al., 1993; Morita et al., 1998). However, nitrite is a highly interactive compound and under certain conditions (heating, lower pH, high temperature) it can act as a nitrosating agent to produce nitroso compounds which confirmed to be carcinogenic not only for children but also for adults (Cammack et al., 1999; Cassens, 1995; Honikel, 2008; Corpet, 2011). Furthermore, adding nitrite showed other high health risks to human such as vasodilator allergenic effects (Marco et al., 2006).
Myoglobin is a globular sarcoplasmic protein with a non-protein iron protoporphyrin IX (known as heme), and is counted as the major responsible for the meat and meat products color (Ajioka et al., 2006). Based on the redox state of the heme iron, color changes may occur in meat, ranging from purplish red deoxymyoglobin (DMb) in anaerobic conditions and cherry red oxymyoglobin (OMb) whereby ferrous iron is bound to oxygen, to brown metmyoglobin (MetMb) if ferrous iron is oxidized to ferric iron (Lindahl, 2005). Therefore, omitting of nitrite is leading to formation of an unacceptable dull brown color of the meat products as a result of heme pigments oxidization to MetMb (Adamsen et al., 2006).
Another critical side in relation to nitrite is it’s categorized as a preservative and therefore it is not permitted by the law in many countries to be used in products marketed as organic (Sebranek and Bacus, 2007; Sebranek et al., 2012). As a result, substitutional methods for meat curing are required to produce natural or organic processed meats that have characteristics similar to nitrite-cured meats (Bázan-Lugo et al., 2012; Sebranek and Bacus, 2007). An alternative to the use of nitrite is to add nitrosylated hemin which is synthesized before and afterwards added with proper natural antioxidant and antimicrobial agents, thereby ensuring the right color (Pegg et al., 1996; Shahidi and Pegg, 1992). Another alternative method primarily described by Sebranek and Bacus, (2007) and later used by other researches called natural curing. This process utilizes natural ingredients such as celery, that contain relatively high levels of nitrate naturally along with a starter culture of bacteria producing nitrate reductase mainly lactic acid bacteria, which in turn reducing nitrate to become nitrite (Terns et al.,2011; Keeton et al., 2012; Deraz, 2017). Another alternative method to produce pink, cured color is by utilizing the ability of some microbial to convert MetMb to NO-Mb through generation of NO from the L-arginine without a nitrification or denitrification pathway, while most of the microorganisms produced NO with reduction of nitrate and nitrite (Arihara et al., 1993; Morita et al., 1997; Xu and Verstraete, 2001). Some lactic acid bacteria (LAB) have showed the ability to turn the muscle color from brown to bright red. Among them, the strain Lactobacillus fermentum (JCM1173) which have been reported to be capable of converting Mb (Fe3+) to cured meat pigment NO-Mb (Fe2+) (Arihara et al., 1993). LAB contribute to the sensory quality and hygienic of fermented meat products mainly through their catabolism of protein and carbohydrate, resulting in pH reduction, sugar depletion, the production of antimicrobial agents, and the generation of flavor compounds (Fadda et al., 2010).
The beneficial effects of food with added live microbes (probiotics) on human health, has been increasingly promoted by health professionals. LAB have positive effect not only on a product but also on a human health through their ability to reduce the risk of tumors, amount of LDL cholesterol fraction and can also balancing the intestinal flora and stimulation immune system (Ferysiuk et al., 2018; Wójciak et al., 2015, Li et al., 2012). Probiotics must be competent to extend their functional benefits on the host through growth and/or activity in the human body (Collins et al., 1998; Morelli, 2000). It is the capability to remain viable and effective at the target site. Such capability should be verified for each potentially probiotic strain through in vitro tests to predict the ability of probiotics to function in humans. Tests such as acid and bile tolerance and antimicrobial product should be performed depending on the intended health benefit (Collins et al., 1998; Havenaar and Huisin’t Veld, 1992).
However, adequate efforts have not been made to either screen for LAB that convert myoglobin to more desirable color derivatives or for selection of probiotic strains for production of natural or organic meat and meat products. This study was designed to test newly isolated LAB strains for their ability to convert metmyoglobin to bright red derivatives and to evaluate their capacity to grow and affect color formation of nitrite-free oriental sausage. Also, the quality of formed color characteristics was quantified. Investigation the probiotic properties of promising LAB isolates was within the scope of our work as well.
MATERIAL AND METHODS
Bacterial Strain and Growth Conditions
Lactic acid bacterial isolates (280 isolates) were isolated from commercially available home-made, raw fermented sausages and karish cheese using MRS agar (De Man et al., 1960) containing 1% CaCO3 and NaCl. After incubation at 37°C, colonies which formed clear zones (due to destruction of CaCO3 by bacterial acid) were selected. Gram-positive, catalase-negative, non-motile, rods or cocci isolates were regarded as LAB and maintained at -80˚C in glycerol stocks. Cultures were purified by successive subculturing on MRS-agar. Isolates subsequently shown to express ability to generate bright red color derivatives from met myoglobin were further identified by physiological and biochemical tests as described by Collins et al. (1989), Schillinger and Lucke (1987) and Stiles and Holzapfel (1997). Carbohydrate fermentation pattern were determined using API 50 CHL and API 20 kits (Biomerieux, France) according to the manufacturer’s instructions. The isolates were then identified using the API LAB Plus software version 3.3.3 (Biomerieux). Prior to use, all LAB strains were propagated at least twice (37°C) in screw-capped test tubes containing MRS broth.
Preparation of Metmyoglobin Solution
A 20 mg/mL horse heart myoglobin (Sigma Chemical Co., St. Louis. MO) solution in 50 m M sodium phosphate buffer (pH 6.5) was heated at 50°C for 30 min to remove any residual enzyme‘s having met myoglobin reducing activity (Hagler et al., 1979). After centrifugation (10,000xg, 10 min, 4°C to remove denatured proteins) the solution was sterilized by filtration (0.45 pm pore size) and stored at 4°C for 2 wk. The resulting solution contained about 95% myoglobin in the met forms determined using the equation:
[Met] = -2.514R1 + 0.777R2+ 0.800R3 + 1.098 Eq (1)
where R1, R2, and R3 are absorbance ratios A572/A525, A565/A525and A545/A525, respectively (Krzywicki, 1982).
