Journal of Animal Health and Production
Research Article
Prevalence, Morphological and Molecular Characterization of Psoroptic Mites in Smallholder Livestock in Egypt
Refaat Ras1*, Samy Shawky2, Nader Sobhy3, Wafaa Mohamed El-Neshwy3
1Department of Parasitology, Faculty of Veterinary Medicine, Zagazig University, 44511-Zagazig, Egypt; 2Department of Parasitology, Animal Health Research Institute, Zagazig, Egypt; 3Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, 44511-Zagazig, Egypt.
Abstract | Psoroptic mange is one of the most common contagious skin conditions causes economic losses in livestock. The present study determine the prevalence of Psoroptes spp. in cattle and buffaloes in Sharkia governorate, Egypt and associated risk factors as season, age and sex. Psoroptes isolates of buffaloes were identified morphologically using light and scanning electron microscope and molecularly using PCR and sequencing against the second internal transcribed spacer (ITS2 gene). The current study revealed that prevalence of Psoroptes spp. in cattle and buffaloes was 4.8% and 27.4% respectively. On the other hand, using sequences analysis showed that the isolates collected from buffaloes were Psoroptes natalensis. Morphometric analysis was compatible with previous studies and discerns that there was no significant variation between the measurements of outer opisthosomal setae from adult males individual sample from different animals. The obtained data enhance the need for a good hygienic and management measures for control of the disease. Availability of genomic information of ITS2 gene may help in future discovering of Psoroptes natalensis sub-species and increase progress in developing of novel acaricidal drug targets.
Keywords | Psoroptes, Cattle, Buffaloes, Prevalence, ITS2, Egypt
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | November 02, 2020; Accepted | November 23, 2020; Published | December 27, 2020
*Correspondence | Refaat Ras, Department of Parasitology, Faculty of Veterinary Medicine, Zagazig University, 44511-Zagazig, Egypt; Email: [email protected]; [email protected]
Citation | Ras R, Shawky S, Sobhy N, El-Neshwy WM (2020). Prevalence, morphological and molecular characterization of psoroptic mites in smallholder livestock in Egypt. J. Anim. Health Prod. 9(s1): 69-76.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.69.76
ISSN | 2308-2801
Copyright © 2020 Ras et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Psoroptic mange caused by Psoroptes mites (Acari: Psoroptidae) is one of the main skin infestations of livestock causing significant economic losses and welfare problems (Pegler et al., 2006).
Psoroptes spp. are an obligatory non-burrowing mites feed superficially on lipid emulsion of lymph, red blood cells, skin cells, exudates and bacteria of skin surface by abrading the outer epidermal layer using toothed chelicerae (He et al., 2016). Hence, it causes severe pruritus, dermatitis, formation of thick scabs on skin and later, animals suffer from scratching, biting, irritation, rubbing against objects and hair loss with subsequent damage fleece or hides (Wildblood and Jones, 2006). The affected animals may suffer from low production, general weakness and death in extreme cases due to dehydration or secondary bacterial infections (Wall, 2012).
In Egypt, 16.35% and 21.3% of water buffaloes were found to be infested with Psoroptes spp. by El-Khodery et al. (2010) and Amer et al. (2015) respectively. High prevalence may attribute to unhygienic husbandry and neglected treatment practices of smallholder farms (Yassin, 2011; Sobhy et al., 2018). Furthermore, Psoroptes infestation mainly influenced by a variety of factors such as age, body conditions seasonal conditions, management systems and rearing with other animals (El-Khodery et al., 2010).
As far as we know, the validity of five Psoroptes spp. based on length of outer opisthosomal setae, infestation specific host locations and host specificity still limited and dialectical because of degree of variance in setae lengths within populations of different hosts, infestation of P. cuniculi to ears and body, successful cross-mating of P. ovis and P. cuniculi in the ears of rabbits and overlapping geographical distribution (Zahler et al., 2000; Juan et al., 2015). Wherefore, the taxonomy of Psoroptes mites based on ribosomal DNA internal transcribed spacers 2 (rDNA ITS2) sequences is much reliable than morphological approaches as the same Psoroptes could be infested more than one host (Pegler et al., 2006). In Egypt, epidemiological and molecular investigations on Psoroptes mites from different livestock in smallholder farms were limited (El-Khodery et al., 2009; Sobhy et al., 2018). As well, epidemiological information details, mange pathology, husbandry management and farmer conception and attitudes are required for applicable control measures (O’Brien, 1999).
