Journal of Animal Health and Production
Research Article
Dox Capsulated Chitosan Nanoparticles Effect as Anti-Carcinogenic Therapeutic Agent in Mice with Ehrlich Carcinoma
Safaa I. Khater1, Rasha M. M. Ezz-eldin1,2, Saydat Saad1, Fatma Gamal1, El-Ayadi D. El-Abed3, Ahmed Hamed Arisha4,5*
1Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 2Central Laboratory, Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 3Faculty of Public Health, Sabratha University, Sabratha, Libya; 4Department of Animal Physiology and Biochemistry, Faculty of Veterinary Medicine, Badr University in Cairo (BUC), Badr City, Cairo, Egypt. 5Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt
Abstract | Drug delivery systems including nanoparticles are used to enhance anticancer drugs therapeutic and pharmacological properties. A biodegradable polymer chitosan (CS) was used in this study for Doxorubicin (DOX) delivery. In Ehrlich ascites carcinoma (EAC) tumor, anticancer activity of these nanoparticles was investigated in-vivo. EAC bearing mice treated with free DOX revealed significantly increase in the levels of blood urea nitrogen (BUN) and creatinine compared with EAC bearing mice group. At the same time, the levels of BUN, creatinine and TNF-α in EAC bearing mice treated with doxorubicin capsulated chitosan nanoparticles (DOX-CNs) at dose (1-3 mg/kg) revealed significant declines when compared with EAC bearing mice treated with free DOX. The expression levels of miR-34a was found to be significantly up-regulated in DOX-treated mice accompanied by non-significantly change in the mRNA expression of renal Sirt1 compared with EAC- bearing mice group. The levels of miR-122 were non-significantly changed in renal tissue of EAC bearing mice given free DOX accompanied by significantly up-regulation in the expression levels of renal FOXO3 gene compared to EAC bearing mice and negative control groups. On the other hand, these levels were reversed in DOX capsulated chitosan nanoparticles treatment at dose (1mg/kg) but not at dose (2 or 3 mg/kg). The study reported the safety of administering DOX capsulated chitosan nanoparticles at dose (1mg/kg) and elicited anti-carcinogenic activity as compared with DOX itself.
Keywords | Ehrlich Ascites Carcinoma; Doxorubicin; Chitosan nanoparticles; Nephrotoxicity; miR 34-a; miR-122; FOXO3, Sirt1
Received | November 17, 2020; Accepted | December 01, 2020; Published | December 27, 2020
*Correspondence | Ahmed Hamed Arisha, Department of Animal Physiology and Biochemistry, Faculty of Veterinary Medicine, Badr University in Cairo (BUC), Badr City, Cairo, Egypt; Email: [email protected]
Citation | Khater SI, Ezz-eldin RMM, Saad S, Gamal F, El-Abed ED, Arisha AH (2020). Dox capsulated chitosan nanoparticles effect as anti-carcinogenic therapeutic agent in mice with ehrlich carcinoma. J. Anim. Health Prod. 9(s1): 110-120.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.110.120
ISSN | 2308-2801
Copyright © 2020 Arisha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer poses a significant threat to human life and health, and has been a leading cause of death in humans in recent years (Stewart and Wild, 2014). In the past half century, chemotherapy has greatly strengthened the treatment for cancer. Unfortunately, traditional chemotherapeutic agents lack selectivity in which tumor cells take up less than 0.1–1 % of the drugs, and the other 99% developing into healthy tissue (van der Veldt et al., 2010). The development of drug delivery systems that are successful and have therapeutic selectivity is therefore one of the main challenges facing chemotherapy today.
The nanoparticle drug delivery system (NDDS) has recently gained significant interest and has evolved rapidly (Wohlfart et al., 2011). Such a drug delivery system has been engineered to achieve a higher partial drug concentration, reduce systemic toxicity, and maintain drug release. Nanoparticles can penetrate tumors by passive targeting because of the nano-size and increased permeability and retention (EPR) effect (Shaikh et al., 2016). In addition, by binding functional ligands to the surface of nanoparticles, NDDS can selectively target tumors to reduce side effects on normal organs (Xing et al., 2017).
The last few decades saw an abundance in publications over the use of chitosan and its derivatives in the pharmaceutical industry. Chitosan is a linear polysaccharide formed by s-(1-4)-2-acetamide-d-glucose and s-(1-4)-2-amino-d-glucose units obtained from partial deacetylation of chitin (Kim and Rajapakse, 2005). The poor solubility of chitosan has been the primary obstacle to its successful use in food and biomedical applications (Ravi Kumar, 2000). Chitosan has numerous biological activities such as antibacterial, immuno-enhancing, antioxidant, inhibition of matrix metalloproteinase (MMP), anti-diabetic, anti-HIV and anti-inflammatory activity (Yang et al., 2010). It also has an antitumor effect by enhancing immune function of the body. Maeda and Kimura (Maeda and Kimura, 2004) showed in S-180-bearing mice that low-molecular-weight chitosan and chitooligosaccharide can inhibit tumor growth. (Torzsas et al., 1996) observed that the occurrence of precancerous lesions in colon cancer caused by azomethane compounds could be decreased by a chitosan-containing diet. In order to build and apply chitosan nanoparticles as drug delivery vehicles, major efforts have been made. The effect of chitosan nanoparticles on tumor cells has been specifically evaluated to interfere with the metabolism of cells, hinder cell growth or induce cell apoptosis (Allen and Martin, 2004).