Screening for Metmyoglobin-Converting Lab Strains
Bacteria were screened for the ability to generate bright red color derivatives from met myoglobin incorporated into MRS (LAB) agar plates (basal media). Met myoglobin solution was added to tempered (50°C) basal media to achieve a final concentration of 2-5 mg of myoglobin per mL. After solidification, agar plates were inoculated with bacterial strains by stabbing. Plates were incubated for l-2 days at 37°C. Isolates generating a bright red color around the stab were retained for further investigation. Cultures showing met myoglobin-converting activity on agar plates were also tested in broth. Overnight cultures (100 µL) were inoculated into 10 mL MRS broth containing met myogoblin (0.5-2 mg/mL). After incubation at 37°C (MRS) for 24 h, cells were removed by centrifugation (10,000xg, 20 min, 4°C), then the supernatant was sterilized by passage through a 0.45 µm filter. Color changes of the resulting culture filtrates were checked visually (Arihara et al., 1993; Morita et al., 1994).
Sausage Preparation
A sausage mix was prepared using a blend of fresh boneless beef and beef fat obtained from a commercial slaughter/fabrication plant. All sausage samples were adjusted to approximately 21% fat. The meat and ingredient quantities per kg meat were as follows 64.55% ground beef meat, 19.36% crashed ice (water), 7.75% wheat flour, 2.29% dry milk, 1.36% salt, 0.85% corn syrup (glucose), 3.24 onion flakes, 0.26% white paper, 0.14% starch and LAB culture at 7 log cfu/g sausages in all treatments. Control sausages were treated with 0.5% sodium nitrite with no LAB cultures added. Sausages were stuffed into sheep natural casing (22-24 mm in diameter). After filling, the sausages were hung on rods for partial draining (a few hours). All patches of sausage were matured for 10 h at 15°C then kept at 20-22°C for 48 h. Two samples from each batch were taken during the 3-days processing as follows: fresh prepared sausage (1 day) and processed sausage (3 days).
Total and Cured Bigmen Analysis
Cured meat pigment and total pigment concentrations were determined chemically after extraction in 80% acetone and acidified acetone, respectively (Hornsey, 1956). The experiment including sample preparation was done under reduced light conditions to reduce pigment fading. Formation of nitroso myoglobin (NO-Mb) pigment of the sausage product (cured pigment) was conducted using a modified method of Hornsey, (1956). Duplicate 10-g samples were mixed with 40mL of acetone and 3 mL of distilled deionized water with a Polytron mixer for 1 min at speed setting 7. The sample was immediately filtered through a Whatman 42 filter paper, and the absorbance 540nm measured on the filtrate. NO-Mb concentration was calculated as follows:
A540× 290 and was recorded in parts per million (ppm). Eq (2)
Total pigment analysis was conducted using a modified method of Hornsey (1956). Duplicate 10-g samples were mixed with 40 mL of acetone, 2 mL of distilled deionized water, and 1 mL of concentrated hydrochloric acid using a Polytron mixer for 1 min at speed setting 7. The samples were allowed to stand for 1 h, then filtered through Whatman 42 filter paper and immediately analyzed. Absorbance was measured at 640 nm. Total pigment concentration was calculated as follow:
A640× 680 and was recorded in ppm.
Eq (3)
The results, reported as percent conversion, are the percent of total heme pigment converted to the cured heme pigment (wet sample basis). NO-Mb percentage was calculated by the following formula:
NO-Mb percentage = (NO-Mb (ppm)/total pigments (ppm)) ×100
Eq (4)
The heme iron content was calculated according to Karwowska and Dolatowski, 2013 as follow:
Heme iron (mg/kg) = total pigment (mg/kg)×8.82/100. Eq (5)
Color Measurments
Color was measured immediately after slicing the sausage lengthwise. Color measurements were conducted using a portable colorimeter (X-Rite, model SP 64, U.S.). The instrument was calibrated using a white standard plate (calibration plate CRA43) and the Hunter Lab color scale was used to measure color. Color was expressed using the CIE as L* (lightness), a* (redness) and b* (yellowness) units, defined by the International Commission on Illumination (CIE) in 1976, together with hue angle (H) and chroma-saturation (C*) obtained from three different cut areas of each sausage. The hue and the chroma were also calculated using the following formulas:
Chroma(C*) = 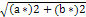 Eq (6)
Eq (6)
Hue (h)= tan−1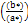 Eq (7)
Eq (7)
Data of culture measurements were recorded and means of three measurements for each treatment were calculated.
pH Measurments
Aliquots of 10 g of homogenized sausage samples were blended with 90 mL of distilled deionized water. pH of the slurry was measured using an Orion 230 A Plus pH meter (Sensorex Co., CA, USA) calibrated with phosphate buffers of 4.0 and 7.0, according to the method of Sebraneket al. (2001). The measurement was carried out in triplicate.
In Vitro evalution Of Potential Probiotic Propertie
Detection of antimicrobial activity: Isolated strains were tested for their antimicrobial potential using the spot on lawn method (Kwon et al., 2002) as follows: an overnight culture of the isolated strain was harvested by centrifugation (6,500×g for 10 min, 4°C) and was adjusted to pH 6.5 by means of 1 M NaOH to exclude antimicrobial effect of organic acid and treated with catalase (1 mg / mL) to exclude the inhibition due to H2O2 production. Indicator lawns were prepared by adding 0.125 mL of 10×diluted overnight culture of the indicator strain to 5 mL of MRS soft agar (0.75%). The contents of the tubes were gently mixed and poured over the surfaces of pre-poured MRS agar plates. To overlaid plates, 10 μL of the cell free supernatant was spotted onto the surface of different indicator lawns. After 24 h of incubation, the plates were checked for the appearance of an inhibition zone.