The present study was pointed to determine the prevalence of the most common Psoroptes spp. in cattle and buffaloes with a potential variable risk factors as age, sex and seasons. Morphological and molecular identification of the most predominant species, using the second internal transcribed spacer (ITS-2) of the rRNA gene.
MATERIALS AND METHODS
Collection of mite isolates
A total of 106 and 84 samples were collected from buffaloes and cattle smallholders respectively in two districts (Diarb Negm and El-Ibrahiemia) in Sharkia governorate of northern Egypt, 120 Km from the capital Cairo, during July 2018 to August 2019.
Skin scrapings were collected from Egyptian buffaloes and closer cattle that showing varying degrees of skin lesions and itching. Each sample was collected from the edges of lesions. Samples were kept in a labelled plastic bottle and then transferred to the laboratory. The samples were collected under consent of animal’s owner with applying a protocol which approved by the Animal Welfare and Research Ethics Committee, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Parasitological examination
Skin scrapings were examined under stereo dissecting microscope to identify different stages of mites. Scrapings with much debris were processed according to (Seid et al., 2016). The recovered mites were stored in 70% ethanol. Mite samples were cleared in lactophenol media, then mounted permanently on clean slide on polyvol and kept in hot air oven at 50°C for 24 hrs (Belding, 1965; Faraji and Bakker, 2008). Sixteen representative mounted specimens of adult males and females pooling mite from different buffaloes were subjected to morphometric analysis using specific characteristic length and width of total body and gnathostoma. The length of leg I ambulacrum, outer opisthosomal and females’ leg III posterior setae were measured.
For scanning electron microscope (SEM) examination, the samples were fixed overnight in fixative (2.5% glutaraldehyde in 0.2 M phosphate buffer pH 7.2) at 4 °C. The fixed samples were rinsed three times with the same buffer, then dehydrated in ethanol using critical point dried instrument. The samples were mounted on cupper stubs with double-sided adhesive tape, coated with gold using Sputter Coater, then observed under SEM (JEOL JSM-5200) at 25 kV and photographed (Fischer et al., 2012).
DNA isolation
DNA were extracted from five representative pooled mite samples, three from different buffaloes and two from cattle using the QIAamp DNA Mini kit (Qiagen, Germany) with modifications from the manufacturer’s recommendations. Briefly, samples were washed with 1% Sodium Dodecyl Sulfate (SDS) reagent, then 180 μl of ATL buffer and 20 μl protease were added to 25 mg of the sample, followed by homogenization in Qiagen tissue Lyser. Disruption was performed in 2 min. high-speed (30 Hz) shaking step, followed by incubation at 56˚C till lysis. Then, 200 µl of the lysate was incubated with 10 µl of proteinase K and 200 µl of lysis buffer at 72°C for 10 min. Thereafter, 200 µl of 100% ethanol was added to the lysate (Lučan et al., 2016). The sample was washed and centrifuged following the manufacturer’s recommendations. DNA was finally eluted with 100 µl of elution buffer.
Molecular identification
ITS-2 gene amplified by protocol provided by (Zahler et al., 1998). The used primers were RIB-3 CCA TCG ATG TGA AYT GCA GGA CA and RIB-2 CGG GAT CCT TCR CTC GCC GYT ACT that can amplify 382 bp. The PCR reaction performed in applied biosystem 2720 thermal cycler. The products of PCR were visualized by electrophoresis on 1.5% agarose gel in 1x TBE buffer at room temperature.
Phylogenetic analysis
Three represented PCR amplicons were purified using QIAquick PCR product extraction kit. (Qiagen, Valencia). Bigdye Terminator V3.1 cycle sequencing kit (Perkin-Elmer, USA) was used for the sequence reaction by Applied Biosystems3130 genetic analyzer (HITACHI, Japan).
The obtained chromatogram was checked and sequences assembled using ‘Sequencher 5.1’ software. Blastn option on GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was selected to match the sequences with GenBank data base. The obtained nucleotide (nt) sequences were submitted in GenBank under accession numbers MT846030, MT846031 and MT846032.
The MEGAX software was used for sequences aligned by Clustal W method and P-distance detection. The phylogenetic analysis was constructed by using maximum likelihood models (T92+G) with the lowest BIC (Bayesian Information Criterion) scores in MEGAX software (Kumar et al., 2016). A phylogenetic tree was constructed to show the relation between the identified species ITS-2 gene sequences and other related reference species.