Doxorubicin (DOX) is a commonly used approach to treatment of solid tumors in clinical practice. DOX takes action by influencing topoisomerase II activity and by inhibiting DNA synthesis to inhibit cell growth (normal as well as cancer cells) (Pranatharthiharan et al., 2016). Therefore, its therapeutic indexes were limited by the serious side effects of DOX, such as cumulative cardiotoxicity and myelotoxicity (Crider et al., 2012). To solve this problem, there has been a great deal of work to expand the delivery of DOX to tumors (Mallick and Choi, 2014). Several DOX-nanoparticles in malignant cancers have been investigated and developed to enhance therapeutic outcomes (Wang et al., 2017). Some DOX-nanoparticles implemented in the formation of liposome or PLGA nanoparticles because of the water solubility of DOX (Yahuafai et al., 2014).
The microRNA (miRNA) negatively controls the expression of genes by base pairing with the target messenger RNA’s 30-untranslated region (UTR) (Bagga et al., 2005). One pathway that describes dysregulated protein expression during the course of kidney disease may be elevated levels of miRNA (Ho and Kreidberg, 2012). While miRNA expression profiles have been investigated in renal diseases such as renal cancer, diabetic nephropathy, fibrosis, and polycystic disease (Chandrasekaran et al., 2012).
Numerous tests have shown that in a number of cancers, including hepatic, breast and renal cancer, miR-122 is dysregulated (Ahsani et al., 2017). It was hypothesised that miR-122 will function by targeting the Forkhead gene family FOXO3 mRNA, encoding the transcription factor FOXO3, which has consistent associations with apoptosis, survival, autophagy and proteostasis, since the expected targets of miR-122 for the Forkhead box O (FOXO) family signalling pathway were enriched (Lee et al., 2014; Kumazoe et al., 2017). It has been shown to induce miR-34a (Ghawanmeh et al., 2011). Additionally, miR-34a is a p53 activation target gene during DNA damage, meaning that the increase in miR-34a can represent the resulting apoptosis (Chang et al., 2007). MiR-34a inhibits deacetylase which activates FOXO3 (Motta et al., 2004).
The goal of this study was therefore to evaluate the toxicity and safety effects of the administration of chitosan nanoparticles as an alternative carrier for doxorubicin and free doxorubicin to mice with Ehrlich carcinoma with particular interest in DOX-induced nephrotoxicity using biomarker, histopathology and molecular changes for evaluation. We hypothesize that such combination would inhibit the progression of the toxicological activity of free doxorubicin.
Materials and Methods
Animals and Experimental Design
A total of 120 adult Balb/c male mice (average 19±1 g in weight) have been acquired from the National Cancer Institute (NCI) Cairo University. All mice were maintained under strict hygienic conditions, both water and feed were available ad-libitum. Animals reared in a 12 h light- dark cycle at a controlled temperature (21-24o C) and humidity (50-60%). They were kept for 15 days without medication for acclimatization before beginning the study in faculty of medicine – Zagzig University. The care and welfare of animals conformed to the guidelines of ZU-IACUC committee of Zagazig University, Egypt. Approval number ZU-IACUC/2/F/8/2020.
Synthesis and preparation of chitosan nanoparticles (Cs-NPs) and Doxorubicin capsulated chitosan nanoparticles (DOX-CNs)
Cs-NPs nanoparticles were prepared by ionic gelation process as previously reported (Liu and Gao, 2009). Similarly, drug loaded nanoparticles (DOX-CNs) were prepared using the drug Doxorubicin (3 mg/ml) in the solution of chitosan. DOX was added into chitosan sample at various drug: polymer ratio (w:w) prior to adding TPP solution. The suspension was centrifuged for 30 min at 15000 rpm and sedimented nanoparticles were washed and lyophilized before storage with deionized water (Ibrahim et al., 2018; Yoncheva et al., 2019).
Transplantation of Ehrlich ascites carcinoma (EAC)
The Ehrlich ascites carcinoma (EAC) cells parent line was provided by the National Cancer Institute, Cairo University, Egypt, and maintained in female Balb/ c mice as tumor cells were collected from moderately growing 7-days old donor. EAC cells were checked for viability. Each mouse was implanted subcutaneously, with 0.2 ml of freshly collected ascites fluid (dilute in 1:5 saline), into the right thigh of the hind limb of a mouse. There were approximately 2.5×106 cells in each inoculum where the tumors were formed in a single, solid form. Post-inoculation tumor development was tracked until the desired volume was around 0.3–0.6 cm3. The day of implantation of (EAC) cells is considered as the zero point (day 0) of the experiment. The total period of this experiment was 37 days (7 days before and 30 days after tumor inoculation) (Faten et al., 2018; Salem et al., 2011).