The antimicrobial activities of LAB isolates was detected against 8 bacterial pathogens viz. Bacillus cereus ATCC 49064, Clostridium perfringens ATCC 13124, Listeria innocua ATCC 33090, Listeria monocytogenes ATCC 19116, Listeria ivanovii Li4 (pVS2), Salmonella enteric ATCC 25566, Yersinia enterocolitica ATCC 23715, Escherichia coli as well as against three LAB strains L. sakei NCDO 2714, Lactococcus lactis subsp. Lactis ATCC 19435, Streptococcus thermophilus CCUG 30577). Listeria ivanovii Li4 (pVS2) was kindly supplied by Dr. Lars Axelsson (Matforsk, Norwegian Food Research Institute, Akershus, Norway). Lactobacillus sakei NCDO 2714 were kindly provided by Prof. Ingolf Nes (Laboratory of Microbial Gene Technology, Agriculture University of Norway). E. coli was isolated and identified at our laboratory. Media used and incubation temperatures applied for all bacterial strains are summarized in Table (5).
Tolerance to simulated gastric juice: The tested LAB isolate was propagated twice in MRS broth (1% v/v) for 16-18 h at 37°C before experimental use. The cells from 100 mL MRS culture were harvested by centrifugation (4300×g, 10 min), and washed three times in sterilized phosphate-buffered saline, pH 7.0. The washed cell pellets were then suspended in (1/10)× cultivation volume in the same buffer, hence obtaining a 10-fold increase in cell density. To 1 mL of the washed cell suspension, 5 mL of simulated gastric juice and 1.5 mL NaCl (0.5 w/v) were added. Simulated gastric juice was prepared fresh by suspending pepsin (3 g/L) in sterile saline (0.5% w/v) and adjusting the pH to 2.0, 2.5, 3.0 and 3.5 with concentrated HCl (Charteris et al., 1998). The materials were vortexed for 10 sec and incubated at 37ºC for 3 h. Aliquots of 0.1 mL were then removed at constant intervals (0, 1, 2 and 3 h) for determination of total viable count. Dilutions (in MRS) were made and cells were spreaded on MRS agar. Plates were incubated at 37ºC for 72 h before enumeration (Deraz et al., 2007).
Bile salts tolerance: Bile containing MRS broth was prepared by adding 0.1, 0.3 and 0.5% (w/v) of ox bile (Sigma-Aldrich, St. Louis, MO, USA). The cells from 100 mL 16-18 h MRS tested culture were collected by centrifugation (3400×g, 10 min), washed twice in saline (8.5 g NaCl/L) and resuspended in 10 mL MRS broth. This suspension was inoculated (1%) into MRS broth lacking or containing bile. After 0, 1, 2 and 3 h of incubation at 37ºC, viable counts on MRS agar plates were determined (Matijasic and Rogelj, 2000). Experiments of acid and bile tolerance were repeated three times in duplicate analysis.
RESULTS AND DISCSSION
The methodology applied led to totally isolation of 280 isolates derived from home-made, raw fermented sausages and karish cheese. Bacterial isolates that gram-positive and catalase-negative, non-motile were considered as presumptive LAB.
Conversion of Metmyoglobin by Lab Isolates
The lactic acid bacterial isolates were subjected to preliminary studies to evaluate their capacity to convert metmyoglobin from a brownish to a bright red derivative. Of 280 isolates tested, 21 isolates changed metmyoglobin to either bright red color around the isolate stab or brownish red rings around colonies on MRS agar plates. The formation of colonies on bright red is an indicator of the conversion of metmyoglob into nitroso myoglobin (Arihara et al., 1993; Morita et al., 1998). Out of 21 isolates, seventeen converted metmyoglobin to more desirable red color forms in MRS broth. Six of the seventeen strains (S4, S6, K2, K3, K4 and K9) consistently exhibited the greatest and most rapid activity in both broth and agar plates. MRS broths containing those bacterial cultures and metmyoglobin had a bright red color after incubation, as observed by visual inspection, whereas in control samples without bacteria culture and with metmyoglobin a light brown color remained. The degree of red derivatives showed a variation depending on the bacteria culture added.
Metmyoglobin (Mbfeiii) Conversion in Sausage Model Sytems
LAB isolates able to form nitrosylmyoglobin were further tested for their abilities enhance color of nitrite-free sausage products. The color of free nitrite sausage samples was determined to evaluate whether “reddening” bacteria in the broth model systems are also effective in the sausage matrix. The sausage production included two batches. The first batch of production investigated the 8 isolates from raw fermented sausages and the second included 9 isolates from raw fermented karish cheese. The sausage colors of different batches were evaluated chemically and instrumentally and both internal (total and cured pigment content) and external (Hunter lab Lab scan spectro colorimeter, X-Rite, model SP 64, USA) color measurements were measured.
Total and Cured Pigment Analysis
Along sausage processing, there were different interactive effects according to type of LAB strains applied on concentration of total and cured pigment (Tables 1 and 2). The general trend indicated that the cured pigment concentration increased over time but to deferent levels depended on type of strain tested. The percent of total heme pigment converted to nitric oxide heme pigment (nitrosylmyoglobin) form that expressed curing efficiency (%) of curing system are shown in Tables 1 and 2. The higher curing efficiencies are related to higher concentrations of nitrosylmyoglobin. At the end of processing time (three days), the control sausage with added nitrite showed a substantial curing efficiency (%) increase from an initial 16.06% to 31.2%. However, treated sausage samples with S4, S7, K2, K3 and K9 had similar or higher conversion levels (52.37, 49.22, 63.53, 30.87 and 32.5%, respectively) compared to control sample (31.2%). Wrolstad (2005) stated that the cured meat products are considered to be acceptable when the pigment conversion ratio is 80% or higher. Cured pigment concentrations increased with increasing of fermentation time. In general, after 48 h of fermentation, pigment conversion can be considered as acceptable because the curing efficiency obtained at that time was in the absence of nitrite addition and it was better than a concentration as low as 50 ppm of nitrite applied in control sausage sample. Zhang et al. (2007) observed similar results with L. fermentum which could cause the formation of nitrosylmyoglobin (MbFeIINO), and therefore the cured meat color in Harbin red sausage without nitrite addition. Arihara et al. (1993) were identified two environmental bacteria (Kurthia sp. K-22 and C. violaceum K-28) that converted metmyoglobin to bright red myoglobin derivatives and they assumed that the reduction of metmyoglobin resulted from the consumption of oxygen and not from the production of a specific extracellular substance. Other studies were stated that the cured pigment nitrosylmyoglobin (MbFeIINO) was considered to be produced by “reddening” bacteria that can synthesis NO from L-arginine through the role of nitric oxide synthase (Ariharaet al., 1993; Li, Shao et al., 2013; Li, Kong et al., 2013). Early studies by Fox and Thompson (1963), Acton and Dick (1977) reported that overall reaction rate in the production of NO pigment increases sharply with decreasing pH, particularly in a pH range of 5.5 to 4.5. As shown in Tables 1 and 2, adding LAB reduced pH from 6 at day-1 to final pHs ranged from 4.2 to 4.9 at the end of sausage processing which was another enhancing factor for production of nitric oxide pigment.