Statistical analysis
Three variables data of age, sex and season conditions were analyzed with Chi-square (χ2) tests using IBM SPSS Statistics for Windows software version 21. P-values ≤ 0.05 were considered statistically significant. The difference between character measurements of individual mite pooling isolates of buffaloes was analyzed using Krustal-Wallis test.
RESULTS AND DISCUSSION
Prevalence of Psoroptes mites
Based on light microscope examinations, 27.4% (29/106) of buffaloes were infested with Psoroptes spp. The prevalence rate was highest in the winter (42.9 %), and lowest in the spring (11.1%) with a significance between the season and infection rate (P-values ≤ 0.05). Animals less than four years had a higher infestation rate (34.8%) than older groups (21.7%). Moreover, the female buffaloes (29.7%) were infected more than males (23.8%). There was no significance between age, sex, and the mite infestation rate (Tables 1 and 2).
The mite’s infestation rate in cattle was 4.8% (4/84). The prevalence in old animals (≥ 4 years) was higher (5.9%) than young calves (< 4 years) (3%) without significance in the infection rate between young and old animals. The prevalence in males and females were 4.2% and 5%, respectively, without significance. The spring and the summer were the most seasons with high infection rates (16.7% and 4%, respectively) without statistical significant between the season and the infection rate (Tables 1 and 2).
Morphometric examinations
Adult male and female mite’s structures and measurements were analyzed with a light microscope, while SEM was used to show the structures and setae. The present findings showed that adult males were slightly oval with sclerotized dorsal propodonotal and opisthosomal plates (Figures 1A; 2A). The mean total length of body including gnathostoma was 385 µm (214 - 429 µm) and the width was ~ 296 µm (286 - 314 µm). Gnathostoma was ~ 144 µm (100 – 286 µm) long and 51 µm (43 – 57 µm) wide (Figure 2B). Furthermore, the males had four pairs of legs which leg I, II, III composed of three segmented pretarsi with segmented pedicle and ended with ambulacral suckers. Leg I ambulacrum length was ~ 56 µm (43 – 71 µm). While, leg IV was much shorter than other legs with absent of pretarsus. The male’s ventral surface had a gonopore with two genital pits on the metapodosomal region with a posterior pair of adanal suckers that attached to the dorsoposterior tubercles of female protonymph (Figure 2C, D and 1C). Posteriorly, (Figure 2D), there was a pair of opisthosomal lobes; each composed of three long and two short setae. The outer opisthosomal setae length was ~ 264 µm (214 – 357 µm). Moreover, one pair of prodosomal setae, five pairs of metapodosomal setae and a pair of adanal setae were found. Male protonymph was seen (Figure 1D) in which leg III has two long setae.
Table 1: The percentage of infested buffaloes and cattle according to seasons.
| Season | Buffaloes | Cattle | ||||
| No. of examined animals | No. of infested animals | % | No. of examined animals | No. of infested animals | % | |
| Winter | 35 | 15 | 42.9 | 34 | 1 | 2.9 |
| Spring | 18 | 2 | 11.1 | 12 | 2 | 16.7 |
| Summer | 36 | 9 | 25.7 | 25 | 1 | 4 |
| Autumn | 17 | 3 | 17.6 | 13 | 0 | 0 |
| Total | 106 | 29 | 27.4 | 84 | 4 | 4.8 |
Table 2: The percentage of infested buffaloes and cattle according to age and sex.
| Animal species | Age groups | Sex | ||||||||||
| < 4 years | ≥ 4 years | Males | Females | |||||||||
| No. of examined animals | No. of infested animals | % | No. of examined animals | No. of infested animals | % | No. of examined animals | No. of infested animals | % | No. of examined animals | No. of infested animals | % | |
| Buffaloes | 46 | 16 | 34.8 | 60 | 13 | 21.7 | 42 | 10 | 23.8 | 64 | 19 | 29.7 |
| Cattle | 33 | 1 | 3 | 51 | 3 | 5.9 | 24 | 1 | 4.2 | 60 | 3 | 5 |
| Total | 79 | 17 | 21.5 | 111 | 16 | 14.4 | 66 | 11 | 16.7 | 124 | 22 | 17.7 |
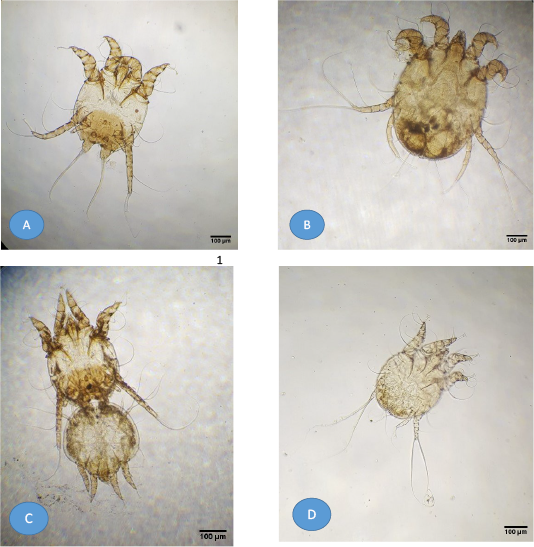
Figure 1: Light microscopy of mite images from buffaloes. (A) Adult Psoroptes natalenesis male; (B) Adult Psoroptes natalenesis female; (C) Adult Psoroptes natalenesis male attached with female tritonymph; (D) Male protonymph Psoroptes natalenesis.
