South Asian Journal of Life Sciences
Research Article
Purification and Properties of Liver Catalase from Water Buffalo (Bubalus bubalis)
Muhammad Shahid Nadeem1*, Jalaluddin Azam Khan1, Bibi Nazia Murtaza2, Khushi Muhammad2, Abdul Rauf3
1Department of Biochemistry, King Abdulaziz University, Jeddah, Saudi Arabia; 2School of Biological Sciences, University of the Punjab, Lahore, Pakistan; 3Department of Zoology, University of Azad Jamu and Kashmir, Muzafarabad, Pakistan.
Abstract | Catalase is ubiquitous enzyme which catalyses the breakdown of hydrogen peroxide (H2O2). The aim of present study was to purify and characterize the liver catalase from Bubalus bubalis, which is an important domestic animal in South Asia. The enzyme was purified to electrophoretic homogeneity by selective acetone precipitations and anion-exchange chromatography based on DEAE-Sephadex Colum. Purified enzyme displayed specific activity of 35753 units per mg of protein with 30.5 % recovery and 123 fold purification. Optimum activity was measured at pH 7 and 30°C. The KM value of purified catalase was calculated as 58mM for H2O2. Gel-filtration chromatography experiments indicate that the purified enzyme was a tetramer with MW of about 240 kDa. There was no effect of 10 to 50mM EDTA on the activity of enzyme after incubation for 30 min at 4°C. Our study reveals the first report on the purification and properties of catalase from river buffalo which is an unexplored species for its proteins. The isolated enzyme with high yield and specific activity, offers an attractive alternate for the bovine and porcine enzymes used in clinical laboratories.
Keywords | Catalase, River buffalo, Ion-exchange chromatography
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | July 08, 2015; Revised | October 21, 2015; Accepted | October 23, 2015; Published | December 23, 2015
*Correspondence | Muhammad Shahid Nadeem, King Abdulaziz University, Saudi Arabia; Email: [email protected]
Citation | Nadeem MS, Khan JA, Murtaza BN, Muhammad K, Rauf A (2015). Purification and properties of liver catalase from water buffalo (Bubalus bubalis). S. Asian J. Life Sci. 3(2): 51-55.
DOI | http://dx.doi.org/10.14737/journal.sajls/2015/3.2.51.55
ISSN | 2311–0589
Copyright © 2015 Nadeem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Catalase (EC 1.11.1.6) which catalyzes the breakdown of hydrogen peroxide to water and oxygen is widely distributed in living organisms, including plants, animals and microorganisms (Abassi and Kushad, 1998; Bailly et al., 2004). It is the most important among antioxidative enzymes involved in the depletion of reactive oxygen species produced during the processes like the β-oxidation and electron transport etc. (Kunce and Trelease, 1986; Havir and McHale, 1987). The typical catalase is active in the pH range from 5 to 10. The optimum pH for the activity of catalase is between pH 6 and 8 and it is mostly stable at temperatures between 10 and 30°C (Aydemir and Kuru, 2003). Catalase from different sources mostly exists as a tetramer with molecular weight ranging from 220 to 270 kDa. Each subunit contains a protoheme molecule as prosthetic group. Dissociation of subunits is observed at extreme pH values and by the action of denaturants (Samejima et al., 1981; Prajapati et al., 1998) which results in the loss of activity. By removing highly reactive hydrogen peroxide, the enzyme inhibits the neuronal damage, apoptosis, inflammation, aging and a wide range of tumours (Vuillaume, 1987; Miyamoto et al., 1996; Esch et al., 1998; Yabuki et al., 1999; Halliwell and Gutteridge, 2011). Clinically it is important as the liver catalase level is decreased in the cancer patients (Falkson and De Jager, 1964). Elevation in plasma level of liver catalase and other antioxidant enzymes is used as an index of the ROS (reactive oxygen species) induced human diseases (Skinner et al., 1984; Lee et al., 2004). Catalase has proven as a possible agent to support the intracellular drug delivery (Siwale et al., 2009) a biosensor for the determination of alcohol (Hnaien et al., 2010), and can be used in an assay for the quantification of cholesterol (Robinet et al., 2010). The enzyme has been purified and characterized from a wide range of organisms like plants, such as tobacco, van apple, parsley and black gram (Garcia et al., 2000; Yoruk et al., 2005; Lokman et al., 2007; Kandukuri et al., 2012), liver of many mammals like goat (Chatterjee et al., 1989), dog (Nakamura et al., 2000), bovine (Prakash et al., 2002), and from bacteria (Zamocky et al., 2011). Most of the literature reports describe the purification of enzyme through selective ammonium sulphate precipitation and chromatographic techniques like gel-filtration chromatography (Goyal and Anjan, 2008), ion-exchange chromatography based on CM (carboxymethyl) Sephadex, DEAE (diethylaminoethyl) Sephadex or DEAE cellulose (Aydemir and Kuru, 2003; Bilal et al., 2006). River buffalo (Bubalus bubalis) is a species of tremendous important in livestock and rural economy in Pakistan where it contributes 27 million tons of milk and 0.68 million tons of meat annually (Li and Schellhorn, 2007; Khan and Iqbal, 2009). Despite of having a great importance in agriculture and livestock, the river buffalo remained unexplored for its proteins, enzymes and related genes. Present study was therefore aimed at the purification and study of physiochemical properties of catalase from the liver tissue of river buffalo.
