Advances in Animal and Veterinary Sciences
Research Article
Evaluation of Superphosphate and Meta-bisulfide Efficiency in Litter Treatment on Productive Performance and Immunity of Broilers Exposed to Ammonia Stress
Essam S. Soliman1*, Rania A. Hassan2
1*Department of Animal Hygiene, Zoonosis & Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt, 41522; 2Department of Animal Wealth Development, Animal Production Division, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt, 41522.
Abstract | Litter treatments used to improve chemical composition; Pest control; reduction of aerial ammonia concentration and enhance productive performance. The efficiency of superphosphate and meta-bisulfide litter treatments on productive performance; biochemical profile; antioxidant assay; immune organs weight and immunoglobulin concentrations were evaluated in broilers exposed to ammonia stress. Three out of four litter trays (4 × 1m3 capacity; superphosphate treated, meta-bisulfide treated and untreated control positive litter trays) and three out of four Ross broiler groups (4 × 100; raised on superphosphate treated; meta-bisulfide treated and untreated control positive litters) were exposed to 60 ppm ammonia for 3 days and 8 – 10 hours daily for 38 days in laboratory and field trials, respectively. A total of 1656 samples (456 air, 200 whole blood, 200 sera, 200 intestinal swabs and 600 organs) were collected. Laboratory trial revealed a highly significant reduction (P ˂ 0.001) in aerial ammonia concentration, litter pH and moisture of superphosphate treated litter tray. Field evaluation revealed a highly significant reduction (P ˂ 0.001) in aerial ammonia concentration; Glutathione Peroxidase (GSH-Px); Total bacterial and Enterobacteriaceae counts, a highly significant increase (P ˂ 0.001) in body weight; performance index; bursa’s weight; globulin and immunoglobulin concentrations (IgG, IgA and IgM) in broilers raised on superphosphate treated litter. Meanwhile, a highly significant reduction (P ˂ 0.001) in Glutathione Reductase (GSH-Rx) and a highly significant increase (P ˂ 0.001) in weight gain; spleen and thymus weights and biochemical parameters (Total Protein; Albumin; ALT; AST and Creatinine) in broilers raised on meta-bisulfide treated litter. In conclusion, superphosphate and meta-bisulfide proved a great efficiency in litter treatment with little differences in between, thus litter treatments are essential to reduce the damage caused by elevated ammonia concentration in broiler houses.
Keywords | Ammonia, Broilers, Litter, Meta-bisulfide, Performance, Superphosphate.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | April 14, 2017; Accepted | June 10, 2017; Published | June 15, 2017
*Correspondence | Essam S. Soliman, Department of Animal Hygiene, Zoonosis & Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt; Email: [email protected]
Citation | Soliman ES, Hassan RA (2017). Evaluation of superphosphate and meta-bisulfide efficiency in litter treatment on productive performance and immunity of Broilers Exposed to Ammonia Stress. Adv. Anim. Vet. Sci. 5(6): 253-259.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.6.253.259
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Soliman and Hassan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Litter as a bedding material is required to maintain warmness in winter; reduce dampness and ammonia levels, as well as act as hindrance against the ground (Bjedov et al., 2013). Quality of litter material is important in poultry industry as it affects their health and production and can be considered a vehicle for potential micro-organisms depending on litter conditions, a state enforces the usage of litter chemical treatments which aim to decrease microbial load and improve performance traits. Management practices in poultry farms essential to control litter pH and moisture content to decrease the microbiota (Lee et al., 2011) and allow natural scratching behavior on floor (Karamanlis et al., 2008; Garcia et al., 2010; Škrbić et al., 2012).
Litter conditioners might reduce ammonia levels inside poultry houses by reducing pH and water activity, which in turn affect the survival of litter microorganisms. Conditioners, phosphoric acid, aluminum chloride, superphosphate and sodium bisulfide have been used successfully to reduce litter pH, ammonia volatilization and inhibit microbial activity (Nagaraj et al., 2007). Litter treatments might be cost-effective and justifiable for many reasons: extremely cold weather; persistent disease challenges; severe vaccination reactions; reduction of ammonia-related stress; prolonged litter reuse and increased bird density.