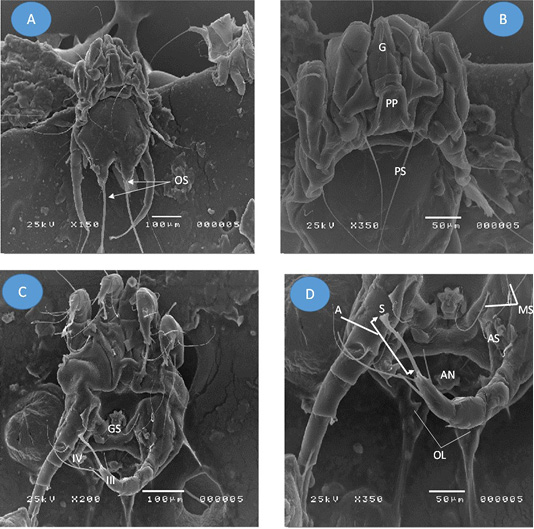
Figure 2: Adult Psoroptes natalenesis male from buffaloes under scanning electron microscope. (A) Dorsal surface; (OS: Opisthsomal setae); (B) Anterior end; Podosoma; (G: Gnathosoma; PP: Propodosomal plate; PS: Propodosomal seta); (C) Ventral surface; (GS: Genital sucker; IV: leg IV (short); III: leg III ended with terminal sucker); (D) Opisthosoma; (MS: Metapodosomal setae; AN: Anus; A: ambulacrum; S: Pulvillus (sucker); OL: Opisthosomal lobes).
On the other hand, the adult female mites were slightly oval without opisthosomal plates (Figures 3A; 1B). The mean length of body including gnathostoma was 521 µm (286 – 643 µm) and ~ 371 µm (314 – 400 µm) wide. Gnathostoma was measured 137 µm (71 – 214 µm) long and ~ 69 µm (29 – 143 µm) wide. Four pairs of legs were with almost the same size. Leg I, II, III had three segmented pretarsi with segmented pedicle and ended with ambulacral suckers, while, leg IV ended with a pair of long setae (Figure 3B). The mean length of Leg I ambulacrum and Leg III posterior setae was 61 µm (43 – 86 µm) and ~ 330 µm (143 – 429 µm), respectively.
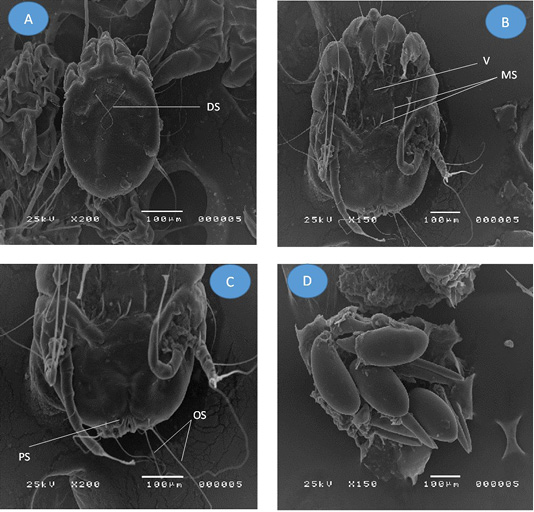
Figure 3: Adult Psoroptes natalenesis female from buffaloes under scanning electron microscope. (A) Dorsal surface; (DS: Dorsal setae); (B) Ventral surface; (V: Vulva; MS: Metapodosomal setae); (C) Opisthosoma; (PS: Perianal seta; OS: Opisthosomal setae); (D) Eggs
The dorsal surface provided with two pair of propodosomal setae, one pair of long dorsal setae, one pair metapodosomal setae and six pairs of idiosomal setae. On the ventral side, a vulva was found with two pairs of cuticular pits in the propodosomal region (Figure 3B) and three pairs of propodosomal setae. Two pairs of idiosomal setae and four pairs of opisthosomal setae were detected. The posterior opisthosomal margin of female is somewhat round without copulatory tubercles and the outer opisthosomal length was ~ 254 µm (129 – 286 µm) (Figure 3C). Some of oval Psoroptes eggs were detected (Figure 3D).