Meat and meat products have been found two forms of iron, heme and non heme iron. The non heme iron (free iron) promotes oxidation and product discoloration (Estevez and Cava, 2004). Furthermore, the degradation of heme iron could have nutritional consequences affecting the properties of sausage. Heme-iron is more available than non-heme iron and degradation of heme iron would reduce the nutritional value of the sausages (Estevez and Cava, 2004). All sausage samples presented higher values of heme iron content compared to control sausage sample with nitrite (Tables 1 and 2). Elias et al. (2008); Abubakr et al. (2012); Shori (2013); Uugantsetseg and Batjargal (2014), stated that the fermentation process by LAB releases a large number of bioactive peptides which known to possess high oxidative inhibitory capacity due to their ability to scavenge free radicals. Therefore our obtained results suggest that the antioxidant activities of lactic acid
Table 1: Total heme pigments, cured pigment, conversion percent and heme iron content of sausages produced with various meat-derived lactic acid bacteria*.
| LAB isolates | Total pigment (ppm)a | Cured pigment (ppm)b | Conversion percent (%)c | Heme iron content (ppm)d | Final pH | ||||
| Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | ||
| Control | 388.28±25.84 | 372.19±19.68 | 54.33±3.94 | 116.27 ±0.53 | 16.06 | 31.2 | 34.25 | 32.82 | 5.9 |
| S1 | 998.69±1.77 | 390.38±15.04 | 14.45±0.33 | 47.39±0.33 | 1.45 | 12.13 | 88.08 | 34.43 | 4.59 |
| S2 | 950.41±1.38 | 313.48±10.02 | 6.64±0.86 | 34.87±0.26 | 0.69 | 11.12 | 83.82 | 27.68 | 4.35 |
| S3 | 728.05±1.81 | 646.70±27.56 | 6.93±0.88 | 73.21±0.26 | 0.95 | 5.75 | 64.21 | 57.03 | 4.43 |
| S4 | 1023.62±1.38 | 447.89±3.77 | 4.05±0.49 | 234.57±0.35 | 0.40 | 52.37 | 90.28 | 39.50 | 4.5 |
| S5 | 805.12±2.39 | 213.07±2.39 | 4.62±0.29 | 41.52±0.26 | 0.57 | 19.48 | 71.01 | 18.79 | 4.36 |
| S6 | 1099.10±3.17 | 488.58±32.98 | 3.95±0.42 | 50.19±0.34 | 0.36 | 11.18 | 96.94 | 43.09 | 4.45 |
| S7 | 755.03±1.77 | 225.08±2.96 | 7.90±0.75 | 110.79±0.25 | 1.05 | 49.22 | 66.59 | 19.85 | 4.43 |
| S8 | 1006.85±31.73 | 517.14±4.42 | 15.60±2.31 | 51.73±0.17 | 1.54 | 10.00 | 88.80 | 45.61 |
4.42 |
*LAB generating bright red color derivatives from metmyoglobin in the absences of nitrite.
a A540× 290 and was recorded in parts per million (ppm).
b A640 × 680 and was recorded in ppm.
c NO-Mb percentage = (NO-Mb (ppm)/total pigments (ppm)) ×100.
d Heme iron (mg/kg) = total pigment (mg/kg)×8.82/100.
Table 2: Total heme pigments, cured pigment, conversion percent and heme iron content of sausages produced with various dairy-derived lactic acid bacteria*.
| LAB isolates | Total pigment (ppm)a | Cured pigment (ppm)b | Conversion percent (%)c | Heme iron content (ppm)d | Final pH | ||||
| Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | ||
| Control | 388.28±25.84 | 372.19±19.68 | 54.33±3.94 | 116.27 ±0.53 | 16.06 | 31.2 | 34.25 | 32.82 | 5.9 |
| K1 | 872.44±25.84 | 1138.09±0.59 | 35.54±0.67 | 80.63±1.26 | 4.07 | 7.08 | 76.94 | 100.3 | 4.2 |
| K2 | 710.6±2.74 | 540.82±3.93 | 22.25±0.44 | 343.62±1.00 | 3.13 | 63.53 | 62.67 | 47.70 | 4.3 |
| K3 | 835.72±5.28 | 983.28±2.35 | 36.41±4.01 | 303.55±1.00 | 4.36 | 30.87 | 73.71 | 86.72 | 4.9 |
| K4 | 784.94±3.05 | 901.68±2.83 | 34.39±0.50 | 139.78±5.12 | 4.38 | 15.50 | 69.23 | 79.53 | 4.8 |
| K5 | 663.45±2.01 | 1113.61±7.07 | 32.56±1.27 | 131.78±0.88 | 4.90 | 11.83 | 58.51 | 98.22 | 4.16 |
| K6 | 797.19±4.41 | 1128.57±0.82 | 35.16±0.63 | 136.98±0.33 | 4.41 | 12.14 | 70.31 | 99.53 | 4.3 |
| K7 | 621.69±19.92 | 1017.73±0.59 | 18.89±1.17 | 144.69±0.95 | 3.03 | 14.21 | 54.38 | 89.76 | 4.5 |
| K8 | 514.31±11.87 | 820.76±14.65 | 32.66±0.83 | 193.82±1.00 | 6.35 | 23.61 | 45.36 | 72.39 | 4.4 |
| K9 | 955.85±17.88 | 463.76±6.67 | 33.24±0.49 | 150.86±1.09 | 3,48 | 32.5 | 84.30 | 40.90 |
4.3 |
*LAB generating bright red color derivatives from metmyoglobin in the absences of nitrite.
a A540× 290 and was recorded in parts per million (ppm).
b A640 × 680 and was recorded in ppm.
c NO-Mb percentage = (NO-Mb (ppm)/total pigments (ppm)) ×100.
d Heme iron (mg/kg) = total pigment (mg/kg)×8.82/100.
bacteria stabilized the porphyrin ring of the heme mole- cule during the course of sausage processing with the same efficiency as curing salt and further inhibiting the oxidative deterioration of the sausages.