Low-cost broiler production required concentrated and confined production systems with birds raised in environmentally controlled houses under uniform management practices. Nitrogen conversion within excreta into ammonia influenced by different factors including temperature, humidity, litter pH and ventilation rate (Miles, 2008). Acidic treatments of poultry litter reduced volatile ammonia through decreasing pH, which in turn changed the NH4+ / NH3 balance towards the formation of more non-volatile NH4+, while ammonia volatilizes for the lack of electrical charges (Moore Junior et al., 2000).
Superphosphate is a compound produced by treating rockphosphate with sulfuric acid or phosphoric acid, or a mixture of both. It is the principal carrier of phosphate, and one of the world’s most important fertilizers. Ordinary superphosphate is a gray powder that is virtually non-caking and of average drill ability; contained about 20% available phosphate. Sodium bisulfide, a dry anhydrous crystalline acidifier, is used widely by the broiler industry to control NH3. It is readily soluble in water, and a 5% aqueous solution has a pH <1. Sodium bisulfide reduces NH3 volatilization through lowering the litter pH, interacting with uric acid, and limiting the growth of microbial populations that generate NH3 gas (Pope and Cherry, 2000). Sodium bisulfide is hygroscopic and binds NH3 to form stable ammonium sulfate.
The aim of this study was evaluating the efficiency of superphosphate and meta-bisulfide in litter treatment against high aerial ammonia concentration (60 ppm) in broiler houses, as well as the influence of these treatments on deviations caused by ammonia stress on broiler performance, biochemical parameters, antioxidant activity, intestinal bacterial load and immunoglobulin concentration.
MATERIAL AND METHODS
Laboratory Trial
Four trays of one cubic meter in capacity were prepared and filled with hay, which was sterilized by auto claving at 121 ºC / 20 min to demonstrate freedom from microbial contamination. Superphosphate was added to the 1st, meta-bisulfide to the 2nd, the 3rd was encountered as control positive (untreated litter exposed to 60 ppm ammonia) and the 4th tray was kept as control negative (untreated non-exposed litter). The four trays were isolated individually in separate fiber chambers, in which superphosphate treated; meta-bisulfide treated and untreated control positive litter trays were exposed to 60 ppm ammonia. Ammonia vapors were emitted every 12 hours for three successive days. Litter was examined every 12 hours for pH and moisture content, while air was examined for ammonia concentration by potentiometric titration (Ndegwa et al., 2009).
Field Trial
Experimental birds: a total of 400 one day old Ross chicks were housed on deep litter system (hay). Broilers were divided into four groups on separate chambers, each group consisted of 100 chicks (ten replicates of ten birds).The birds were brooded at 35 °C that was gradually decreased up to 25 °C by the end of 3rd week. The chambers were supported with both artificial ventilation (supplying and suction fans) and natural ventilation (windows). Artificial light was supplied for at least 18 hour a day. Birds were given ad libitum access to water and a standard corn-soybean diet containing approximately 21.5% crude protein (Hu and Guo, 2008).The experiment was designed to last for five weeks (38 days). Mortalities, indoor temperature and relative humidity were monitored and recorded daily during the experiment.
Vaccination: Birds were vaccinated in drinking water at 7th day for Infectious Bronchitis and Newcastle disease using live attenuated virus vaccine of IB-H120 103.5 EID50; at 14th and 21st day for Infectious Bursal disease using live attenuated virus vaccine of VMG91 103.0 TCID50 and at 18th and 28th day for Newcastle disease virus using live lentogenic ND virus vaccine of LA SOTA 106.0 EID50.