Material and methods
Materials
All reagents and chemicals used in this study were of analytical grade. DEAE Sephadex, Hydrogen peroxide and other related reagents were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Fresh B. bubalis liver was obtained from the main slaughter house in Lahore, Pakistan.
Extraction and Purification
Fresh liver tissue (1200 g) was minced and homogenized in 1800mL of ice chilled 20mM sodium phosphate buffer pH 7 (buffer-A), in a laboratory waring blender at high speed for 2-3 min. The homogenate was centrifuged at 12000x g and 4°C for 20 min, the precipitate discarded. The supernatant was cooled down to 0°C in the ice box at below 0°C and ice cold acetone was added up to 25%. The sample was centrifuged as above and precipitate was discarded. Supernatant from previous step was brought to 70% acetone and centrifuged at 9,000x g and 4°C for 10 min, precipitate was dissolved in 200mL of buffer-A and dialyzed in the same buffer at 4 to 10°C. The dialysate was loaded onto 2.5 x 36 cm column packed with DEAE-Sephadex gel and equilibrated with buffer-A. Sample was applied at the flow rate of 3mL per min, column with bound proteins was washed with ice cold buffer-A. The bound proteins were eluted with 0.5M sodium chloride. Fractions with high specific activity were combined.
Measurement of Activity
Enzyme activity was measured by a procedure modified from the method described in literature (Laemmli, 1970). The spectrophotometer (SHIMADZU BioSpec-1601) was adjusted at λ–240nm and 25°C. Buffer solution containing 40-50mM hydrogen peroxide was added to the experimental and control cuvettes followed by incubation in spectrophotometer for 4-5 min. Enzyme dilution (10µL) was added to the experimental cell and change in the absorbance at 240nm was measured for 3 to 5min. The amount of hydrogen peroxide used per min was calculated by using Beer-Lambert law. One unit is the amount of enzyme which decomposes one micromole of H2O2 per min at 25°C.
Determination of Molecular Weight
Purified recombinant enzyme was analysed on SDS-PAGE (Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis) to determine purity and molecular weight (Srivastava et al., 1971). The quaternary structure of purified enzyme was determined by FPLC (Fast Performance Liquid Chromatography) based gel-filtration chromatography which was carried out with Superdex 200 10/300 GL column (GE Healthcare). The column, with a total volume of 25mL, void volume (VO) 8mL, bed volume 24mL, and particle size of 13μm was used. Sodium phosphate buffer (50mM), pH 7 containing 150mM NaCl was used as an elution buffer with a flow rate maintained at 300µL per min.
Physiochemical Properties
The effect of pH variation on the activity of catalase was determined by using buffer solutions of specific pH as assay medium (50mM sodium acetate buffer adjusted to 4 to 5.5 pH, 50mM sodium phosphate buffer adjusted to 6 to 8 pH, 50mM Tris-HCl buffer ranging from 8.5 to 10 pH). Temperature stability of enzyme was determined by incubation of enzyme dilution for 5 min at different temperatures ranging from 25 to 65°C followed by instantaneous measurement of activity. Effect of temperature on the enzyme activity was determined by adjusting the temperature of reaction mixture from 20 to 65°C. Variation in activity of enzyme was measured by linear increase in H2O2 concentration in the reaction mixture. KM (Michaelis constant) value of purified liver catalase was calculated by using Lineweaver-Burk plot. The purified enzyme was incubated with 10 to 50mM EDTA for 30min at 4°C to determine the effect of EDTA on the activity of enzyme.