Experimental design
One hundred and twenty adult male Balb/ c mice were assigned randomly into six groups (20 mice each). Group (1) included mice were kept as negative control. Groups (2-6) included mice that were implanted subcutaneously with 0.2 ml of freshly drawn ascites fluid (diluted in saline solutions at a ratio of 1:5), into the right thigh of the hind limb of a mouse, each inoculum contained approximately 2.5×106 cells where the tumors. Group (2) included EAC- bearing mice were kept as positive control. Group (3) included EAC- bearing mice received intraperitoneal (i.p.) injection of 0.3 ml DOX (3 mg/kg body weight) once weekly for one month (Yoncheva et al., 2019). Group (4) included EAC- bearing mice received intraperitoneal (i.p.) injection of 0.3 ml doxorubicin capsulated chitosan nanoparticle (1 mg/kg body weight) once weekly for one month (Verma et al., 2018). Group (5) included EAC-bearing mice received intraperitoneal (i.p.) injection of 0.3 ml doxorubicin capsulated chitosan nanoparticle (2 mg/kg body weight) once weekly for one month (Verma et al., 2018). Group (6) included EAC- bearing mice received intraperitoneal (i.p.) injection of 0.3 ml doxorubicin capsulated chitosan nanoparticle (3 mg/kg body weight) once weekly for one month (Yoncheva et al., 2019).
Sample collection
At the end of the experiment, mice in each groups were used for blood collection from orbital venous plexus. Blood samples were taken without anticoagulant in sterile test tube for serum separation that were collected and kept for a time, centrifuged at 3000 r.p.m for 15 minutes, the resulting supernatant were collected and stored at -20oC till analysis of biochemical parameters. Immediately after scarifying, kidneys and tumor tissues were taken, weighted from all groups. Every sample was divided into two parts: One of them was wrapped in aluminum foil and put immediately in liquid nitrogen container to make snap-freezing of tissue and minimize the action of endogenous RNase, for real time-PCR analysis. The other sample was fixed at 10% buffered formalin solution at room temperature and processed for histopathological analysis.
Biochemical measurements
The serum levels of blood urea nitrogen (BUN), creatinine and serum determination of Tumor Necrosis Factor alpha (TNF-α) concentration were performed as previously reported (Saber et al., 2020).
Histopathological examination
Kidney and tumor specimens were obtained and fixed in a 10% buffered neutral formalin solution, processed and five microns thick paraffin sections were prepared, stained with hematoxylin and eosin (H&E) dyes and analysed microscopically (Layton and Suvarna, 2013; Yan et al., 2019).
Real time RT-PCR
Total RNA extraction was performed as previously described (Abu Zeid et al., 2021; Khamis et al., 2020; Mansour et al., 2020). For mRNA, 500 ng of that total RNA was reverse-transcribed as previously described (Arisha and Moustafa, 2019; Hussein et al., 2020), while for miRNA, 10 ng were reverse-transcribed using the TaqMan™ Small RNA Assays (ThermoFisher Scientific, Waltham, MA, USA) following manufacturer instructions. The Stem-loop RT and miR specific primers, and the universal reverse primer were designed using the (http://genomics.dote.hu:8080/mirnadesigntool) assay design software (Czimmerer et al., 2013). Sangon Biotech synthesised all the primers used (Beijing, China) (Table 1). The real-time PCR was performed using Maxima SYBR Green/Rox qPCR Master Mix (2X) (ThermoFisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. The relative expression of each gene normalized to either housekeeping β-actin (for mRNA) or U6 (for miRNA) was reported as fold change by a 2−ΔΔCT relative to control (Livak and Schmittgen, 2001).
Data analysis and statistics
Statistical analysis was applied by SPSS (IBM SPSS Statistics for Windows) version 24 and figures were generated using GraphPad prism 8 software (GraphPad Software Inc., San Diego, CA, United States). Data
Table 1: Specific real time PCR primers for examined genes.
| Gene | Primer | |
| miRNA 34a | Forward primer | GTGTTTTTTGGCAGTGTCTTAGC |
| Stem loop | GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAAC ACAACC | |
| miRNA 122 | Forward primer | GGTGTGGAGTGTGACAATGG |
| Stem loop | GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAAC CAAACA | |
| U6 | F | CCAACTCCTGCCACTAGAGC |
| R | TCATACTTCAGGCCCTTCTCC | |
| Sirt1 | F | CGGCTACCGAGGTCCATATAC |
| R | CAGCTCAGGTGGAGGAATTGT | |
| FOXO3 | F | CTCTCAGGCTCCTCACTGTA |
| R | ATGAGTTCACTACGGATGAT | |
| ß-actin | F | CACTGTCGAGTCGCGTCC |
| R | CGCAGCGATATCGTCATCCA | |
were presented as means ± SEM. Statistical comparisons between groups were performed using ANOVA followed by post hoc Tukey test. A P-value < 0.05 was presented as statistically significant.