Furthermore, Table 2 shows that sausage samples processed using LAB strains isolated from dairy products contain higher concentration of heme iron at the end of processing time compared to samples processed with meat-derived isolates as indicated in Table 1. LAB were generally considered to be a rare bacterial group having no iron requirement (Bruyneel et al., 1989; Weinberg, 1997). However, recent investigations provided evidences that meat-borne lactic acid bacterium have heme and iron requirements restricted to iron sources present in the meat environment, such as myoglobin, hemoglobin, and transferrin, confirming the tight adaptation of this species to meat (Makarova et al., 2006; Duhutrel et al., 2010).
Table 3: CIE attributes of lightness (L*), redness (a*), yellowness (b*) intensity, chroma-saturation (C*) and hue angle (hº) of cured oriental sausages produced with various meat-derived lactic acid bacteria*.
| LAB isolates | CIE color values | |||||||||
| Day 1 | Day 3 | |||||||||
| L* | a* | b* | C* | h | L* | a* | b* | C*a | hºb | |
| Control | 62.75±0.55 | 14.77±0.65 | 14.51±0.68 | 20.70 | 44.9 | 61.12±0.76 | 15.81±1.00 | 13.50±0.37 | 20.79 | 40.49 |
| S1 | 61.11±0.52 | 11.28±0.41 | 12.15±0.82 | 16.51 | 47.13 | 57.26±0.74 | 12.69±0.13 | 18.29±0.34 | 22.26 | 55.24 |
| S2 | 54.77±1.21 | 10.87±0.69 | 17.46±0.50 | 20.56 | 58.09 | 55.45±0.39 | 11.04±0.42 | 17.76±0.03 | 20.91 | 58.13 |
| S3 | 58.7±0.64 | 8.11±0.17 | 14.29±0.57 | 16.43 | 60.42 | 55.2±0.73 | 10.41±0.18 | 17.89±0.18 | 20.69 | 59.80 |
| S4 | 54.55±1.15 | 10.81±0.13 | 17.96±0.18 | 20.96 | 58.96 | 54.80±0.19 | 15.28±0.39 | 16.54±0.08 | 22.51 | 47.26 |
| S5 | 56.08±0.66 | 11.52±0.11 | 18.68±0.07 | 21.94 | 58.33 | 55.44±0.94 | 12.93±0.21 | 17.98±0.44 | 22.14 | 54.28 |
| S6 | 56.38±0.84 | 10.33±0.16 | 17.74±0.26 | 20.52 | 59.79 | 56.06±0.64 | 11.52±0.11 | 18.69±0.09 | 21.95 | 58.35 |
| S7 | 65.03±1.06 | 12.79±0.57 | 14.35±0.24 | 19.22 | 48.29 | 62.63±0.27 | 15.95±0.54 | 13.12±0.27 | 20.65 | 39.43 |
| S8 | 61.96±0.87 | 12.30±0.21 | 12.66±0.05 | 17.65 | 45.82 | 59.79±0.67 | 13.96±0.55 | 13.17±0.16 | 19.19 |
43.33 |
L* (brightness, 0 to 100); a* (redness); b* (yellowness); C*: Chroma (saturation) and H* (Hue angle, tone) CIE 1976.
a Chroma (C*) = 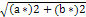
b Hue (h)= tan−1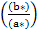
Table 4: CIE attributes of lightness (L*), redness (a*), yellowness (b*) intensity, chroma-saturation (C*) and hue angle (hº) of cured oriental sausages produced with various dairy-derived lactic acid bacteria*.
| LAB isolates | CIE Color Values | |||||||||
| Day 1 | Day 3 | |||||||||
| L* | a* | b* | C* | h | L* | a* | b* | C*a | h*b | |
| Control | 62.75±0.55 | 14.77±0.65 | 14.51±0.68 | 20.70 | 44.9 | 61.12±0.76 | 15.81±1.00 | 13.50±0.37 | 20.79 | 40.49 |
| K1 | 56.52±1.23 | 9.88±0.53 | 16.46±0.69 | 19.19 | 59.02 | 56.39±0.84 | 10.33±0.16 | 17.74±0.26 | 20.52 | 59.78 |
| K2 | 63.91±0.10 | 12.28±0.26 | 14.63±0.23 | 19.10 | 49.99 | 61.83±0.40 | 16.04±0.21 | 12.84±0.34 | 20.54 | 38.67 |
| K3 | 56.07±0.49 | 11.37±0.14 | 18.21±0.23 | 21.46 | 58.02 | 53.60±0.65 | 13.44±0.19 | 17.48±0.25 | 20.04 | 52.44 |
| K4 | 54.55±1.15 | 10.81±0.13 | 16.54±0.08 | 19.75 | 56.83 | 56.55±0.95 | 11.37±0.09 | 17.28±1.65 | 20.68 | 56.65 |
| K5 | 56.55±0.94 | 11.22±0.09 | 17.28±1.65 | 20.60 | 57.0 | 55.44±0.95 | 12.93±0.21 | 18.35±0.39 | 22.44 | 54.83 |
| K6 | 56.20±0.38 | 11.19±0.19 | 17.14±0.60 | 20.46 | 56.86 | 53.92±0.69 | 11.83±0.59 | 19.51±0.06 | 22.81 | 58.77 |
| K7 | 56.38±0.97 | 10.80±0.43 | 17.21±0.73 | 20.31 | 57.88 | 51.55±1.60 | 12.35±0.24 | 19.24±0.75 | 22.86 | 57.30 |
| K8 | 55.89±0.32 | 11.94±0.69 | 18.01±0.32 | 21,60 | 56.45 | 55.77±0.67 | 13.73±0.28 | 18.32±0.36 | 22.89 | 53.15 |
| K9 | 55.20±0.73 | 10.41±0.18 | 17.86±0.21 | 20.67 | 59.76 | 54.550.30 | 14.50±0.65 | 17.74±0.07 | 22.91 |
50.73 |
L* (brightness, 0 to 100); a* (redness); b* (yellowness); C*: Chroma (saturation) and H* (Hue angle, tone) CIE 1976.