Litter treatment: litters in the 1st and 2nd broiler’s chambers (G1 and G2) were treated with superphosphate (0.5 kg / m3) and sodium meta-bisulfide (0.05 g / m3), respectively. Meanwhile, litters in the 3rdcontrol positive (G3) and the 4thcontrol negative (G4) broiler’s chambers were kept untreated.The treatments application on litter were carried out once at the beginning of the experiment to ensure maximum absorbance abilities of the examined treatments and measure the long term effectiveness of these treatments.
Ammonia Exposure: broilers raised on superphosphate treated (G1), meta-bisulfide treated (G2) and untreated control positive (G3) litters were subjected to 60 ppm ammonia vapors using vaporizers for 8 - 10 hours per day for the entire duration of the experiment.
Sampling and Measurements
Sampling: a total of 1656 samples (456 air, 200 whole blood, 200 sera, 200 intestinal swabs and 600 organ samples including bursa; spleen and thymus) were collected during the entire period of the study. Air samples were collected using impinger sampler 50 cm above the ground level. Three replicates of air samples per chamber were collected twice (at starting point of ammonia vapors release; P1 and 12 hours latter; P2) on a daily basis starting from day one of the experiment. Whole blood samples were collected on EDTA tubes centrifuged at 4000 rpm / 10 min; then aspirate off the plasma. The erythrocytes were washed 3-4 times using 5 mL sodium chloride 0.9% solution and centrifuged after each wash. The washed erythrocytes were re-suspended in cold distilled water and mixed; the lysate was stored at -20 oC for anti-oxidant assay. Serum blood samples were collected and kept for overnight at 4 °C; centrifuged at 3000 rpm for 20 minutes. A clear non-hemolyzed serum was divided into 4 equal parts in eppendorf tubes, stored at -20 °C for biochemical analysis (Coles, 1986). Birds were slaughtered after blood sampling, in which bursa, spleen and thymus were removed; weighed, and expressed as (g / kg body weight). Swab samples were collected from birds’ intestine on phosphate buffer saline, preserved in ice box and transferred to the laboratory for bacteriological assessment. Blood, organs and swab samples were collected on a weekly basis starting from 7th day old.
Performance indices: Average live bird body weight was detected weekly by weighting representative samples of birds; as well as their feed intake. On the basis of live body weight and feed intake of birds; number of indices were weekly calculated as indicators for broiler performance including: Body Weight Gain (Brady, 1968); Feed Conversion Ratio and Performance Index (Yamani et al, 1997).
Ammonia Quantification: ammonia concentrations were measured in air samples by potentiometric titration (Ndegwa et al., 2009) using methyl red indicator and 0.05M sulfuric acid solution (APHA, 2012).
Bio-chemical analysis: serum samples were examined for Total Protein (TP); Albumin (Alb); Alanine aminotransferase (ALT); Aspartate aminotransferase (AST); Urea and Creatinine (Creat) calorimetrically (Young, 2001) using UV1100 spectrophotometer. Serum IgG, IgA, IgM concentrations were measured using immunoturbidimetric assay (Wang et al., 2010).
Antioxidant assay: Erythrocyte lysate was examined for Superoxide Dismutase; SOD (Nishikimi et al., 1972), Glutathione Peroxidase; GSH-Px (Paglia and Valentine, 1967) and Glutathione Reductase; GSH-Rx (Goldberg and Spooner, 1983).
Bacteriological examination: swabs were prepared according to APHA (2012).Tenfold decimal serial dilution up to 10-6 were prepared. Bacterial counts (Total Bacterial Count TBC and Total Enterobacteriaceae Count TEC) were applied using drop plate technique (Zelver et al., 1999; Herigstad et al., 2001). Standard Plate Count Agarwas used for Total aerobic Bacterial Count at 37 ºC for 24 - 48 hrs and Eosine Methylene Blue Agar (EMB) for Total Enterobacteriaceae Count at 37 ºC for 24 - 48 hrs. Plates showed 30 - 300 CFU were counted (Cruickshank et al., 1980).