Results
Histopathological examination of the entire Ehrlich carcinoma tumor sections
Confirmation of the presence of Ehrlich carcinoma tumor in mice infected with Ehrlich Ascitic Carcinoma (EAC) via examination of different sections from the solid masses collected at necropsy before and after starting the different treatments under light microscope revealed tumor tissue that massively infiltrated the subcutaneous layer and formed solid sheets of undifferentiated tumor cells which separated by necrotic areas admixed with inflammatory cells. The tumor cells showed malignancy criteria which include high grades of anaplasia, pleomorphism, and abnormal mitotic activity as shown in Figure 1 A & B.
Effect of free DOX and DOX-CNs at different doses on BUN (mg/dl), serum creatinine (mg/dl) and TNF-α (pg/ml) level
As shown in figure (2A), the obtained results revealed that significant increase in blood urea nitrogen level in EAC bearing mice given free DOX compared to negative control group. However, BUN level is significant decrease in groups treated with different doses of DOX-CNs compared to EAC bearing mice given free DOX. Treatment with DOX-CNs at 2mg/kg and 3 mg/kg were significant decrease in BUN level when compared with EAC bearing mice given free DOX. While treatment with DOX-CNs (1 mg/kg) could improve BUN level compared to EAC bearing mice given free DOX.
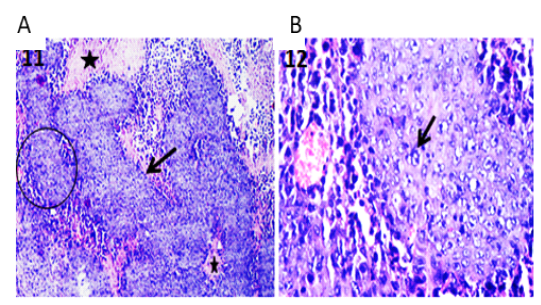
Figure 1: Photomicrographs of tumor sections of Ehrlich Ascetic Carcinoma (EAC) bearing mice. (A) Tumor tissue showing solid sheets of undifferentiated tumor cells (arrow) and surrounding necrotic areas (stars) admixed with inflammatory cells. H&E X100. (B) High magnification of the previous figure to show solid tumor sheet with malignancy criteria (arrow) and surrounding round cells. H&E X400.
As shown figure (2B), the results showed that the serum creatinine levels were significantly higher after administration of free DOX compared to their levels in EAC bearing mice and negative control groups. On the other hand, there has been a significant decline in in serum levels of creatinine of EAC bearing mice given DOX-CNs at different doses compared to EAC bearing mice given free DOX. Treatment with DOX-CNs at (2mg/kg) and (3 mg/kg) were significant decrease in creatinine level when compared with EAC bearing mice given free DOX. While treatment with DOX-CNs (1mg/kg) improved serum creatinine activity compared EAC bearing mice given free DOX.
Cytokine (TNF-α) is critical for tumor growth. EAC bearing mice showed significant elevation in TNF-α level during tumor progression when compared to normal mice. Treatment with free DOX showed significant decrease in TNF-α level when compared to EAC bearing mice group. Whereas DOX-CNs treated group in a dose (1mg/kg) showed significant decreased in the levels of TNF-α compared to EAC bearing mice given free DOX and EAC bearing mice groups as shown in figure (2C).
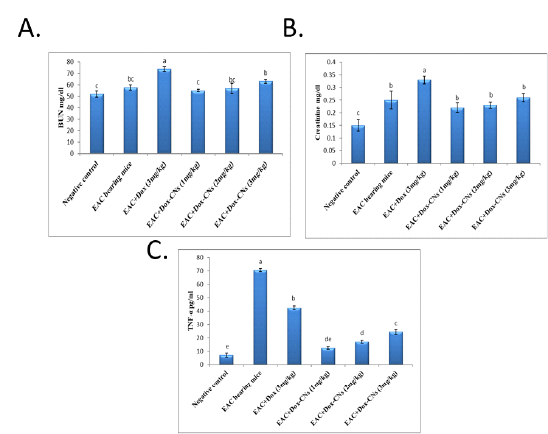
Figure 2: Effect of free DOX and DOX-CNs at different doses on (A) BUN (mg/dl), (B) serum creatinine (mg/dl) and (C) TNF-α (pg/ml) level. Values are mean ± SEM of 10 animals per experimental group. Means bearing different superscripts were significantly different at P < 0.05.