a Chroma (C*) = 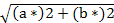
b Hue (h)= tan−1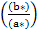
Color Cie l*, a* and b*, Chroma-Saturation and Hue Measurement
Absorption values previously used to generate the total pigment and cured pigment concentration could have some error due to chemical extraction of the pigment as stated by Martín-Sánchez et al. (2010). However, the reflectance method excludes errors due to the extraction, since the analysis is done without destroying the sample (Martín-Sánchez et al., 2010).Three-dimensional spatial models with defined coordinates of each model such as those of the Hunter L, a, b and CIE L*,a*,b* color spaces is an excellent tool to describe the meat and meat products color appearance on surface which also closely related to the visual perception (Kartika et al., 2013). Color formation during processing of sausages depends largely on nitrosomyoglobin formation (Møller et al., 2007). Changes involved in this process include decreasing the lightness (L*) as a result of drying, the increase in red green coordinate (redness) (a*) due to the formation of nitrosomyoglobin and decreasing values yellow blue coordinate (yellowness) (b*) (Perez-Alvarez et al., 1999). Processed sausages made with different LAB with nitrosomygloblin formation ability experienced various degree of the normal color development expected for traditional cured sausages. Detailed results for color attributes are depicted in Table 3 and 4. Almost all variables, L*, a*, b*, chroma-saturation (C*) and Hue angle, tone (H*) were differently affected by the type of strain added. Data presented in Tables 3 and 4 show a continuing trend in the most of the samples with L* and a* values after 3 days of processing. Generally, L* values decreased while a* values increased but to different levels after 3 days (Tables 3 and 4). All sausages showed increased L* values at the beginning of ripening with mean values ranged from 44.55 to 65.03. This trend is in agreement with those reported by Chen et al. (2016) who noticed significant increased lightness within the first hours of ripening. Sindelar et al. (2010) reported that a lighter color suggests the existence of more darkish-colored metmyoglobin pigment and less reddish-colored nitrosylmyoglobin pigment. Lightness depends on various factors, among them pH and water content. The higher the pH and water content the higher of lightness attribute (Fernandez–Lopez et al., 2000). Tables 3 and 4 show that most sausage samples in both batches had decreases lightness (L*) over the processing times. Hutchings (1994) suggested that a decrease in L* corresponds to a decrease in whiteness. According to Slinde and Nordal (1978), the dissociation of the heme group would result in a decreased lightness, i.e. darkening and thus an L* value decline.
According to Hutchings (1994), an increase in a* value is correlated to an increase in the red color of what it is being measured. a* value is usually the value which more highly correlated to visual scores than either L*- and b*-values (Precise color communication, 1998). Consumers are supposedly able to perceive a difference in a* value of 0.6 – 0.9 depending on the light source (Zhu and Brewer, 1998).The most important color parameter for nitrite-free meat products is the redness (a*) value. As shown in Tables 3 and 4, the highest redness was detected in control sample with nitrite (15.81) as well as sausage samples supplemented by LAB isolates S4, S7 and K2 with values of 15.28, 15.95 and 16.04, respectively. The data suggest that the samples inoculated with the aforementioned LAB species became much redder in color. The rest of sausage samples showed a* values ranged from 10.41 to 14.50. Increases in redness during fermentation are ascribed to a typical reaction of color development in cured sausages, in which nitrosomyoglobin is produced and favored at a lower pH (Moller et al., 2007; Nediani et al., 2017).
The yellowness (b*) values increased from day 1 to day 3 in most sausage samples of both batches, except for sausages samples supplemented with S4, S7 and K2 tended to be rather decreased (Table 3 and 4). Similar results reported during fermentation of salami sausage (Demeyer et al., 1986), and they are probably due to the different oxygen consumption levels by different microorganisms during their exponential growth phase and the decrease in oxymyoglobin, which contributes to the b* values (Cavalheiro et al., 2013). However, other studies suggested that oxidation of meat and fat present in the sausages induced by metabolites produced by microorganisms also contributing to the decrease in b* value (Demeyer et al., 1986, Sarasibar et al., 1989).The general processing sequence of the sausage samples supplemented with S4, S7 and K2, slightly changed the internal color attributes decrease of ‘ L*’, showing a darkened of the sausages. The ‘ a*’ values increased, denoting positive movement toward redness while the ‘ b*’ values decreased, indicating a decrease in yellowness.
The color can be described on the basis of hue (H*) and color intensity (C*), which are directly related to psychological color characteristics. Color intensity (C*) is the strength of a color and is that quality by which we distinguish strong and weak colors or how different the color is from gray of the same lightness, also known as the chroma or the degree of color saturation. The hue (H*) is a color attribute which describes a diminution of a color as red, yellow, green, blue, or an intermediate between any contiguous pair of these colors. Also is expressed as the descriptive color identified using an angle (Ɵ) within a “color circle (Hunter and Harold, 1987; Hunt et al.,1991; Fernandez–Lopez 2000). In this study, we observed that C* values of most LAB sausages increased along its processing to similar or higher compared to those of control nitrite-sausages. H* values also showed a more rapid development in almost all sausages (except for S7 and K2 sausages) compared to control samples. Most sausage samples showed H* values around 50 which indicating an orangish color, however S7 and K2 sausage samples showed H* values around 30 indicating a reddish color (Tables 3 and 4). At 3-days processing, C* values of S7, K2 and control samples indicated approximately equivalent color intensity with values of 20.65, 20.54 and 20.79, respectively. However, lower H* angles S7 and K2 sausages showed a better redness development (Tables 3 and 4). The obtained results of C* and H* values were consistent with increased levels of NO heme pigment produced by S7 and K2 isolates as indicated in Tables 1 and 2. Perez–Alvarez, (1996) stated that color intensity (C*) might be related to the state of the myoglobin, and would diminish as the proportion of MetMb increased.