STATISTICAL ANALYSIS
Statistical analysis was carried out using statistical package for social sciences (SPSS, 2001) and statistical analysis system (SAS Institute, Inc., 2002). The obtained data were analyzed statistically using multifactorial Analysis of Variance (ANOVA) with general linear model procedures (GLM) for broilers raised on treatedand untreated litters, time and their interactions in both laboratory and field trials, Levesque, (2007).
RESULTS
Aerial ammonia examination in the laboratory experiment as shown in Table 1, revealed the ability of superphosphate treatment to reduce ammonia concentration with a highly significant difference (P ˂ 0.001) compared to meta-bisulfide. A highly significant reduction (P ˂ 0.001) was recorded in litter pH and moisture content of superphosphate treated litter compared to those of meta-bisulfide treated litter as appeared in Table 1. The same results confirmed by the field trial as shown in Figure 1, which revealed a highly significant reduction (P ˂ 0.001) in aerial ammonia concentration of chamber in which broilers raised on superphosphate rather than those raised on meta-bisulfide treated litter.
Broilers’ performance traits were greatly improved (Table 2) in groups raised on treated litter. Body weight, feed conversion ratio and performance index revealed a highly significant improvement (P ˂ 0.001) in group raised on superphosphate treated litter. Meanwhile, weight gain showed a highly significant improvement (P ˂ 0.001) in group reared on meta-bisulfide treated litter compared to the other groups.
Meta-bisulfide litter treatment contributed a highly significant enhancement (P ˂ 0.001) in spleen and thymus weights compared to superphosphate treatment and control groups as revealed in Table 3. On the other hand, superphosphate litter treatment enabled a highly significant increase (P ˂ 0.001) in bursa’s weight compared to the other groups (Table 3).
Table 1: Aerial ammonia concentration and chemical characteristics (Mean ± SE) of laboratory treated and untreated litter.
|
Litter Treatments |
Ammonia ppm |
Litter pH |
Moisture % |
|
Superphosphate |
1.580c± 0.063 |
7.49c± 0.011 |
39.35c± 0.026 |
|
Meta-bisulfide |
2.225b ± 0.015 |
7.70b± 0.014 |
40.73b ± 0.032 |
|
Control positive |
48.89a ± 0.194 |
10.89a ± 0.028 |
59.36a± 0.689 |
|
Control negative |
1.426c ± 0.001 |
7.23d ± 0.005 |
30.03d± 0.057 |
|
P value |
0.001 |
0.001 |
0.001 |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01); Means carrying the same superscripts in the same column are non-significantly different at (P<0.05).
Table 2: Performance traits (Mean ± SE) of different broilers groups reared on treated and untreated litter.
|
Litter Treatments |
Body weight g |
Weight gain g |
FCR % |
PI |
|||||
|
Superphosphate |
838.9b ± 2.92 |
348.3c ± 4.02 |
1.78a ± 0.020 |
4.57b ± 0.069 |
|||||
|
Meta-bisulfide |
795.0c ± 3.01 |
320.5b ± 5.19 |
1.72b ± 0.031 |
4.32c ± 0.124 |
|||||
|
Control positive |
438.8d± 3.24 |
201.0d± 1.56 |
1.61c± 0.061 |
1.97d± 0.166 |
|||||
|
Control negative |
870.9a ± 2.94 |
363.6a ± 3.76 |
1.76ab ± 0.018 |
4.86a ± 0.097 |
|||||
|
P value |
0.001 |
0.001 |
0.076 |
0.001 |
|||||
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01); Means carrying the same superscripts in the same column are non-significantly different at (P<0.05).