Female protonymph has four legs, leg III ended with two long setae and a body with a broad characteristic shoulders and copulatory tubercles at opisthosoma region (Figure 4).
The current study revealed no significant difference between males and females characters between different buffaloes isolates.

Figure 4: Female protonymph of Psoroptes natalenesis from buffaloes under scanning electron microscope showing dorsoposterior tubercles (PT).
Molecular analyses
Psoroptes isolates of buffaloes and cattle were successfully molecularly identified. The sequences of the obtained samples represent complete ITS2 gene sequence and partial sequence of 5.8S rRNA, and 28S rRNA genes. Blasting of the sequences on NCBI (blastn) reveals that sequences related to Psoroptes natalensis reference sequences. Phylogenetic analysis showed sequences in the same clade with other Psoroptes natalensis references strains and closely related to the clade of other Psoroptes spp. (P. cervinus, P. cuniculi, P. ovis and P. Cuniculi) (Figure 5). Identity between the identified sequences was 99-100% with two nt substitution (T to A and A to G at position 253 and 281, respectively) while identity with previous identified Egyptian strains (AB968084 and AB968081) was 99% with 5 nt substitution at positions 2, 7, 12, 253 and 281. Identity with Chinease P. natalensis strain (EF025929) was 96% with 10-12 substituted nt. Identity with other psoroptes types (P. cuniculi, P. ovis and P. cervinus) was 92% while identity with other mites species was 85% and 55% in Choriptes and Dermatophagoides, respectively.
Psoroptic mange is a highly contagious chronic skin infestations caused by Psoroptes mites in many wild and domestic animals worldwide (He et al., 2016). The present study revealed that prevalence rate of Psoroptes spp. mites in buffaloes was 27.4%. These findings are close to results published by (Amer et al., 2015; Haggag et al., 2018) who recorded 21.3% and 33.33% prevalence rate in Egyptian water buffaloes respectively, and lower than data obtained by Elkhtam and Mousa (2016) who recorded 53.90% prevalence rate in Menofia governorate, Egypt. High prevalence rate may attributed to exposure of animals to unhygienic measures, bad housing conditions, and ignoring the important veterinary care and therapeutic control by smallholders farmers (Yassin, 2011; Elkhtam and Mousa, 2016; Sobhy et al., 2018).
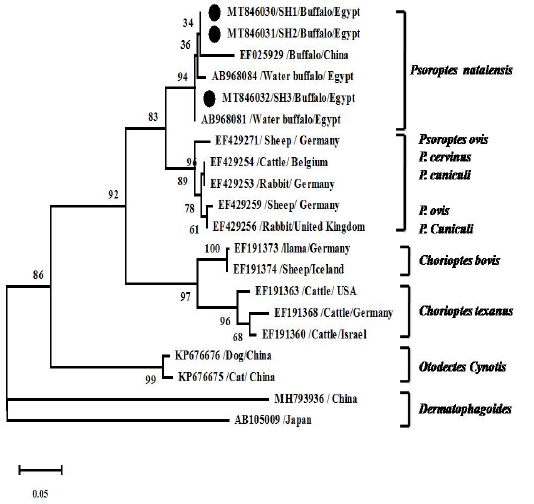
Figure 5: Phylogenetic analyses using Tamura 3-parameter +G as a maximum likelihood models with the lowest BIC scores showing the relationship of the obtained ITS2 gene nt sequences with others closest mite species references. Numbers at nodes show the percentage in 1000 bootstrap replicates.