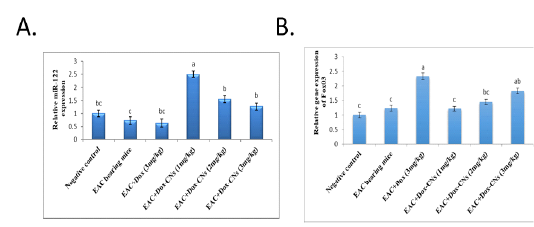
Figure 3: Effect of free DOX and DOX-CNs at different doses on relative expression of miR-34a (A) and its target Sirt1 (B) in renal tissue. Values are mean ± SEM. Means bearing different superscripts were significantly different at P < 0.05
Effect of free DOX and DOX-CNs at different doses on Relative expression of miR-34a and its target Sirt1 in renal tissue
The results presented in figure (3A) confirmed that the expression levels of miR-34a were significantly up-regulated in the renal tissue of free DOX-treated mice compared to EAC bearing mice and negative control groups. As treatment with DOX-CNs (1 mg/kg) was significantly decreased in the expression of miR-34a levels compared to EAC bearing mice given free DOX. However, EAC bearing mice treated with at dose DOX-CNs (3 mg/kg) was non significantly decreased in the expression of miR-34a levels compared to EAC bearing mice given free DOX. The results presented in figure (3B) detected the expression of Sirt1 in renal tissue of mice received free DOX or DOX-CNs treatments. Results revealed that free DOX-treated group non-significant down-regulated in the expression levels of Sirt1 gene in renal tissue of mice compared with EAC- bearing mice and negative control groups. However treatment with DOX-CNs at dose (2mg/kg) and (3mg/kg) were non-significant up-regulated in renal Sirt1 gene expression when compared with EAC- bearing mice received free DOX. But, renal Sirt1 gene expression was significant up-regulated in EAC- bearing mice post treatment of DOX-CNs (1mg/kg) compared with EAC- bearing mice received free DOX and EAC- bearing mice groups.
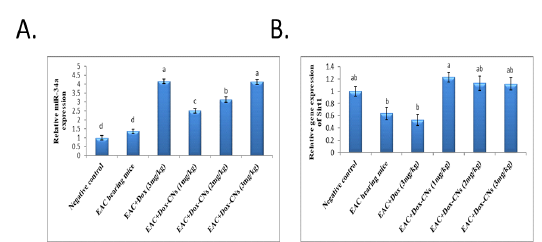
Figure 4: Effect of free DOX and DOX-CNs at different doses on relative expression of miR-122 (A) and its target FOXO3 (B) in renal tissue. Values are mean ± SEM. Means bearing different superscripts were significantly different at P < 0.05.
Effect of free DOX and DOX-CNs at different doses on Relative expression of miR-122 and its target FOXO3 in renal tissue
The results presented in figure (4A), revealed that the expression levels of miR-122 was non- significantly down-regulated in renal tissue of EAC bearing mice given free DOX group compared to negative control and EAC bearing mice groups. Whereas mice bearing EAC treated with DOX-CNs at dose (1mg/kg) showed significantly up-regulated the expression levels of miR-122 when compared with EAC bearing mice given free DOX. However, mice bearing EAC treated with DOX-CNs at doses (2mg/kg) and (3mg/kg) showed non significantly up-regulated of expression levels of miR-122 when compared with EAC bearing mice given free DOX. As shown in figure (4B), the expression levels of FOXO3 gene in renal tissue of mice were significantly up regulated in mice treated with free DOX compared to EAC bearing mice and negative control groups. The obtained results revealed that significant down-regulation of expression levels of renal FOXO3 gene in EAC bearing mice given DOX-CNs (1mg/kg) compared to EAC bearing mice received free DOX group. However, the expression levels of renal FOXO3 gene in EAC bearing mice given DOX-CNs at dose (3mg/kg) was non significantly decreased when compared to EAC bearing mice received free DOX group.
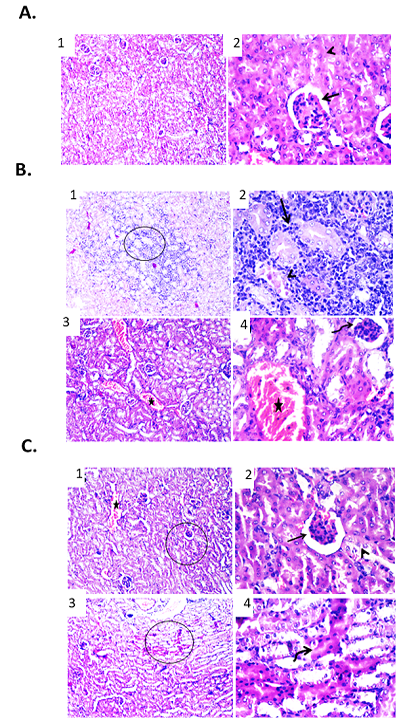
Figure 5: Effect of free DOX at different doses on renal tissue of male mice infected with Ehrlich Ascetic Carcinoma (EAC). (A) Photomicrographs of kidney sections of negative control group. (1) Kidney sections showing normal renal parenchyma. H&E X100. (2) High magnification of the previous figure to show normal tubular epithelium (arrowhead) and glomerular structures (arrow). H&E X400. (B) Photomicrographs of kidney sections of EAC bearing mice group. (1) Kidney showing necrotic renal tubules and metastasized neoplastic cells admixed with mononuclear inflammatory cells between renal tubules. H&E X100. (2) High magnification of the previous figure to show necrotic renal tubules (arrowhead) and metastasized neoplastic cells admixed with mononuclear inflammatory cells between renal tubules (arrow). H&E X400. (3) Kidney showing shrinkage of some glomerular structures and congestion of renal blood vessels. H&E X100. (4) High magnification of the previous figure to show shrinkage of glomeruli (curved arrow) and congestion of renal blood vessels (star). H&E X400. (C) Photomicrographs of kidney sections of EAC bearing mice treated with free DOX (3 mg/kg). (1) Kidney showing congested renal blood vessels (star) and shrunken glomeruli. H&E X100. (2) High magnification of the previous figure to show degenerative changes of some renal tubular epithelium (arrowhead) and shrunken glomeruli (arrow). H&E X400. (3) Kidney showing apoptosis of some renal tubules. H&E X100. (4) High magnification of the previous figure to show apoptotic renal tubular epithelium with deeply eosinophilic cytoplasm and pyknotic nuclei (curved arrow). H&E X400.