Table 3: Immune organs’ weight (Mean ± SE) of different broilers groups reared on treated and untreated litter.
|
Litter Treatments |
Bursa g / kg |
Spleen g / kg |
Thymus g / kg |
|
Superphosphate |
3.792a ± 0.022 |
3.494c ± 0.027 |
3.364b ± 0.025 |
|
Meta-bisulfide |
2.434c ± 0.041 |
4.102a ± 0.096 |
3.474a ± 0.091 |
|
Control positive |
0.861d ± 0.007 |
1.022d ± 0.001 |
2.012d ± 0.021 |
|
Control negative |
3.402b ± 0.012 |
3.668b ± 0.058 |
3.508a ± 0.022 |
|
P value |
0.001 |
0.001 |
0.001 |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01); Means carrying the same superscripts in the same column are non-significantly different at (P<0.05).
Table 4: Serum Biochemical parameters (Mean ± SE) of different broilers groups reared on treated and untreated litter.
|
Parameters |
Super-phosphate |
Meta-bisulfide |
Control positive |
Control negative |
P value |
|
Total Protein g / dl |
4.39c ± 0.018 |
4.99b ± 0.019 |
10.21a ± 0.002 |
3.59d ± 0.019 |
0.001 |
|
Albumin g / dl |
2.37c ± 0.043 |
3.87b ± 0.042 |
9.18a ± 0.005 |
1.87d ± 0.043 |
0.001 |
|
Globulin g / dl |
2.02a ± 0.0276 |
1.11c ± 0.0270 |
1.03d ± 0.001 |
1.71b ± 0.0271 |
0.001 |
|
ALT IU / L |
25.7d ± 0.083 |
27.4b ± 0.087 |
38.89a ± 0.118 |
27.1c ± 0.087 |
0.001 |
|
AST IU / L |
34.8d ± 0.159 |
36.6b ± 0.154 |
49.87a ± 0.214 |
36.2c ± 0.156 |
0.001 |
|
Urea mg / dl |
73.6c ± 1.601 |
75.7c ± 1.590 |
118.21a ± 3.143 |
81.7b ± 1.584 |
0.001 |
|
Creatinine mg / dl |
0.49d ± 0.0239 |
0.84b ± 0.032 |
1.91a ± 0.007 |
0.59c ± 0.0325 |
0.001 |
Means carrying different superscripts in the same row are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01); Means carrying the same superscripts in the same row are non-significantly different at (P<0.05).
Biochemical analysis of broilers’ sera in Table 4 revealed a great ability of both litter treatments to reduce the influence of high ammonia concentration on blood parameters. A highly significant improvement (P ˂ 0.001) in total protein, albumin, ALT, AST and creatinine in birds raised on meta-bisulfide treated litter rather than those of broilers raised on superphosphate treated litter. Meanwhile, broilers reared on superphosphate treated litter revealed a highly significant increase (P ˂ 0.001) in globulin (Table 4) and immunoglobulin concentrations (IgG, IgA and IgM, Figure 2 compared to those for birds raised on meta-bisulfide treated litter and control groups.
Antioxidant activity as shown in Figure 3, revealed a highly significant reduction (P ˂ 0.001) in Glutathione Peroxidase (GSG-Px) of birds reared on superphosphate treated litter, and a significant reduction (P ˂ 0.001) in Glutathione Reductase (GSH-Rx) of birds reared on meta-bisulfide treated litter. Superoxide Dismutase (SOD) revealed a non-significant difference between birds raised on treated and untreated litters.
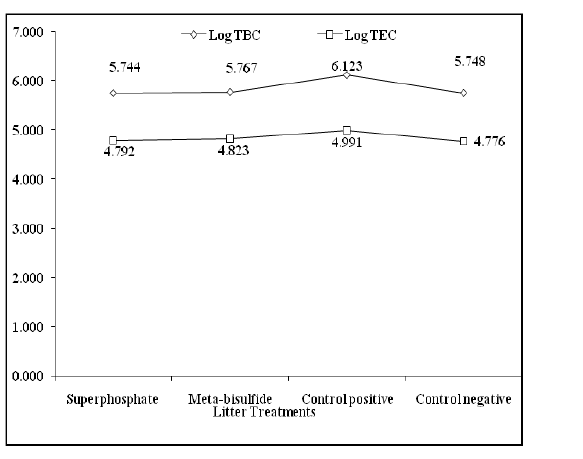
Figure 1. Aerial Ammonia concentration in different broilers groups’chambers reared on treated and untreated litter.