Results
Catalase was purified from fresh liver tissue by selective acetone precipitations and ion-exchange chromatography. Most of the enzyme was precipitated between 25 to 65% acetone at 0°C. After complete dialysis, the enzyme was bound to the equilibrated DEAE-Sephadex column, when buffer-A was used as mobile phase. The combined purified
Table 1: Specific activity, percentage yield and fold purification of catalase at different stages of purification from the liver tissue of water buffalo (Bubalus bubalis)
|
Enzyme purification steps |
Total Units |
Total Protein content (g) |
Specific activity (U per mg) |
Percentage Yield |
Fold Purification |
|
|
Precipitations |
Crude Extract |
19338000 |
66.0 |
293.0 |
100 |
1 |
|
Supernatant of 25% acetone saturation |
18360000 |
56.1 |
327.2 |
94.9 |
1.11 |
|
|
Precipitate of 70% acetone saturation |
17700000 |
43.2 |
409.7 |
91.0 |
1.4 |
|
|
Dialysis |
Dialysate |
16033500 |
37.7 |
425.3 |
82.9 |
1.48 |
|
Chromatography |
Combined Fractions |
5899350 |
0.165 |
35753 |
30.5 |
123 |
One enzyme unit is the amount of enzyme which can catalyze the breakdown of one micromole of H2O2 per min at 25°C
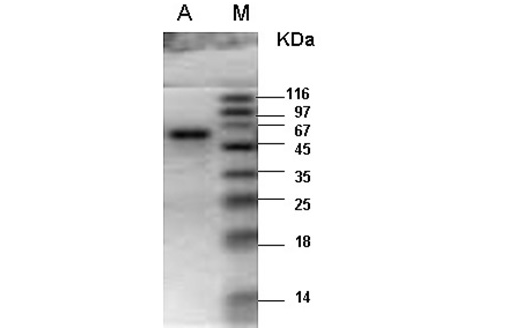
M- Protein marker; A- Catalase purified from the liver tissue of river buffalo displaying a single band at 60kDa.
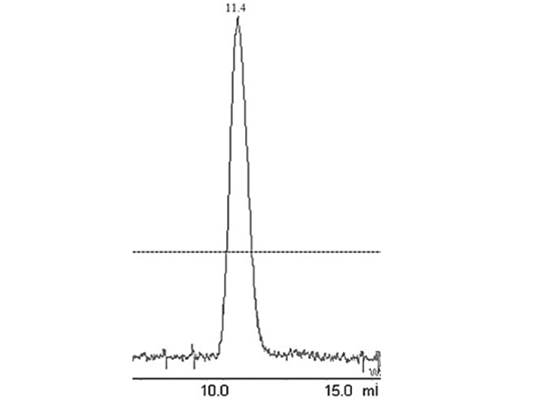
Figure 2: Elution peak of purified liver catalase on a gel-filtration Colum (G-200 Superdex performed with FPLC (AKTA purifier GE-Healthcare)
enzyme fractions had specific activity of 35753U per mg of enzyme sample. Final recovery was 30.5% with 123 folds purification (Table 1). The combined purified fractions displayed a single band at 60kDa on SDS-PAGE (Figure 1). Gel filtration column chromatography was used for the determination of quaternary structure of purified catalase.
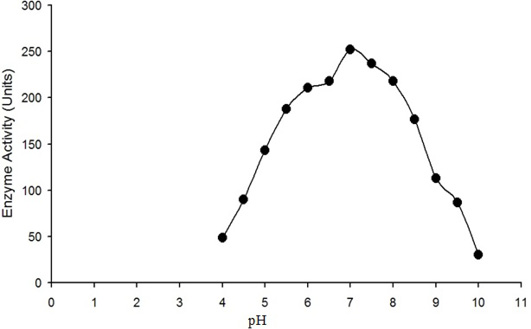
The enzyme activity (micromole of H2O2 converted into product per min) is plotted against the pH of reaction mixture. Maximum activity of purified water buffalo liver catalase was measured at pH 7.