Effect of free DOX on renal tissue of male mice infected with Ehrlich Ascetic Carcinoma (EAC)
Renal sections examination of the various groups revealed marked differences in the cellular features. In normal mice, kidney sections showed normal renal tubular epithelium and glomerular structures as shown in Figure 5A. While, in mice infected with Ehrlich Ascetic Carcinoma (EAC) showed metastasized neoplastic cells admixed with mononuclear inflammatory cells between renal tubules. Furthermore, some renal tubules showed necrotic change. Other examined sections exhibited shrinkage of glomeruli and congestion of some renal blood vessels as shown in Figure .5B. Kidney sections of EAC bearing mice treated with free DOX (3 mg/kg) revealed degenerative changes within some renal tubular epithelium. Congested renal blood vessels and shrunken glomeruli were also detected. Other examined field showed apoptotic renal tubular epithelium that appeared shrunken with deeply eosinophilic cytoplasm and pyknotic nuclei as shown in Figure. 5C.
Effect of DOX-CNs at different doses on renal tissue of male mice infected with Ehrlich Ascetic Carcinoma (EAC)
In comparison with free DOX, EAC bearing mice treated with DOX-CNs (1 mg/kg) showed improvement of renal architecture of most examined sections with dilated lumen of some tubules. Other examined section showed degenerative changes and apoptosis of some renal tubular epithelium as shown in Figure. 6A. Renal sections from DOX-CNs (2 mg/kg)-treated EAC bearing mice revealed apparently improvement of kidney tissues architecture. However, edema and extravasated erythrocytes were seen between some shrunken renal tubules. Other examined field showed degenerative changes and apoptosis in some renal tubular epithelium as shown in Figure. 6B. However, the kidney tissue sections from DOX-CNs (3 mg/kg)-treated EAC bearing mice showed necrotic some renal epithelium and apoptosis of clusters of epithelial lining other renal tubules which appeared shrunken with deeply eosinophilic cytoplasm and pyknotic nuclei. Other examined field revealed dilated renal blood vessels with perivascular edema and protein casts in the lumen of renal tubules as shown Figure. 6C.
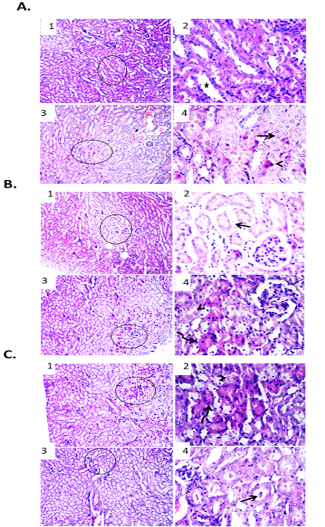
Figure 6: Effect of DOX-CNs at different doses on renal tissue of male mice infected with Ehrlich Ascetic Carcinoma (EAC). (A) Photomicrographs of kidney sections of EAC bearing mice treated with DOX-CNs (1 mg/kg). (1) Kidney showing normal renal parenchyma with dilated lumen of some tubules. H&E X100. (2) High magnification of the previous figure to show dilated lumen of some tubules (star). H&E X400. (3) Kidney showing degenerative changes and apoptosis of some renal tubular epithelium. H&E X100. (4) High magnification of the previous figure to show degenerative changes (arrow) and apoptosis (arrowhead) of some renal tubular epithelium. H&E X400. (B) Photomicrographs of kidney sections of EAC bearing mice treated with DOX-CNs (2 mg/kg). (1) Kidney showing apparently normal renal parenchyma with shrunken some renal tubules. H&E X100. (2) High magnification of the previous figure to show edema and extravasated erythrocytes between some shrunken renal tubules (arrow). H&E X400. (3) Kidney showing degenerative changes and apoptosis in some renal tubular epithelium. H&E X100. (4) High magnification of the previous figure to show degenerative changes (arrowhead) and apoptosis (curved arrow) in some renal tubular epithelium. H&E X400. (C) Photomicrographs of kidney sections of EAC bearing mice treated with DOX-CNs (3 mg/kg). (1) Kidney showing necrotic some renal epithelium and apoptosis of clusters of epithelial lining renal tubules. H&E X100. (2) High magnification of the previous figure to show necrotic some renal epithelium (arrowhead) and apoptosis of clusters of epithelial lining renal tubules (curved arrow). H&E X400. (3) Kidney showing protein casts in the lumen of renal tubules. H&E X100. (4) High magnification of the previous figure to show protein casts in the lumen of renal tubules (arrow). H&E X400.