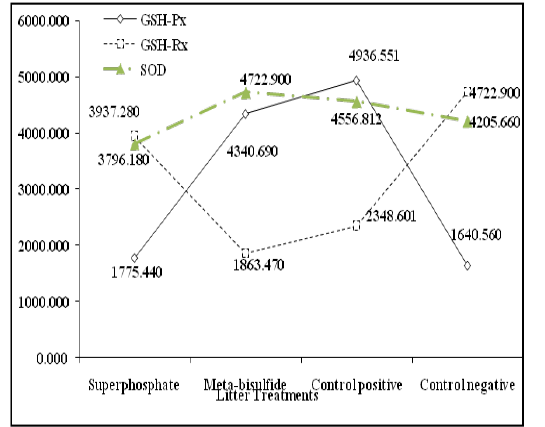
Figure 2: Immunoglobulin concentration of different broilers groups reared on treated and untreated litter.
Bacterial counts (Total bacterial, Total Enterobacteriaceae) as revealed in Figure 4 were reduced with a highly significant difference (P ˂ 0.001) in birds reared on superphosphate treated litter compared to birds reared on meta-bisulfide treated litter and untreated litter.
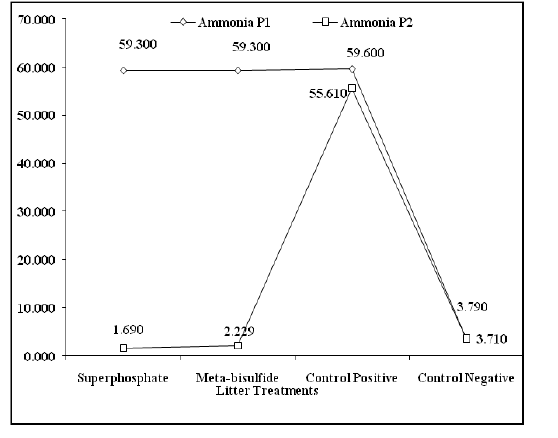
Figure 3. Logarithmic Bacterial counts of different broilers groups reared on treated and untreated litter.
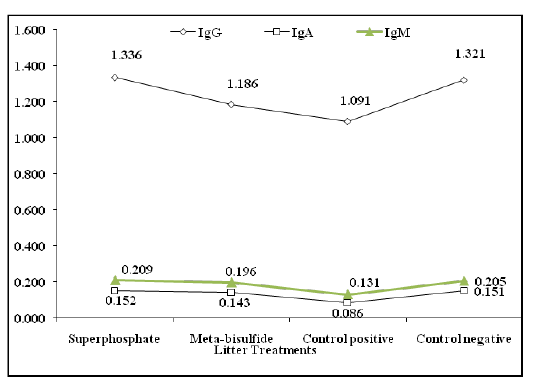
Figure 4. Antioxidant enzymatic changes of different broilers groups reared on treated and untreated litter.
s
DISCUSSION
Control, preventive and management practices are important tools in broilers houses for strict biosecurity programs.Litter in poultry houses is essential to absorb moisture, protect from lesions and for thermal insulation (Hernandes and Cazetta, 2001). Broiler farms between flocks naturally activate litter decomposition producing heat that caused subsequent inactivation of viruses and reduction of aerobic bacterial count in litter (Macklin et al., 2006; Wilkinson, 2007), although the generated heat from decomposition was able to minimize the amount of ammonia at the inner parts of the compost (Miles, 2008).
The type of litter material can influence growth rate and broilers performance as recorded by Anisuzzaman and Chowdhury, (1996), who found that among rice husk; saw dust; paddy straw and sand, the highest growth rate and feed intake with higher feed conversion ratio were recorded in broiler raised on rice husk. On the other hand, Swain and Sundaram, (2000) detected no changes in weight gain, feed conversion ratio and mortalities between broilers raised on rice husk, saw dust and choir dust.