Among the 17 isolates used in the preparation of nitrite free oriental sausage, only isolates of S4, S7 and K2 showed CIE attributes that were similar to control with added nitrite. Sausages processed with S4, S7 and K2 developed pinkish color characteristic of nitrite-cured meats (Tables 3 and 4).
Table 5: Antimicrobial activity of a cell-free pH-neutralized supernatant of Pediococcus acidilactici S7 and Lactobacillus plantarum K2
| Indicator strains | Medium and growth temperature | Antimicrobial activity | |
|
Pediococcus acidilactici S7 |
Lactobacillus plantarum K2 |
||
| Food spoilage and pathogenic bacteria | |||
|
Listeria innocua ATCC 33090 |
BHI, 37 ◦C |
- | - |
|
Listeria monocytogenes ATCC 19116 |
BHI, 37 ◦C |
++ | - |
|
Listeria ivanovii Li4 (pVS2) |
BHI, 37 ◦C |
++ | - |
|
Salmonella enteric ATCC 25566 |
Nutrient Broth, 37 ◦C |
- | +++ |
|
Bacillus cereus ATCC 49064 |
Nutreint Broth, 37 ◦C |
+ | + |
|
Yersinia enterocolitica ATCC 23715 |
Tryptose Broth, 37 ◦C |
++ | ++ |
|
Clostridium perfringens ATCC 13124 |
Trypticase Soya Broth, 37 ◦C |
- | - |
|
Escherichia coli wild |
LB Broth, 37 ◦C |
+ | |
| Other lactic acid bacteria | |||
|
Lactobacillus sakei NCDO 2714 |
MRS, 37 ◦C |
- | - |
|
Lactococcus lactis subsp. Lactis ATCC 19435 |
MRS, 37 ◦C |
- | - |
|
Streptococcus thermophiles CCUG 30577 |
MRS, 37 ◦C |
- |
- |
Moreover, formation of heme containing pigment mainly nitrosomyoglobin followed the same trend as in control sample. The low pH values has also a strong influence on the heme groups dissociated values in many fermented sausages and color is primarily attributed to nitrosomyoglobin (Slinde and Nordal, 1978). Similar results were obtained by Morita et al. (1997) and Moler et al. (2003) using L. fermentum JCM1173 and L. fermentum IFO3956 which exhibited the ability to synthesize NO from guanidine nitrogen of L-arginine.
It can be concluded that isolates S4, S7 and K2 contributed to the formation of NO-Mb in cured sausages without adding extraneous nitrite. This conclusion was supported by the measurable correlation between pink color intensity in sausages with the degree of curing efficiencies obtained by tested isolates. The data also suggested that those three isolates were capable to increase the pink color intensity comparable to that produced by 50 mg/kg of nitrite. However, using LAB isolates as a particular pink color-generating starter in free-nitrite sausage products requires further optimization of processing and fermenting conditions.
Identification of Efficient Metmyoglobin-Converting Isolates in Meat System
A couple of isolates (S7 and K2), were selected based on their ability to convert MetMb to red Mb derivatives on the oriental sausage samples. Based on obtained morphological characteristics, gram staining, catalase activity, gas production from glucose, growth at different temperatures in MRS and Carbohydrate fermentation patterns using API 50 CHL and API 20 kits, isolates S7 and K2 were identified as Pediococcus acidilactici S7 and Lactobacillus plantarum K2, respectively.
In Vitro Evaluation of Potential Probiotic Properties
This study was designed to develop a new healthier oriental sausage. Therefore, in vitro studies was conducted to establish the health benefits of promising strains viz. Pediococcus acidilactici S7 and Lactobacillus plantarum K2 as potential probiotic supplements for oriental sausages.
Detection of antimicrobial activity: One of the study targets was using probiotics in preventing and/or inhibition of food-borne pathogens. Therefore, culture free supernatant of Pediococcus acidilactici S7 and Lactobacillus plantarum K2 were tested for their antimicrobial activities against various food borne pathogenic bacteria using the modified spot-on-lawn methods. As shown in Table 5, both LAB strains showed antimicrobial activities against various gram positive and gram negative food borne pathogens. Pediococcus acidilactici S7 strain showed activity against Salmonella enteric ATCC 25566, Bacillus cereus ATCC 49064, Yersinia enterocolitica ATCC 23715 and E.coli. However, Lactobacillus plantarum K2 showed activity against Listeria monocytogenes ATCC 19116, Listeria ivanovii Li4 (pVS2), Bacillus cereus ATCC 49064 and Yersinia enterocolitica ATCC 23715. On the other hand, both strains did not show any antibacterial activity towards several species of LAB such as Lactococcus lactis subsp. Lactis ATCC 19435, Lactobacillus sake NCDO 2714 and Streptococcus thermophilus CCUG 30577 (Table 5). Based on the aforementioned results, both strains exhibited potential preservation activities since they are active against food-borne pathogenic bacteria without antagonism activity against other LAB.