Discussion
Nanotechnology is one of most critical areas of science research especially used in the creation of a new model of drug delivery system with greater selectivity targeting and improved delivery performance, capable of achieving limitations and improving the overall pharmacological properties of anticancer drugs by taking advantage of tumor microenvironmental implications. Doxorubicin accumulates in healthy tissues like the lung, kidney, testis and liver, leading to structural and functional changes in these tissues (Pugazhendhi et al., 2018; Shivakumar et al., 2012). Doxorubicin has significant adverse kidney effects (Saad et al., 2001). Doxorubicin tends to accumulate in the glomerulus and triggers the kidney to undergo significant damage. Nevertheless, several previous experiments have shown that reactive oxygen species and free radicals are significant factors leading to the nephrotoxicity caused by DOX (Liu et al., 2007). The current research is critically examining doxorubicin’s therapeutic potential in the form of chitosan nanoparticles against EAC tumor.
In the current study, serum creatinine and BUN levels, as kidney injury markers, were assessed. A sensitive indicator of renal disorders is BUN. The level of creatinine in serum is considered to be more sensitive test of kidney function than BUN by way of kidney injury is the only cause of elevated creatinine as reported by (Chauhan et al., 2016). The effect of free doxorubicin and doxorubicin capsulated chitosan nanoparticles on the blood concentration of these markers was recorded. In the free Doxorubicin treated mice, the serum levels BUN and creatinine were significantly higher compared their levels with EAC bearing mice and negative control groups. Furthermore, there was significant reduction in serum levels of BUN and creatinine of EAC bearing mice given Dox-CNs at different doses compared to EAC bearing mice given free DOX.
Treatment with DOX-CNs at (2mg/kg) and (3 mg/kg) were significant decrease in BUN and creatinine levels when compared with EAC bearing mice given free DOX. While treatment with DOX-CNs (1 mg/kg) could improve BUN and creatinine levels compared to EAC bearing mice given free DOX. These changes in the BUN and creatinine levels, may be due to the elevated clearance by the kidney of various drug formulations. Interestingly, other studies have reported that renal degradation and apoptosis have been observed in DOX-administered animals, but serum creatinine levels are within normal range (Weening et al., 1983). This finding was fully agreed with (Chauhan et al., 2016) which considered BUN and creatinine levels to be more than normal reference levels. Therefore, it is proposed that chitosan nanoparticles capsulated with doxorubicin have protective effects on glomerular toxicity caused by doxorubicin. These findings were similar to liposomal DOX tumor treatment in which the toxicity of the drug to the liver, heart and kidney is reduced due to lower cumulative dose of DOX in these tissues compared to that of free drug (Goyal et al., 2005).
TNFα is a critical mediator of apoptosis under physiological conditions (Twomey et al., 2015). The present study shows that EAC bearing mice showed significant increase in TNF-α level during tumor progression when compared to normal mice. Treatment with free DOX showed significant decline in TNF-α level when compared to EAC bearing mice group. Whereas treatment with doxorubicin capsulated chitosan nanoparticles at different doses (1mg/kg), (2mg/kg) and (3mg/kg) of the experimental mice-bearing EAC provided a significant decrease in levels of TNF-α against the Ehrlich carcinoma-bearing group when compared to EAC bearing mice given free DOX and EAC bearing mice groups. TNF-α was the principal inflammatory response mediator (Nasti et al., 2009). The decrease in levels of TNF-α suggests an anti-effect that could delay the progression of cancer (Agrawal et al., 2011). In EAC carrying mice, oxidative stress can be related to the elevated cytokine (TNF-α) level. This may have stimulated the immune response and triggered the transcriptional factor NFπB which in turn increased the levels of TNF-α. Treatment with doxorubicin-capsulated chitosan nanoparticles reduced EAC bearing mice’s TNF-α which rejuvenated the scavenging process to remove any ROS or free radical forms (Jin et al., 2016).