Increasing prevalence seem to be seasonal and buffaloes were more infected in winter than other seasons, in agreement with El-Khodery et al. (2010) and Yassin (2011). Contrasty, Elkhtam and Mousa (2016) reported a highest infestation rate (65.97%) was in the spring. The bad management systems including poor housing with low temperature during winter obligate animal to warm themselves by close contact giving the mites chance for transmission (Smith et al., 1999). It can be also explained by the capability of female mites to produce few eggs or because of physiological changes in host skin as a form of a summer latency (Strong and Halliday, 1992). There was no statistically significance between age, sexes and infestation rate in buffaloes. However, our findings asserted with reports that young animals had a higher infestation rate than adult ones (Hayat et al., 1996) and the females were to be infected more than males (Yassin, 2011). Increase infestation rate in young age attributed to soft tender skin, dense hair coat, overcrowding, and poor immunity (Hazarika et al., 1995).
The current study showed that the Psoroptes infestation rate of cattle was 4.8%. The rate was much lower than previously reported by Haggag et al. (2018) who found the prevalence rate of Psoroptes in cattle was 41.67% respectively. Low infestation rate in cattle despite probable closer contact with infested buffaloes may indicate predilection of Psoroptes natalensis to infest buffaloes over cattle, even some studies counted it as a rare parasite for cattle (Heath, 1994). We assumed that the difference in cattle and buffaloes skin structure may contribute to mites habits and host preferences which in buffaloes the average thickness of skin is thicker with subepidemal blood capillaries and lymph vessels branches are more frequent and superficial level than in cattle (Hossain et al., 2016; Debbarma et al., 2018). However, increasing preference of Psoroptes natalensis to infest buffaloes need further investigation. While, decreasing rate with time may reflect the improvement in farms housing, rearing, and management condition in concurrently with improvement of veterinary care (Qadoos et al., 1995; Sarre et al., 2012).
In agreement with Onu and Shiferaw (2013), no association was seen between age, sex of cattle and mite infestations. Although high prevalence rate was seen in spring, no association between mite infestations and seasons was detected. Conversely, Vieira et al. (2014) suggested that increasing in infestation rate among cattle during winter and reducing during summer and spring as the animals back to pastures.
Morphometric analysis referred to presence of Psoroptes natalensis. Overall, these morphological findings correspond to the results reported by (Sanders et al., 2000; Zahler et al., 2000; Amer et al., 2015). However, in the present study there was no significant variation between the measurements of outer opisthosomal setae from adult males individual sample from different animals. To our knowledge, only one study has explored the morphological and molecular description of P. natalensis in buffaloes in Kafr El Sheikh governorate in Egypt was performed by Amer et al. (2015) who reported that outer opisthomal setae lengths in adult were close to our findings. Contrary to our findings, Pegler et al. (2006) who demonstrated that there are too many significant differences in lengths of the outer opisthosomal seta lengths L4 setae of adult males between the same species of mites within the same host which it is intricate to designate mites on specific hosts based exclusively on setae lengths but perhaps referred to adaptation to the local microenvironment and not speciation.
As the rRNA genes are relatively conserved in Eukaryotes evolution, it did not used in identification (Navajas et al., 1999). The genomic comparison of ITS2 for mite species identification found to be useful in P. natalensis identification and phylogenetic study. The marked variation of the obtained P. natalensis sequences over other Psoroptes spp. in phylogenetic tree refers to species specific feature of P. natalensis ITS2 gene. The same phylogenetic feature observed by Amer et al. (2015). The P-distance and nt assembly refer to identity with previously detected two different Egyptian strains (AB968084 and AB968081) with same two nt substitution in same positions as detected by Amer et al. (2015). The significance of substitution in these positions need further investigation. Detection of ITS2 variation among the sequenced samples may refer to presence of sub-species that could not distinguish morphologically. The Intra-isolate variations that could be related to evolutionary separated entities is common in Eukaryotes more likely than difference between isolates itself (Lohse et al., 2002).
The results analysis refers that P. natalensis correlated well with its morphology, host and not necessary geographical origin. Inability to detect other mites species in buffaloes morphologically and molecularly, supporting hypothesis that, P. natalensis species has preference to infest buffaloes (Juan et al., 2015). The taxonomic significance of this study remains to be explored. So, in Egypt, further studies should be conducted to determine the subtype of hosts derived mite populations using morphological and molecular approaches.
CONCLUSIONS AND RECOMMENDATIONS
The observation of the current study might contribute to better understanding of the epidemiology of Psoroptes mites affecting livestock animals in Egypt and thus building control strategy measures can be suggested.
AUTHOR’S CONTRIBUTION
RR, WE, NS contributed in design and conceived of the study. Sampling and data collection were carried by SS. RR and NS have performed laboratory examinations, data analysis and interpretation. RR, SS, WE and NS contributed to preparation of the manuscript. All authors approved the final version of manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