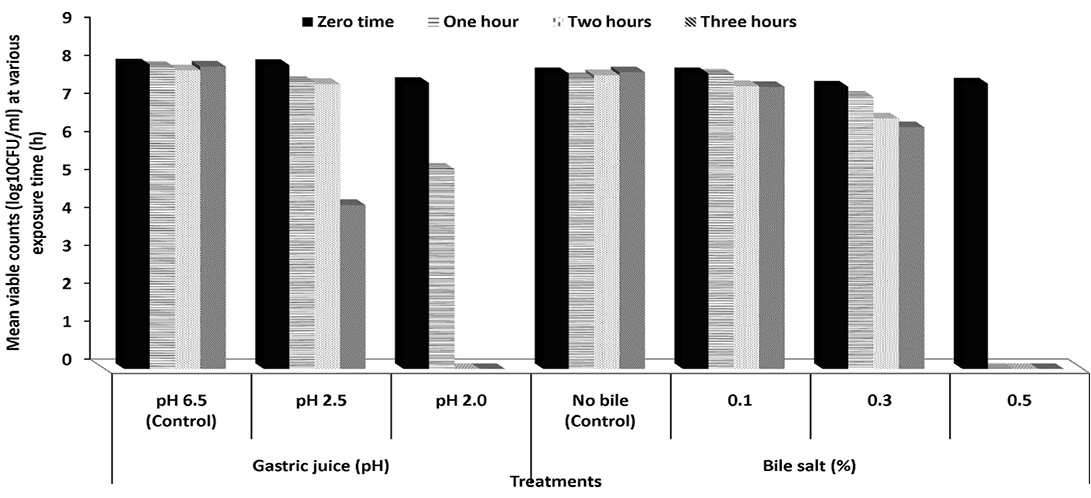
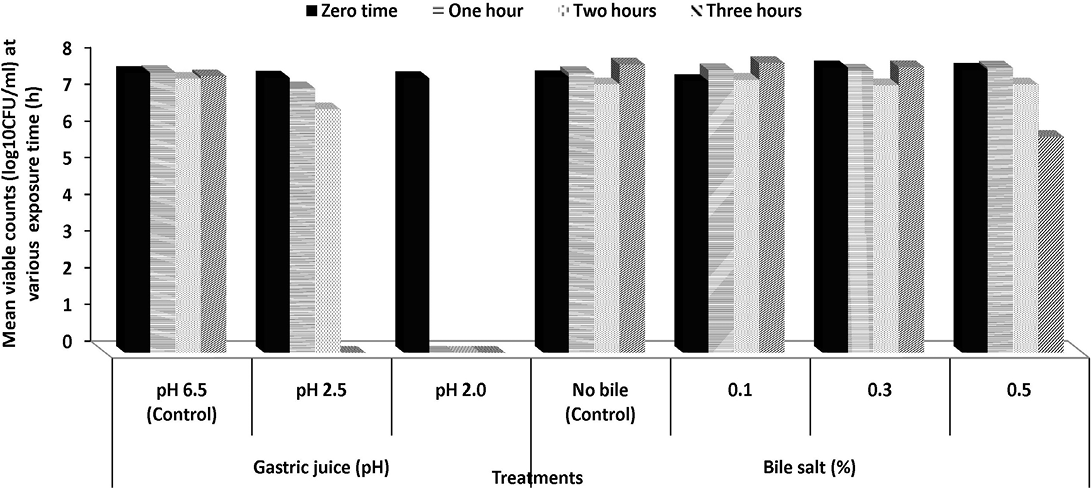
Figure 1: Survival capacities of Pediococcus acidilactici S7 to pHs of gastric juice and increment concentrations of bile salts (%) at 37 ºC
Tolerance to simulated gastric juice: To reach the intestinal tract, strains must first survive through the stomach, where a gastric acid constitutes (hydrochloric acid and enzyme) is secreted with a pH as low as 1.5 providing a powerful barrier to the entrance into the gut of ingested microorganisms as probiotic supplement (Henriksson et al., 1999).The effect of simulated gastric juice at pH 2.5 and 2.0 on the viability of Pediococcus acidilactici S7 and Lactobacillus plantarum K2 is presented in Figures 1 and 2. Both strains showed a good viability along simulated gastric juice experiments (treatments were all replicated twice). After 1 and 2 h on gastric simulation at pH 2.5, the counts of viable cells of Pediococcus acidilactici S7 was slightly decreased to 94.38 and 93.75% while counts of Lactobacillus plantarum K2 decreased to 96.13 and 88.53%, respectively, compared to the initial viable count. However, after 3 h, only 53.93% of initial viable count of Pediococcus acidilactici S7 survived. No viable cells of both strains were detected after incubation with gastric juice at pH 2.0. The obtained results suggested that Pediococcus acidilactici S7 and L. plantarum K2 may successfully transit the human stomach and would be capable of reaching the intestinal environment and functioning effectively there. Although the pH in the stomach can be as low as pH 1.5, a high survival rate at pH 2.5 for at least 3 h, is often considered satisfactory, especially as probiotic strains can be buffered by food or other carrier molecules and in fact are not directly exposed to such a low pH in the stomach (Prasad et al., 1998).
Bile salts tolerance: When evaluating the potential using of LAB as effective probiotics it is generally considered necessary to evaluate their ability to resist the effects of bile acids (Havenaar et al., 1992). Bile acids are synthesized in the liver from cholesterol and are secreted from the gall bladder into the duodenum in the conjugated form (500–700 mL/d) (Hoffman et al., 1983). The average intestinal bile concentration is around 0.3%, and may range up to an extreme of 2.0% during the first hour of digestion (Gowri and Ghosh, 2010).The ability of isolated Pediococcus acidilactici S7 and L. plantarum K2 to survive in the presence of bile salts in concentration ranged from 0.1 to 0.5% for 3 h was examined (Figures 1 and 2). Pediococcus acidilactici S7 showed tolerance to bile salts ranged from 0.1 to 0.3% along the three hours. However, after 2 and 3 h, at bile concentration of 0.3%, there was significant reduction in the viability of this strain. The reduction rates were 88.71 and 85.61% of the initial viable cells after 2 and 3 h, respectively (Figure 1). On the other hand, L. plantarum K2 showed full tolerance to all tested concentrations of bile salts. Cells not only were able to resist different bile concentrations but also slight increase was observed in their growth after 3 h of incubation in the presence of 0.1% bile salts (Figure 2). Previous studies have suggested that a common bile resistance mechanism in LAB was strongly correlated to the presence of bile salt hydrolase activity, which probably exerts a detoxification effect by catalysing the hydrolysis of glycine or taurine-conjugated bile salts into amino acid residues and unconjugated bile salts (Yin et al., 2011).
CONCLUSION
In this study, it has been found that using probiotic Pediococcus acidilactici S7 and Lactobacillus plantarum K2 can be healthier alternatives to nitrate and nitrite application in organic oriental sausage. However, application of those strains either as a particular pink color-generating starter or probiotic supplementation in nitrite-free sausage products requires further optimization with respect to the processing and fermenting conditions.
ACKNOWLEDGMENTS
The authors express their gratitude to lab technicians at Natural Products Lab, Department of Protein Research, City of Scientific Research and Technological Applications for helping in manufacturing of sausages during this study.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
AUTHOR CONTRIBUTIONS
Sahar Deraz conceived the idea, planned the study, conducted practical work and prepared early draft of manuscript. Ashraf Khalil provided technical support for the practical work and helped in compiling and paper write up.
Refrences