Litter moisture was affected by different factors including type of diet; water intake; type of drinkers, ambient temperature and ventilation system in the farm (Oliveira et al., 2004). In the present study, litter treatments with both superphosphate and meta-bisulfide were able to significantly reduce pH and litter moisture and this was recorded with more pronounced appearance in litter treated with superphosphate rather than that treated with meta-bisulfide. The reduced ability of meta-bisulfide in lowering moisture content was recorded in (Nagaraj et al., 2007). Meanwhile, (Eleroglu et al., 2005) recorded a greater reduction in litter moisture using zeolite 25, 50 and 75%, while (Hüseyin Yalçin and Hasan Eleroğlu, 2005) found that using only 25% zeolite in litter consisted of wood shaving produced the same reduction ability in moisture content of litter with a positive effect on broiler performance.
(Maria C de Oliveira et al., 2015) compared the efficiency of litter treatment using aluminum sulfate; quick lime; dolomitic limestone; zeolite; charcoal with untreated litter and in-house composted litter, and found no influences of these treatments on male broiler performance but only increased the cost of production.
Superphosphate and meta-bisulfide litter treatments usually minimize ammonia concentrations and maintain normal litter pH compared to ancient and traditional methods including ferrous sulfate that lack a long term effectiveness, paraformaldehyde that failed in the field trials, lime that interfere with litter quality and acids that were used with extreme cautions as sulfuric acid. The success of these treatments usually depends on the reduction of litter pH and thus the formation of non-volatile NH4+ (Moore Junior et al., 2000).The current research found that superphosphate was able to reduce aerial ammonia concentration from 60 ppm in both laboratory and field trails up to 1.580 and 1.690 ppm; respectively. Although, superphosphate was able to enhance broiler’s immunity and performance traits up to great levels, others found that the addition of superphosphate to litter significantly reduced bird viabilities as recorded by (Ferreira et al., 2004).
Previous studies found that litter treatment using meta-bisulfide was able to minimize ammonia concentration via a chemical reaction to produce ammonium sulfate and acidifies litter contributing a reduction in bacterial population especially Salmonella and Campylobacter. (Nagaraj et al., 2007) found that ammonia levels were smaller in treated litters with sodium bisulfate in comparison with those untreated until 35th day of breeding. Litter treatment with sodium meta-bisulfide (PLT) revealed a great ability in reducing atmospheric ammonia concentration, thorasic lesion and tracheal mucosal injuries with significant increase in body weights as recorded by (Terzich et al., 1998).The present study also recorded nearly similar abilities of meta-bisulfide, which was able to minimize high aerial ammonia concentration (60 ppm) in the laboratory trial up to 2.225 ppm and in the field trial up to 2.229 ppm.
CONclusion and Recommendation
Litter treatments in broiler houses are highly recommended to provoke the litter chemical composition and its quality as a fertilizer, its uses in pest control programs and minimize the influences of elevated microclimatic ammonia in broilers houses.
Superphosphate and meta-bisulfide litter treatment showed a great efficiency in reducing aerial ammonia concentration either in laboratory or in field trials and revolved all biological parameters via improving bird performance, immune organs’ weight, immunoglobulin concentration, antioxidant enzymatic activity as well as a great reduction of bacterial counts. The choice of the chemical used for litter treatment is usually left for its economic value.
ACKNOWLEDGMENT
Sincere thanks to Prof. Dr. Mohamed A. Sobieh for his guidance and tremendous direction throughout the scheme of work.
AUTHOR CONTRIBUTIONS
All authors contributed equally in all steps of laboratory and field trials.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICAL APPROVAL
All applicable international, national, and/or institutional guidelines for the care and use of birds were followed.
REFERENCES