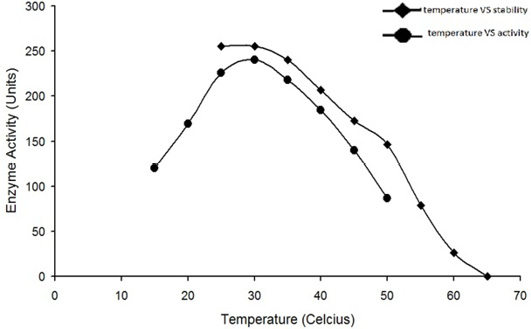
Optimum temperature for the activity of water buffalo liver catalase is at 30°C. The enzyme was quite stable while incubated at temperature up to 35°C for 5min; the enzyme activity was decreased with an increase in temperature beyond 35°C and it was completely lost by incubation at 65°C for 5min.
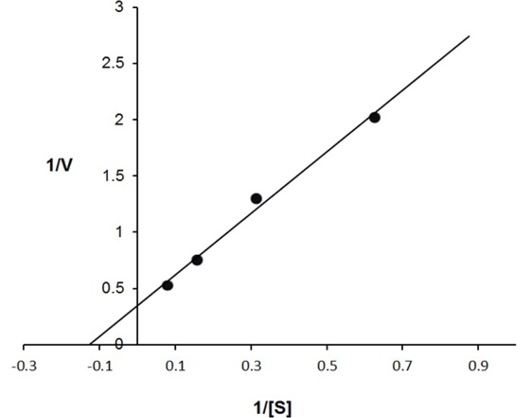
The enzyme eluted as a single peak with a retention volume of 11.4mL (Figure 2). The molecular weight calculated on the basis of these results was 240kDa indicating that the enzyme exists as a tetramer. Purified buffalo liver catalase retains activity at a wide range of pH with maximum activity at pH of 7 (Figure 3). Maximum activity of enzyme was measured at 30°C, it was rapidly inactivated by incubation at temperatures above 45°C and activity was completely lost by incubation at 65°C for 5 min (Figure 4). There was no significant change in the activity when the enzyme was incubated in 10 to 50mM EDTA for 30min at 4°C. The KM value of enzyme for hydrogen peroxide was calculated as 58mM by Lineweaver-Burk plot (Figure 5).
Inverse values of rate of reaction (V) and substrate concentration (mM of H2O2) are plotted against each other. The calculated value of KM is 58 mmoles of H2O2 for buffalo liver catalase.
Discussion
Catalase, purified from the liver tissue of river buffalo has been characterized for various physiochemical properties. A comparison of its characteristics has been made with that of enzyme from different species reported in the literature. Molecular weight of catalase investigated in the present study (240kDa) is similar to that reported from different species e.g. 240kDa from chicken erythrocytes (Aydemir and Kuru, 2003), 230kDa from dog liver (Nakamura et al., 2000), 240kDa from bovine liver (Prakash et al., 2002) and pig liver 241kDa (Arabaci et al., 2013). The specific activity of catalase described in the present study is comparable with that of enzyme reported in these studies from pig, bovine and dog liver. However, the purification method used in the present study provides better percentage recovery.
The purified enzyme has shown optimum activity at pH 7 at 30°C. Similar pH and temperature conditions have been reported for the maximum activity of catalase isolated from different species e.g. enzyme from goat liver exhibited an optimum pH 6.8 (Chatterjee et al., 1989), camel liver enzyme and the enzyme from black gram have shown highest catalysis rate at pH 7 (Kandukuri et al., 2012; Al-Bar, 2012), enzyme isolated from apple have exhibited an optimum pH 7.5 and that isolated from pig liver has an optimum pH 8 (Arabaci et al., 2013). The catalase from van apple has shown maximum activity at 50°C (Yoruk et al., 2005). The KM value of river buffalo liver catalase (58mM) is lower than that from goat liver (110mM) and bovine liver (93mM) (Kandukuri et al., 2012; Arabaci et al., 2013) indicating its greater affinity towards hydrogen peroxide. Present study reveals the first report on the purification and properties of liver catalase from river buffalo which is an unexplored species for its proteins and related genes. The enzyme isolated from the river buffalo offers a substitute for the bovine and porcine enzymes used in clinical laboratories.
Acknowledgements
Authors are grateful to the Higher Education Commission of Pakistan for funding this research.
Conflict of interest
The authors have no conflict of interests.
Authors’ Contribution
Nadeem MS being the main author, conducted the major research work in lab. Khan JA contributed in writing of the manuscript. Murtaza BN helped in sample processing and chromatography. Muhammad K contributeed in the spectrophotometric analysis of enzyme properties. Rauf A assisted in the article writing.
References