Hyperemia in the kidneys was found at the histological examination in EAC carrying mice group and EAC carrying mice treated with free doxorubicin, while there were no major pathological lesions in other groups. In mice infected with EAC showed metastasized neoplastic cells admixed with mononuclear inflammatory cells between renal tubules. Furthermore, some renal tubules showed necrotic change and exhibited shrinkage of glomeruli and congestion of some renal blood vessels. Whereas kidney sections of EAC bearing mice treated with free DOX (3 mg/kg) revealed degenerative changes within some renal tubular epithelium and congested renal blood vessels and shrunken glomeruli. In comparison with free DOX, EAC bearing mice treated with DOX-CNs (1 mg/kg) showed improvement of renal architecture of most examined sections with dilated lumen of some tubules and showed degenerative changes and apoptosis of some renal tubular epithelium. And also, renal sections from DOX-CNs (2 mg/kg)-treated EAC bearing mice revealed apparently improvement of kidney tissues architecture. However, edema and extravasated erythrocytes were seen between some shrunken renal tubules. However, the kidney tissue sections from DOX-CNs (3 mg/kg)-treated EAC bearing mice showed necrotic some renal epithelium and apoptosis of clusters of epithelial lining other renal tubules which appeared shrunken. Srdjenovic et al. (2010) and Lui et al. (1986) reported similar findings of nephropathy in rats treated only with doxorubicin. Shivakumar et al. (2012) stated that, as observed in this study, DOX has the potential to cause injury to the kidney tissue from glomerulonephritis. In comparison with free DOX, EAC bearing mice treated with DOX-CNs at dose (1 mg/kg) in the present study have alleviated the toxic effect of doxorubicin on mice’s kidneys. This finding, agreed with MGNPs-DOX administration, demonstrated the highest therapeutic anticancer efficacy and the lowest systemic toxicity relative to free DOX in the presence of external magnetic fields (Elbialy et al., 2015).
Histopathological analyses demonstrated the high growth rate of the EAC bearing mice group. Tumor section of EAC bearing mice group revealed a neoplastic characteristic with normal focal and diffuse necrosis percentage. Sections from the solid masses collected at necropsy before and after starting the different treatments under light microscope revealed tumor tissue that massively infiltrated the subcutaneous layer and formed solid sheets of undifferentiated tumor cells which separated by necrotic areas admixed with inflammatory cells. The tumor cells showed malignancy criteria which include high grades of anaplasia, pleomorphism, and abnormal mitotic activity.
In this study, the expression levels of miR-122 was non- significantly down-regulated in renal tissue of EAC bearing mice given free DOX group compared to negative control and EAC bearing mice groups. Whereas mice bearing EAC treated with DOX-CNs at dose (1mg/kg) showed significantly up-regulated of expression levels of miR-122 when compared with EAC bearing mice given free DOX. However, mice bearing EAC treated with DOX-CNs at doses (2mg/kg) and (3mg/kg) showed non significantly up-regulated of expression levels of miR-122 when compared with EAC bearing mice given free DOX. Our results represent the first evidence that miR-122 decrease triggers FOXO3 induction during AKI. Our findings have consistently reported the expression levels of FOXO3 gene in renal tissue of mice were significantly up regulated in mice treated with free DOX compared to EAC bearing mice and negative control groups. However, the obtained results revealed that significant down-regulation of expression levels of renal FOXO3 gene in EAC bearing mice given DOX-CNs (1mg/kg) compared to EAC bearing mice received free DOX group. However, the expression levels of renal FOXO3 gene in EAC bearing mice given DOX-CNs at dose (3mg/kg) was non significantly decreased when compared to EAC bearing mice received free DOX group
FOXO3 induction was also detectable by PCR suggesting that FOXO3 may serve as a marker of tubular cell injury. As FOXO3 was an important target of the regulatory defined. Since FOXO3 was a key target of the established miR-122 (Coomans de Brachène and Demoulin, 2016). FOXO3 is a crucial gene for human survival and participates in various processes in human physiology, including homeostasis of glucose, apoptosis, immunity, homeostasis of stem cells, autophagy and suppression of tumors (Lee and Dong, 2017; Martins et al., 2016).
Our data indicate that the exposure to free DOX showed significantly up-regulated in the expression levels of miR-34a in the renal tissue compared to EAC bearing mice and negative control groups. As treatment with DOX-CNs (1 mg/kg) was significantly decreased in the expression of miR-34a levels compared to EAC bearing mice given free DOX. However, EAC bearing mice treated with at dose DOX-CNs (3 mg/kg) was non significantly decreased in the expression of miR-34a levels compared to EAC bearing mice given free DOX. And also results revealed that free DOX-treated group non-significant down-regulated in the expression levels of Sirt1 gene in renal tissue of mice compared with EAC- bearing mice and negative control groups. However, treatment with DOX-CNs at dose (2mg/kg) and (3mg/kg) were non-significant up-regulated in renal Sirt1 gene expression when compared with EAC- bearing mice received free DOX. But, renal Sirt1 gene expression was significant up-regulated in EAC- bearing mice post treatment of DOX-CNs (1mg/kg) compared with EAC- bearing mice received free DOX and EAC- bearing mice groups.
Conclusions
In conclusion, this work confirmed that chitosan nanoparticles loaded with DOX have fewer nephropathic effects compared with the free DOX in EAC bearing mice. The study thus indicates that DOX-loaded chitosan nanoparticles can reduce the off-target effects of DOX in mice. This study disclosed the safety and resistance of repeated dose administration of the DOX-loaded chitosan nanoparticles in EAC-bearing mice due to low DOX exposure to vital organs, decreasing the risk of nephrotoxicity as a result.
conflict of interest
No conflicts of interest were declared by the authors, financial or otherwise.
Authors contribution
All authors performed the experiments, analyzed the data, interpreted the results of the experiments, and drafted the manuscript.
References






